Back to Journals » International Journal of General Medicine » Volume 15
Subtotal Laryngectomy with Epiglottic Reconstruction for Glottic Carcinoma: A Single Institutional Experience
Authors Le Minh K , Nguyen Dinh P, Doan Thi Hong N, Pham Van H, Nguyen Xuan Q, Nguyen Xuan H, Nguyen Thi To U
Received 22 November 2021
Accepted for publication 8 February 2022
Published 1 March 2022 Volume 2022:15 Pages 2321—2328
DOI https://doi.org/10.2147/IJGM.S350624
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Ky Le Minh,1 Phuc Nguyen Dinh,2 Nhat Doan Thi Hong,3 Huu Pham Van,4 Quang Nguyen Xuan,4 Hoa Nguyen Xuan,5 Uyen Nguyen Thi To2
1Vietnam National University, Hanoi (VNU), National Otorhinolaryngology Hospital of Vietnam, Hanoi, Vietnam; 2ENT Department, Hanoi Medical University, Hanoi, Vietnam; 3ENT Department, Vinh Medical University, Nghe An, Vietnam; 4Department of Head and Neck Surgery, National Otorhinolaryngology Hospital of Vietnam, Hanoi, Vietnam; 5ENT Department, Vietnam University of Traditional Medicine, Hanoi, Vietnam
Correspondence: Ky Le Minh, Department of Otolaryngology, Head and Neck Surgery, Vietnam National University, Hanoi (VNU), National Otorhinolaryngology Hospital of Vietnam, 144 Xuan Thuy-Cau Giay District, Hanoi, Vietnam, Tel +84 4-37450188, Fax +84 4-37450146, Email [email protected]
Aim: Laryngeal cancer is a common form of head and neck cancer in Vietnam where the current treatment is surgery. Subtotal laryngectomy with epiglottic reconstruction, a conservative surgery, allows removal of anterior commissure including thyroid cartilage and paraglottic space and provides a maximum restoration of the anatomical structure of the larynx.
Purpose: To evaluate the results, the safety and effectiveness of patients who were treated with subtotal laryngectomy with epiglottic reconstruction.
Material and Method: From January 2012 to July 2017, 42 patients (41 male, 1 female, median age 55.6 years, range 38– 75 years) were diagnosed with glottic carcinomas at Vietnam National ENT Hospital, where they underwent a subtotal laryngectomy with epiglottic reconstruction.
Results: Thirty-one patients (73.8%) had T2 glottic carcinoma, 4 (9.5%) T3 glottic carcinoma, and 12 (25.6%) had neck dissection. The arytenoid cartilage on the tumor-bearing side was resected in 11 patients (26.2%). Functional ipsilateral neck dissection was performed in 30 patients. Positive lymph node of stage T2 was 1/31 (3.2%). Postoperative histopathologic examination showed a tumour free of resection margin in 41 patients (97.6%). Only one post-operative complication occurred with bleeding 24 hours after surgery. There was no mortality. The 3- and 5-year overall survival rates were 97.6% and 85.7%, respectively. The rate of local control was 92.9%.
Conclusion: Subtotal laryngectomy with epiglottic reconstruction was performed mostly for T2 and certain T3 glottic carcinomas when there is difficult to safely remove the tumour with transoral laser microsurgery. This surgery appears to be effective for the overall survival and has potential in clinical practice for treating moderate glottic carcinoma.
Keywords: partial laryngectomy, subtotal laryngectomy, moderate glottic carcinoma, epiglottic reconstruction
Introduction
Laryngeal cancer is a common form of head and neck cancer in Vietnam, where smoking and drinking is highly prevalent, especially in mountain and rural areas.
