Back to Journals » Local and Regional Anesthesia » Volume 13
Successful Dental Treatments Using Procaine Hydrochloride in a Patient Afraid of Local Anesthesia but Consenting for Allergic Testing with Lidocaine: A Case Report
Authors Ayuse T, Kurata S , Ayuse T
Received 19 June 2020
Accepted for publication 6 August 2020
Published 20 August 2020 Volume 2020:13 Pages 99—103
DOI https://doi.org/10.2147/LRA.S268498
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Stefan Wirz
Terumi Ayuse,1 Shinji Kurata,2 Takao Ayuse1,2
1Nagasaki University Hospital, Department of Special Care Dentistry, Nagasaki, Japan; 2Nagasaki University Hospital, Department of Dental Anesthesiology, Nagasaki, Japan
Correspondence: Takao Ayuse
Nagasaki University Graduate School of Biomedical Science, Department of Translational Medical Sciences, Division of Clinical Physiology, Nagasaki, Japan
Tel +81-95-849-7714
Fax +81-95-849-7715
Email [email protected]
Background: We report a case in which effective dental anesthetic management was achieved using procaine hydrochloride for a patient who had an unknown history of allergic reactions to lidocaine.
Case Presentation: Because the patient refused to undergo screening tests using any of the amide-type local anesthetics because of her extreme fear against local anesthetics that she had been administered previously, procaine hydrochloride, which is an ester-form local anesthetic, was the only agent to be tested on this patient at the department of dermatology. Consequent to a negative allergy test, we performed complete dental treatment using procaine hydrochloride after additional chairside drug challenge tests using minimum test dose under vital sign monitoring.
Conclusion: The success of dental treatment using procaine hydrochloride may have relieved the patient’s fear of local anesthesia. We discuss an important aspect of treatment planning for patients with a history of complications during local anesthesia.
Keywords: allergic reaction, amide-type local anesthesia, procaine hydrochloride, dental fear, dental anxiety, local anesthesia, complication
Background
Immediate or delayed allergic reactions can occur against amide local anesthetics, such as lidocaine and propitocaine (prilocaine),1–5 although allergic reactions to amide-type local anesthetics are extremely rare. Ester-type local anesthetics are more likely to cause an allergic reaction compared to amide-type local anesthetics and are therefore not usually the first choice in dentistry.6,7 However, a previous study suggested that individuals allergic to lidocaine could tolerate ester-type anesthetics.8
Herein, we report a case in which effective dental anesthesia was achieved using procaine hydrochloride in a patient who had an unknown history of allergic reactions to amide-type local anesthetics, ie, lidocaine and propitocaine (prilocaine). Furthermore, we discuss the effective control of pain perception in patients with an unknown history of allergy to amide-type local anesthetics during dental treatment.
Case Presentation
In this case presentation, approval from the Nagasaki University Hospital review board was not required and written informed consent was obtained from the patient for publication of this case report.
A 30-year-old woman was referred to the department of special care dentistry at Nagasaki University Hospital for dental treatment because she had experienced frequent episodes of nausea and unconsciousness during dental treatment after the administration of widely used amide-type local anesthetics at dental clinics. When the patient visited the department of special care dentistry at the Nagasaki University Hospital, a medical examination and screening test were required to identify an available non-allergic local anesthetic through the algorism of clinical guidelines for patients with a history of allergic reactions because the possibility of allergic reaction to local anesthetics could not be completely ruled out. However, as she refused to undergo screening tests using any of the amide-type local anesthetics she had been administered previously, procaine hydrochloride, which is an ester-form local anesthetic, was the only agent tested on her at the department of dermatology. At the completion of the test, she was informed that the test with 1% procaine hydrochloride had not shown any abnormal reaction. After obtaining informed consent, she was scheduled to undergo a drug challenge test using minimum test dose (0.1 mL) for gingival mucosa and dental treatment using 1% procaine hydrochloride under careful monitoring of vital signs.
Course of Treatment (Preparation of Specially Made Drug)
Because of the lack of commercially available prefilled cartridges of the desired agents, we consulted with the hospital pharmacy on the method of preparation of procaine hydrochloride 10 mg/mL with 0.005 mg of adrenaline (adrenaline equivalent to a concentration of 1:200,000). As suggested by the pharmacy staff, we added 1 drop of adrenaline to 1 mL of procaine hydrochloride (10 mg/mL) using a 27-G needle to add 5 μg of adrenaline immediately before administering it. After confirming no side-effects of the drug challenge test using a minimum test dose (0.1 mL) for gingival under mucosa topical anesthesia using ethyl aminobenzoate, 5 minutes later, we provided an infiltrated initial dose of 0.5 mL of procaine hydrochloride into the gingival mucosa for resin restoration of the anterior teeth. Treatment was started after confirming the analgesic effect, and if pain was reported, an additional dose was planned to be added as appropriate. However, dental treatment was completed because the first initial dose of total 0.6 mL provided a sufficient analgesic effect.