According to statistics published by the WHO Cancer Country Profile-Viet Nam in 2014, 45.5% of all males in Vietnam are smokers and 1.7% of females.1
There are various treatment modalities for early moderate glottic cancer including radiotherapy, transoral microsurgery with or without laser, or open partial laryngectomy. In the context of laryngeal cancer, partial laryngectomy intends to provide complete surgical resection in compliance with oncological principles while maintaining deglutition, phonation, and respiration.
For moderate glottic cancer with impaired vocal fold mobility of paraglottic space extension or anterior commissure extension, it is difficult to safely remove the tumour with transoral laser microsurgery. In these cases, we performed subtotal laryngectomy with epiglottic reconstruction.
We performed subtotal laryngectomy with epiglottic reconstruction employing two different techniques of Tucker’ frontal-anterior partial laryngectomy2 and supracricoid laryngectomy with cricohyoidoepiglottopexy (Majer-Piquet surgery).3 We performed a preservation of posterior part of the laryngeal structure and a reconstruction of the framework of larynx in this technique which is indicated for T1b, T2 and certain T3 glottic carcinomas. In these cases, there was a moderate extension into the anterior commissure or the paraglottic space or susglottis, subglottis, which meant that this type of tumour could no longer be safely removed using transoral laser microsurgery.
The purpose of this study was to evaluate long-term survival, safety, and effectiveness of using this surgical approach to treat patients with glottic carcinoma.
Materials and Methods
We performed a retrospective study of 42 patients who were diagnosed with glottic carcinoma between January 2012 and July 2017 and underwent subtotal laryngectomy with epiglottic reconstruction. There were 41 males and 1 female patient with a median age of 55.6 years (range 38–75 years).
All patients were preoperatively examined by suspension laryngoscopy and were endoscopically examined using 30° and 70° telescopes to assess the possible involvement of the ventricles, the anterior commissure, and the subglottic region. No patient had received any previous radiation therapy or surgical procedure.
Patients were staged according to TNM staging of head and neck cancer and neck dissection classification, established by the American Joint Committee on Cancer (AJCC) seventh edition.4
Indications for subtotal laryngectomy with epiglottic reconstruction:
• glottic tumours classified as T1b or T2 based on extension into the ventricle, false vocal cord, petiole of epiglottis, anterior aspect of the arytenoid cartilage, without or with impaired true vocal cord.
• selected glottic tumors classified as T3 based on extension to paraglottic space, impaired mobility or fixation of the true vocal cord without fixation of the arytenoid cartilage.
We only included patients with squamous cell carcinoma.
Contraindications for subtotal laryngectomy with epiglottic reconstruction:
• Patients older than 75 years old.
• Patients with poor cardiac or pulmonary function.
Surgical Procedure
Patients were intubated with a tracheal tube, and a nasogastric feeding tube was passed. First, we performed a tracheostomy at the third tracheal ring while exposure of the larynx was carried out by a separate horizontal skin incision above the cricothyroid membrane. If a neck dissection needed to be performed ipsilaterally, a J-shaped incision was made; and if a bilateral dissection of the neck needed to be performed, then a U-shaped incision was made. The skin flap was raised in the subplatysmal plane to the level of the hyoid bone. The soft tissues in front of the larynx were removed while the Delphian lymph node was removed and sent for histopathological analysis. The strap muscles were separated from the midline. The attachments of the sternothyroid and the thyrohyoid muscles on the oblique line of the thyroid cartilage were preserved.
Prior to surgery, all patients were reassessed using rigid endoscopy (viewing angles of 0° and 30°) to check tumour involvement and identify a safe location to cut the cartilage while maintaining maximal cartilage frames.