Blood pressure, heart rate, and arterial oxygen saturation were monitored to evaluate her overall general status during the treatment. No abnormalities of the vital signs were evident 10 minutes after inducing local anesthesia. No abnormalities were identified before the end of the treatment, and the sensation of paralysis in the anesthetized region lasted for 55 minutes. The patient was subsequently scheduled for approximately ten treatments under anesthesia with procaine hydrochloride to complete the initial treatment plan (Table 1).
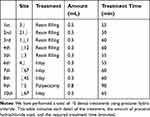 |
Table 1 Detail of All Dental Treatment with Procaine Hydrochloride |
At the 6-month follow-up of the treatment at the outpatient center of the department of special care dentistry, the patient complained of the failure to conceive as a result of the inability to use local anesthesia for possible surgical treatments. We explained the mechanisms of allergic reactions to local anesthetics and recommended that she undergo another intradermal test at the dermatology department. She understood that amide-type local anesthetics could be used for the allergic screening test because ester-type local anesthetics, such as procaine hydrochloride, which we had used, are generally considered more likely to cause allergic reactions based on medical reports.
One year after the initial treatment, the patient requested a screening test using Patch test and scratch testing at the dermatology department of the hospital. Lidocaine hydrochloride (Xylocaine cartridge®; lidocaine hydrochloride 36 mg/1.8 mL with adrenaline [0.0125 mg/mL] 1:80,000) and propitocaine hydrochloride (Citanest Octapressin®; prilocaine 30 mg/mL with feripressin [0.045 unit]) and Methyl 4-hydroxybenzoate showed only mild erythema on scratch testing, but adrenaline did not show erythema symptoms. Therefore she was informed that lidocaine hydrochloride (lidocaine injection as a single agent) without any vasoconstrictor plus preservative or procaine hydrochloride would be safe for her. The dermatologist also pointed out that she showed hypersensitivity to pain because of vagotonia during the test.
Seven years later, she re-visited our hospital for the treatment of pain in the mandibular right second molar. At the time, an improved form of lidocaine hydrochloride was prepared after removing the preservative methyl-4-hydroxybenzoate (methylparaben) had become available. Therefore, we conducted screening tests for four types of lidocaine hydrochloride among amide-type local anesthetics. All results from scratch and intradermal tests for lidocaine hydrochloride, except prilocaine hydrochloride, were negative. After she acquired sufficient information on the features of each agent, she opted to undergo local anesthesia using mepivacaine hydrochloride. However, infiltration of 7 mL of mepivacaine hydrochloride failed to provide a pain-free condition. After obtaining her consent, we added 2 mL of the new type of lidocaine hydrochloride (ORA injection cartridge®), allowing successful hemi-section of the mandibular right second molar.
Discussion and Conclusion
When an abnormality of the overall general status is found during local anesthesia in dentistry,3,7,9 the patient is often referred to the university hospital with the suspicion of allergic reactions to the local anesthetic. Although we carefully recorded the patient’s medical history, it was difficult to obtain a precise diagnosis for her allergic reactions to local anesthetics. In this case, vasovagal syncope could be diagnosed based on her symptoms, such as transient nausea and fainting. Medical examination and screening tests are required to identify an available non-allergic local anesthetic through the algorism of clinical guidelines for patients with a history of allergic reactions because the possibility of allergic reactions to local anesthetics could not be completely ruled out. Based on her request, we could only test for the rarely used ester-type local anesthetics, so procaine hydrochloride was the only option. Ester-type local anesthetics are not usually used in dental clinics, but might be considered for pain relief during dental treatment. Individuals allergic to lidocaine can tolerate ester-type local anesthetics.8 Our experience in this case suggests that ester-type local anesthetics are useful for patients with a history of allergic reactions to amide-type local anesthetics.
The risk of allergic reactions to added injection solutions might be greater compared to the local anesthetic alone.10 If the patient has a history of suspected allergic reactions, ordinary local anesthetics should be avoided because most agents contain an antioxidant agent and a bacteriostatic agent for unstable added vasoconstrictors. When we used procaine hydrochloride, we added adrenaline immediately before infiltration into the mucosa, avoiding the need for preservative agents. We added adrenaline to procaine hydrochloride at a concentration of 1:200,000, achieving infiltration anesthesia with 0.3–0.5 mL for crown restoration. An effect time of approximately 90 minutes was obtained with the infiltration of 0.8 mL for pulpectomy, which takes approximately 60 minutes.