The laryngotomy began with an incision of the cricothyroid membrane at the superior edge of the cricoid. At this stage, after checking that the tumour did not extend into the subglottis up to 5 mm free from cricoid cartilage, the thyrohyoid membrane and epiglottis were sectioned with an electric knife along the superior margin of the thyroid cartilage. The resection of the thyroid cartilage began on the less affected side. The cartilage was cut with scissors or a saw at the middle third of the thyroid cartilage that location was calculated at least 3 mm free from the margin of tumour. The vocal cord and the ventricular fold were cut in same plane with thyroid cartilage cut (Figure 1). Then, the thyroid cartilage was retracted with a hook, allowing complete visualization of the endo-laryngeal structures. On the more affected side, the cartilage section was cut more posteriorly. The paraglottic space was dissected and removed (Figure 1). The arytenoid on the affected side was removed if tumour involvement was present. Histological examination was performed to confirm that the resection margins were disease free.
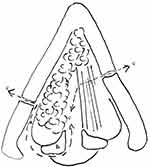 |
Figure 1 Limits of the resection. (a) Paraglottic space. (b) Arytenoid cartilage. (c) Excision planes. |
Epiglottis was released by cutting the hypoepiglottic and lateral glosso-epiglottic ligaments via blunt scissor dissection. When the epiglottis was pulled down to the upper border of the cricoid arch, the lower part of the dissected epiglottis was sutured to the upper edge of the cricoid. Crico-epiglottopexy required three 1/0 vicryl sutures, looped around the cricoid. Cut mucosa on the lateral sides of the epiglottis was sutured to the remaining perichondria of the resected lateral section of the thyroid cartilage. The strap muscles were sutured in the midline, a drainage was placed beneath the platysma, and the skin was closed in two layers. A cuffed tracheostomy tube was inserted and a dressing applied.
Antibiotics were given post-operatively and the drainage was removed on the second day. A non-cuffed tracheostomy tube was inserted on day 2, allowing the patient to breathe through mouth. On day 5, postoperative rehabilitation began including speech and swallowing therapy. Once swallowing motion was satisfactory, the nasogastric feeding tube was removed and decannulation achieved via progressive plugging of the fenestrated tube until it could also be removed. Voice rehabilitation was conducted at home.
Kaplan–Meier analysis was used to evaluate overall survival, local control, and nodal control. A parametric Chi squared test was used for the analysis of qualitative and quantitative variables. A p-value below 0.05 was considered statistically significant. All functional results are expressed as mean ± one standard deviation.
Results
Forty-two patients were included in the analysis (41 men (97.6%), 1 woman (2.4%), mean age of 56.60 years old, range 38–75y).
The majority of patients had a history of both smoking tobacco and drinking alcohol (57.14%), while 90.5% were only smokers and 61.9% were only consuming alcohol. Two patients (4.76%) were neither smokers nor drinking alcohol.
For most patients (n = 31, 73.8%), this type of surgical indication was for T2N0M0 tumors, ie, tumours extending into the ventricle, the false vocal cord, the petiole of epiglottis, the vocal process of the arytenoid cartilage, with or without the impaired true vocal cord. There were seven cases at the stage T1bN0M0 (16.7%) involving the anterior commissure, partially reaching the opposite cord or covering both cords, or extending from the anterior commissure without impairing the movement of the cords. There were four cases of T3N0M0 tumors (9.7%) with paraglottic extension and impaired mobility or fixation of the true vocal cord without fixation of the arytenoid cartilage.
The arytenoid cartilage on the tumor-bearing side was resected in 11 patients (26.2%), and both arytenoid cartilages were preserved in the remaining 31 patients (73.8%). A functional ipsilateral neck dissection was performed on 30 patients and one case with nodal involvement in neck dissection specimen was noted. All patients had squamous cell carcinoma with glottic localization. Nodal involvement in T1b tumours was 0/7 (0%), in T3 it was 0/4 (0%), and in T2 it was 1/31 (3.2%). Postoperative histopathological examination showed that 41 patients (97.6%) had tumour-free margins, while one patient presented a positive surgical margin.
The overall functional results of subtotal laryngectomy were satisfactory, with all our patients being decannulated, a preserved swallowing function. All patients underwent successful decannulation. The average time until decannulation was 21,21+-8,8 days ranging from 12 to 54 days. No patient was limited in their performance of daily activities due to breathing difficulties; however, 2 patients needed laser treatment to resect mucous flap; one patient underwent laryngeal edema after radiation, has a temporary tracheostomy and lately decannulated. Postoperative adjuvant radiotherapy was performed in 9 cases due to either positive margin, a positive cervical lymph node, a significant tumoral extension in the subglottic or paraglottic space observed during procedure.
No patient died from complications in post-operative period. No patient had aspiration pneumonia. One case of bleeding occurred within 24 hours of the procedure requiring emergency surgery.
The mean follow-up period was 70.1 ± 2.4 months (95% ranging from 65.3 to 75.0 months).
The 3- and 5-year overall survival rates were 97.6% and 85.7%, respectively (Figure 2).
 |
Figure 2 Kaplan-Meier analysis for overall survival of the whole population. |
The stage of the glottic carcinomas had no statistically significant effect on overall survival (Table 1).
 |
Table 1 Overall Survival by Tumor Stage |
Comparing the overall survival rates of patients with and without adjuvant radiotherapy, there was a significant difference on the mean survival time, the mean survival times was of 57.0 ±7.7 and 73.5 ± 1.7 months, respectively (p = 0.011) (Table 2).
 |
Table 2 Overall Survival for Surgery vs Surgery Plus Adjuvant Radiotherapy |
There was one patient with a local recurrence and one with a nodal recurrence, achieving a local control rate of 95%. One patient had distant metastasis without local recurrence (33.33%).
Discussion
According to Yağız et al5 the average age of patients undergoing Tucker’s laryngectomy is 56.5 years old (range 40–78y). A patient age above 75 y, poor cooperation, and cardiac or pulmonary disease all are contraindications for Tucker’s laryngectomy.2,6 This surgery can also be performed on older patients, but their respiratory and cardiovascular parameters must be taken into account. Indeed, for T2 glottic carcinomas, our therapeutic choice depended on the age of the patient. In patients older than 75y, we either performed a transoral laser microsurgery as this procedure avoids some of the postoperative complications encountered with subtotal laryngectomy, or we referred them to radiotherapy. For younger patients, whose functional recovery is more rapid and complete, subtotal laryngectomy with epiglottic reconstruction may be preferable.
T2 glottic squamous cell carcinomas form a very heterogeneous group of lesions in terms of extension. As a result, they differ greatly in treatment indications and prognosis. In our 42 cases, indication was mostly for T2N0M0 tumours (n = 31, 73.8%) with only few T3 (n = 4, 9.5%). For Tucker’s laryngectomy, Pech et al7 reported a total of 12 cases, 6 of which were T2N0M0, 1 case for T1bN0M0, and 1 case for T3N0M0. In a study by Zanaret et al,8 68 out of 137 cases were T2N0M0 with no case of T3N0M0. For conventional Mayer-Piquet’s laryngectomy, Guerrier et al9 reported a total of 58 patients, with 19 T2N0M0 and 3 T3N0M0. A study by Laccourrey et al10 included 36 patients, most were T2N0M0 and only 1 case was T3N0M0.
In 1979, Tucker et al described the anterior partial laryngectomy with epiglottic reconstruction for treatment of early glottic carcinoma.2 This technique allows removal of the anterior commissure with thyroid cartilage, anterior part of both true and false vocal cords, and 1 cm of subglottis, conventionally applied for T1 glottic carcinoma. According to Yağız et al5 the presence of impaired vocal mobility, subglottic extension being over 1 cm anteriorly and 0.5 cm laterally, and over-involvement of ventricular folds or anterior commissure are contraindications for Tucker’s technique.
In the Majer-Piquet’s procedure the resection includes the entire thyroid cartilage, requiring freeing of the mucosa of the piriform sinus and reconstruction is made using fixed knots of cricoid cartilage, hyoid bone and epiglottis (CHEP) so laryngeal framework no longer exists.