Notably, mepivacaine hydrochloride (Scandonest cartridge 3%®) has gained popularity and recognition for use with fewer allergic reactions for clinicians because of the lack of vasoconstrictor agents. Dermatologists often select mepivacaine hydrochloride in screening tests for local anesthesia for patients with a history of allergic reactions to the conventionally available agents in both medical and dental surgical procedures. We have encountered other patients who have previously undergone dental treatment without local anesthesia because of systemic symptoms of allergic reactions; the use of mepivacaine hydrochloride for these patients enabled the provision of comfortable dental treatment under safer local anesthesia. Intradermal testing is performed in the department of dermatology for the precise detection of potential allergens. A patient with a history of allergic reactions to a particular ester-type local anesthetic would have a higher possibility of allergic reactions to other ester-type local anesthetics.11
An important aspect of treatment planning for patients with a history of complications of local anesthesia should be considered if the patient would sufficiently cooperate during the planned treatments to obtain acceptable treatment results. Because she was satisfied with the safe management and was confident for future dental treatments with higher motivation, we could successfully propose better management with other local anesthetics, including lidocaine. Several commercially available amide-type local anesthetics without methyl-4-hydroxybenzoate, including lidocaine hydrochloride, are now available in Japan. With several choices and updated knowledge on local anesthesia in dentistry and medicine, clinicians can avoid mental distress to patients, such as issues of risk from pregnancy or medical procedures involving severe pain. However, well-trained dentists should take responsibility for performing drug challenge tests under monitoring of vital signs because interpretation of results obtained from screening tests for the desired clinical safety may be difficult. In consideration of a history of complications or unpredictable allergic reactions to local anesthesia, we should carefully consult with patients and try to find out better proposals by reducing patients’ fear and anxiety against local anesthesia.
In this case, we observed that infiltration of 7 mL of mepivacaine hydrochloride failed to provide a pain-free condition. We do not explain why adding 2 mL of 2% lidocaine hydrochloride (ORA injection cartridge®) on initial dose of 7 mL of 3% mepivacaine hydrochloride can produce an adequate pain free condition. If we consider potent of four local anesthesia used in this case, potent would be similar level depend on concentration. We speculate that the supplementation of adrenaline for local anesthesia without preservative might be appropriate and an effective drug type for greatly invasive and painful procedure (Table 2).
 |
Table 2 Overview of Local Anesthetics Used in This Case |
Conclusions
The success of dental treatment using procaine hydrochloride may have relieved the patient’s fear against local anesthesia. We should strongly consider how it is important to make a treatment plan for patients with a history of complications during local anesthesia.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Fuzier R, Lapeyre-Mestre M, Mertes PM, et al. Immediate- and delayed-type allergic reactions to amide local anesthetics: clinical features and skin testing. Pharmacoepidemiol Drug Saf. 2009;18(7):595–601. doi:10.1002/pds.1758
2. Noormalin A, Shahnaz M, Rosmilah M, Mujahid SH, Gendeh BS. IgE-mediated hypersensitivity reaction to lignocaine - a case report. Trop Biomed. 2005;22(2):179–183.
3. Sambrook PJ, Smith W, Elijah J, Goss AN. Severe adverse reactions to dental local anaesthetics: systemic reactions. Aust Dent J. 2011;56(2):
4. Evans LA, Pointing J, Wills EJ, Adelstein S, Michalopoulos J. Recurrent facial swelling following dental procedures. Med J Aust. 2002;177(9):522. doi:10.5694/j.1326-5377.2002.tb04928.x
5. Mackley CL, Marks JG
6. Sidhu SK, Shaw S, Wilkinson JD. A 10-year retrospective study on benzocaine allergy in the United Kingdom. Am J Contact Dermat. 1999;10(2):57–61. doi:10.1016/S1046-199X(99)90000-3
7. Speca SJ, Boynes SG, Cuddy MA. Allergic reactions to local anesthetic formulations. Dent Clin North Am. 2010;54(4):655–664. doi:10.1016/j.cden.2010.06.006
8. Melamed J, Beaucher WN. Delayed-type hypersensitivity (type IV) reactions in dental anesthesia. Allergy Asthma Proc. 2007;28(4):477–479. doi:10.2500/aap.2007.28.3020
9. Tomoyasu Y, Mukae K, Suda M, et al. Allergic reactions to local anesthetics in dental patients: analysis of intracutaneous and challenge tests. Open Dent J. 2011;5:146–149. doi:10.2174/1874210601105010146
10. Eggleston ST, Lush LW. Understanding allergic reactions to local anesthetics. Ann Pharmacother. 1996;30(7–8):851–857. doi:10.1177/106002809603000724
11. Harper NJ, Dixon T, Dugue P, et al. Suspected anaphylactic reactions associated with anaesthesia. Anaesthesia. 2009;64(2):199–211. doi:10.1111/j.1365-2044.2008.05733.x
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