In subtotal laryngectomy with epiglottic reconstruction, the resection is modified, so that the posterior part of the thyroid cartilage is retained. The amount of thyroid cartilage attached to the tumor that is resected, differs for each patient to ensure maximal preservation of the safe parts of the larynx, reconstruction by epiglottic flap is performed so laryngeal framework is preserved. In our study, the most frequent indication of subtotal laryngectomy with epiglottic reconstruction was stage T2N0M0, which is similar as for conventional Majer-Piquet surgery.8,10
Organ-preservation laryngeal surgery has been defined as a combination of procedures that remove a portion of larynx while maintaining the physiological functions of speech, swallowing, and respiration without compromising local control and cure rates. Many surgical techniques were reported such as frontolateral laryngectomy, hemi-glottectomy, reconstructive supracricoid laryngectomy and transoral CO2 laser microsurgery.
Laser microsurgery has been widely used in T1 tumours. However, for T2 tumours, the indication is more controversial, especially in the cases of the tumour extending to the anterior commissure or the paraglottic space. Several authors suggested that the involvement of the anterior commissure is a contraindication for CO2 laser surgery.3,6 This is supported by the considerable difficulties encountered when seeking a correct endoscopic exposure of this region. Furthermore, this is exacerbated by these tumours’ ability to infiltrate the thyroid cartilage at the anterior commissure, which is without perichondrial protection, and to invade the supraglottic and/or subglottic regions. Invasion of the anterior commissure, arytenoids, or paraglottic space decreases local control rate to approximately 75%.3 Peretti found a significant decrease in local control with transoral laser resection for T2 tumours extending into the paraglottic space.11 These findings support the use of open partial laryngectomy as the best means of preserving laryngeal function in selected patients. Nowadays in our department, preservation laryngeal surgery for glottic cancer is focused on two techniques: transoral laser microsurgery for early glottic carcinoma and subtotal laryngectomy with epiglottic reconstruction for moderate glottic carcinoma in general, indications for each surgery became clearer.
We agree with previous study that in order to achieve favourable survival outcomes associated with laryngeal preservation surgery as either primary or salvage therapy, the surgeon must have an in-depth understanding of the indications and contraindications for each procedure. Thus, a careful preoperative laryngeal examination is paramount.12 However, we do not use stroboscopy to evaluate tumour spread. Performing a CT scan and tumour assessment in the operation room just before surgery using rigid endoscopy (viewing angles of 0° and 30°) helps to determine the extension of the tumour and decide whether this surgery can be performed and to calculate the proper location of thyroid cartilage section (Figure 1). In fact, the preoperative CT scan plays an important role in assessing invasion to thyroid cartilage at the anterior commissure and paraglottic space, while direct endoscopy yields the actual tumour extension.
We have performed a concurrent functional ipsilateral neck dissection in those cases with supraglottic or subglottic involvement even when lymph nodes were not palpable because of the tendency for lymphatic spread in this region. The neck dissection was performed prior to subtotal laryngectomy.
The overall functional results of subtotal laryngectomy in our study were satisfactory, with all our patients being decannulated and the recovery of swallowing was achieved, but the possibility of aspiration pneumonia and significant difficulties in swallowing for older patients must be considered. Recently, Spirano described minimal invasive lateral cervical approach among open partial laryngectomy, the anterior myocutaneous (AMC) flap is performed, to maintain the trophism of the anterior region of the neck.13,14 This is the trend of minimal invasive surgery that can improve the outcomes of functional results.
The 5-year overall survival rates for Tucker’s and Majer-Piquet’s techniques range between 82% and 95%, respectively.7,12,15,16 The 3-year and 5-year overall survival rates at our study were 97.6% and 85.7%, respectively. Several studies have shown that lymph node metastasis or presence of unclear surgical margin decreases survival and that patients with no clear surgical margins need a close follow-up.6 The tumoral margins in our series were relatively safe, with only one patient having had positive surgical margin.
Currently, although there is a tendency to use transoral laser surgery and radiotherapy as an initial treatment for early glottic carcinoma, in case of anterior commissure involvement this approach should be considered with caution due to the risk of local recurrence. In 2009, Rödel et al6 reported that anterior commissure involvement caused a decrease in 5-year local control rates for T1a tumors 73% vs 89% and T1b tumors 68% vs 86%.
The advantage of this surgery as well as Tucker’s surgery is the complete removal of the anterior commissure and the anterior thyroid cartilage, hence the marginal section is safe in case the tumour invaded the anterior commissure. In the series by Yağız,5 11 out of 39 T1 tumors had reached the anterior commissure, while the remaining 28 involved the anterior commissure indicating Tucker’s procedure.
When comparing survival rate of T2N0 laryngeal cancer patients treated with radiotherapy or surgery, the 5 years survival rate for radiation was 59% and 68% for preserving laryngeal surgery.17 In a study of Pantel including 73 patients with T2 laryngeal cancer treated by surgery with or without radiotherapy, 2-year overall survival was 85.1% dropping to 56.5% after 5 years.18
The occurrence of postoperative complications not only depends on the general health status of patients, the stage of the disease, the level of experience of the surgeon, but also on the surgical methods and facilities. A retrospective study by Ganly et al19 including 150 patients with haemodialysis due to anaemia and operated squamous cell carcinoma of the larynx showed postoperative complication rates of up to 11%.
Generally, patients with subtotal laryngectomy are closely monitored for complications after surgery. In our study, we only noted one type of early complication, namely laryngeal bleeding on day 10 (n = 1) due to laryngeal pseudomembrane flaking off. Respiratory and bleeding complications are the most dangerous complications encountered with subtotal laryngectomy and other techniques of partial laryngectomy. Laryngeal bleeding can cause obstruction of the airways, which needs to be managed properly and promptly, requiring good post-op monitoring and well-trained medical staff.
Other complications (eg, dyspnoea, subcutaneous emphysema, infection, aspiration pneumonia) did not occur in our study.
Conclusion
Subtotal laryngectomies with epiglottic reconstruction can be performed for glottic cancer with moderate extension to the anterior commissure or the paraglottic space when there is difficult to safely remove the tumour with transoral laser microsurgery. There is a good functional recovery with acceptable morbidity and oncologic outcome when strict selection criteria are applied. This study demonstrated that subtotal laryngectomy with epiglottic reconstruction is effective, safe, and may be suitable for managing T2 and certain T3 glottic cancers.
Statement of Ethical Approval
I confirm I have obtained ethical approval from an ethics review board of National Otorhinolaryngology Hospital of Vietnam, to conduct the study, as well as permission from the dataset owner to use the information in databases/repositories for the purposes of the research.
This is a retrospective review, I confirm that the patient data was anonymized or maintained with confidentiality and that this study was conducted per the declaration of Helsinki.
Acknowledgments
The authors would like to express sincere thanks to the participants in this study. The authors also thank the medical and nursing staff for their assistance in performing this surgery. Thanks to Dr. Stefanidis Constantin and Dr. Anh-Dung Hoang, Erasme Hospital, Bruxelles, for their helpful discussion.
Disclosure
The authors declare that they have no potential conflicts of interest for this work.
References
1. World Health Organization. Cancer Country Profile-Viet Nam. Vol. 2; 2014.
2. Tucker HM, Wood BG, Levine H, Katz R. Glottic reconstruction after near total laryngectomy. Laryngoscope. 1979;89(4):609–618. doi:10.1288/00005537-197904000-00010
3. Piquet JJ, Darras JA, Berrier A, Roux X, Garcette L. Functional subtotal laryngectomies with cricohyoidopexy. Technics, indications, results. Ann Otolatyngol Chir Cervicofasc. 1986;103(6):411–415. PMID: 3538977.
4. American Joint Committee on Cancer. Cancer Staging Handbook.
5. Yağız R, Taş A, Uzun C, et al. Frontal anterior laryngectomy with epiglottic reconstruction (Tucker’s Operation): oncologic and functional results. Balkan Med J. 2012;29:77–83. doi:10.5152/balkanmedj.2011.025
6. Rödel RM, Steiner W, Müller RM, Kron M, Matthias C. Endoscopic laser surgery of early glottic cancer: involvement of the anterior commissure. Head Neck. 2009;31(5):583–592. doi:10.1002/hed.20993
7. Pech A, Cannoni M, Abdul S, Zanaret M, Thomassin JM, Goubert JL. Reconstructive anterior frontal laryngectomy. Ann OtonaryngolChirCervicofac. 1982;99(3):141–146. PMID: 6484339.
8. Zanaret M, Giovanni A, Gras R, Bonnefille E, Robert D, Cannoni M. Reconstructive anterior frontal laryngectomy. Long-term results in T2 glottic cancers. Ann Otolaryngol Chir Cervicofac. 1995;112(5):205–210. PMID: 7503499.
9. Guerrier B, Lallemant JG, Balmigère G, Bonnet P, Arnoux B. Our experience in reconstructive surgery in glottic cancers. Ann Otolaryngol Chir Cervicofac. 1987;104(3):175–179.
10. Laccourreye H, Fabre A, Menard M, Brasnu D. Partial surgery of epithelioma of the glottic area. Ann Otolaryngol Chir Cervicofac. 1988;105(6):449–452. PMID: 3358605.
11. Peretti G, Piazza C, Mensi MC, Magnoni L, Bolzoni A. Endoscopic treatment of cT2 glottic carcinoma: prognostic impact of different pT subcategories. Ann OtolRhinolLaryngol. 2005;114(8):579–586. doi:10.1177/000348940511400801
12. Holsinger FC, Nussenbaum B, Nakayama M, et al. Current concepts and new horizons in conservation laryngeal surgery: an important part of multidisciplinary care. Head Neck. 2010;32(5):656–665. doi:10.1002/hed.21208
13. Spriano G, Mercante G, Cristalli G, Pellini R, Ferreli F. Lateral cervical approach for supracricoid partial laryngectomy. Am J Otolaryngol. 2017;38(5):598–602. doi:10.1016/j.amjoto.2017.06.011
14. Spriano G, Mercante G, Anelli A, Cristalli G, Ferreli F. Lateral cervical approach for open laryngeal surgery: technical notes.. Head Neck. 2018;1–8. doi:10.1002/hed.25475
15. Mallet Y, Chevalier D, Darras JA, Wiel E, Desaulty A. Near total laryngectomy with epiglottic reconstruction. Our experience of 65 cases. Eur Arch Otorhinolaryngol. 2001;258(9):488–491. doi:10.1007/s004050100374
16. Ambrosch P, Fazel A. Functional organ preservation in laryngeal and hypopharyngeal cancer. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2011;10(Dec):02. doi:10.3205/cto000075
17. Chen AY, Pavluck A, Halpern M, Ward E. Impact of treating facilities’ volume on survival for early stage laryngeal cancer. Head Neck. 2009;31(9):1137–1143. doi:10.1002/hed.21072
18. Pantel M, Wittekindt C, Altendorf-Hofmann A, et al. Diversity of treatment of T2N0 glottic cancer of the larynx: lessons to learn from epidemiological cancer registry data. Acta Otolaryngol. 2011;131(11):1205–1213. doi:10.3109/00016489.2011.603136
19. Ganly I, Patel SG, Matsuo J, et al. Analysis of postoperative complications of open partial laryngectomy. Head Neck. 2009;31(3):338–345. doi:10.1002/hed.20975
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
