Back to Journals » Journal of Pain Research » Volume 12
Substance P modulates electroacupuncture analgesia in humanized mice with sickle cell disease
Authors Wang Y, Lei J, Jha RK, Kiven S , Gupta K
Received 27 March 2019
Accepted for publication 5 June 2019
Published 1 August 2019 Volume 2019:12 Pages 2419—2426
DOI https://doi.org/10.2147/JPR.S210196
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael Schatman
Ying Wang, Jianxun Lei, Ritu K Jha, Stacy Kiven, Kalpna Gupta
Vascular Biology Center, Division of Hematology, Oncology and Transplantation, Department of Medicine, University of Minnesota, Minneapolis, MN, USA
Purpose: Chronic pain is a major comorbidity of sickle cell disease (SCD). Acupuncture, a non-opioid and non-addictive therapy to treat pain, was found to reduce pain in the majority (80%) of SCD patients in an earlier retrospective review. We observed that electroacupuncture (EA) decreased hyperalgesia in transgenic mice with SCD with varied analgesia from high to moderate to no response. Interestingly, poor responders exhibited high levels of substance P (SP), a mediator of chronic pain, as well as active p38 MAPK in spinal cords. The present study aimed to investigate the roles of inhibition of SP and SP-activated p38 MAPK in chronic pain in sickle mice that are poorly responsive to EA intervention (moderate/non-responders).
Materials and methods: Humanized mouse model with SCD defined as moderate- and non-responders to EA were intraperitoneally administered with antagonist of SP receptor NK1R (netupitant, 10 mg/kg/day, i.p.) or p38 MAPK inhibitor (SB203580, 10 mg/kg/day, i.p.) alone or in combination with EA (acupoint GB30, every 3rd day until day 12). Hyperalgesia to mechanical, thermal and cold stimuli, as well as deep tissue were measured. Phosphorylated p38 MAPK (phospho-p38 MAPK) in the lumbar spinal cord was quantified using western blotting. Phospho-p38 MAPK nuclear translocation in spinal dorsal horn was examined using immunohistochemical staining and confocal microscopy.
Results: In EA poor-responders, combined treatment with EA and netupitant significantly enhanced the analgesic effects of EA in poor-responders on mechanical, heat, cold, and deep tissue pain, and decreased phosphorylation of p38 MAPK in lumbar spinal cords and its nuclear translocation in the spinal dorsal horn. Furthermore, combined treatment with EA and SB203580 significantly improved analgesic effects of EA on mechanical and heat hyperalgesia, but not cold or deep tissue hyperalgesia. However, additional EA treatment only, or administration of either netupitant or SB203580 alone did not lead to analgesic effects.
Conclusions: These results suggest a pivotal role of SP in maintaining the chronic pain in SCD via spinal phospho-p38 MAPK signaling, which may hinder the effect of EA in poor responders. Inhibition of SP signaling pathway or activity of p38 MAPK significantly improved the EA analgesia In EA poor-responders with SCD, which provides a promising way to treat the chronic pain in patients with SCD.
Keywords: acupuncture, pain, neuromodulator, p38 MAPK, neurokinin 1 receptor, neuroinflammation
Introduction
Chronic pain remains a major comorbidity in sickle cell disease (SCD) requiring long-term opioid treatment.1 Due to the side-effects of opioids including addiction, non-opioid treatments are needed for the long-term treatment of chronic pain in SCD. Strategies such as acupuncture have fewer side-effects than pharmacological agents. Limited evidence to date suggests that acupuncture offers pain relief in patients with SCD2,3 and in humanized HbSS-BERK (BERK) transgenic mice with SCD.4 In a previous study, we administered electroacupuncture (EA) treatment in awake, free-moving mice devoid of the potential influence of anesthetics or restraint-induced stress.4 EA led to variable analgesia in BERK mice, with high, moderate, and no effect.4 Importantly, we observed that the varied responses to EA analgesia in SCD mice correlated with levels of spinal substance P (SP), phospho-p38 mitogen-activated protein kinase (MAPK), and several inflammatory cytokines.4
It is likely that p38 MAPK activation is critical in the analgesic response because it is constitutively activated in the spinal cord of sickle mice in association with central sensitization.5 SP is an activator of p38 MAPK because inhibition of the SP receptor, neurokinin 1 receptor (NK1R), had an inhibitory effect on p38 MAPK in human intervertebral disc cells.6 Circulating SP levels are elevated in patients with SCD.7,8 BERK mice also exhibit higher SP in the blood, dorsal root ganglion, skin secretagogue, and spinal cord.9,10 EA treatment reduced spinal SP and p38 MAPK phosphorylation in BERK mice showing higher analgesic response but not in moderate- and non-responders.4 These findings suggest that the inadequate analgesic effect of EA in moderate- and non-responders might be attributed to the hypersensitivity resulting from the higher levels of inflammatory signaling via SP and p38 MAPK. We, therefore, hypothesized that targeted inhibition of SP or p38 MAPK signaling may improve EA analgesia in BERK mice.
Materials and methods
Animals
Transgenic homozygous HbSS-BERK (BERK) sickle mice (5–7 month, both gender) expressing >99% human sickle hemoglobin were used for this study.11 All animal studies were performed following guidelines established by the International Association for the Study of Pain and protocols approved by the Institutional Animal Care and Use Committee of the University of Minnesota.
Treatments
As described earlier, homozygous BERK mice without anesthesia or restraint were treated with EA.4 Analgesic responses to EA were defined as high- (nociceptive threshold increase >200%), moderate- (threshold between 100% and 200%), and non-responders (threshold increase ≤100%) according to the change in pain threshold as described previously.4
Netupitant (Sigma-Aldrich), an inhibitor of the SP receptor neurokinin 1 (NK1R),12 and SB203580 (Tocris/BioTechne),13 a p38 MAPK inhibitor, was used in this study. EA-treated moderate- and non-responders were randomly assigned to untreated control, EA-only treatment, netupitant-only treatment, SB203580-only treatment, or combination treatment with EA. Mice were treated (i.p., 10 mg/kg for both drugs) with netupitant or SB203580 daily from day 1, alone or concomitantly with EA (frequency: 4 Hz for the first three sessions followed by 10 Hz for the last session, pulse width: 100 μs, duration: 30 min/session) on day 0 and subsequently on days 3, 6, and 9.
Measurements of hyperalgesia
Mice were assessed for mechanical hyperalgesia determined by paw withdrawal frequency evoked by calibrated 1-g von Frey (Semmes-Weinstein) monofilaments (PWF-VF, 1 g fiber) or paw withdrawal threshold assessed by a series of von Frey monofilaments (PWT), heat sensitivity determined by paw withdrawal latency evoked by a heat stimulus applied to the plantar surface of the hind paw (PWL-heat), cold hyperalgesia determined by paw withdrawal frequency on a 4°C cold plate recorded over a 2-min period (PWF-cold, 2 mins), and deep tissue hyperalgesia determined by the forepaw grip force in gram measured with a computerized grip force meter (grip force, g). Behavioral measurements were performed in a quiet, temperature- and humidity-controlled room as described.4 The experimenter was blinded to the treatment for each mouse. Mice were acclimated to each behavioral testing paradigm in the behavioral room prior to the experiments. Evaluation of the baseline of each pain behavioral test was performed 24 hrs before the start of the study. Only BERK mice that displayed mechanical hyperalgesia response of 7 or higher out of 10 applications (70%) in PWF-VF were selected for further studies. EA was applied every 3rd day prior to other subsequent treatments.
Quantification of SP by ELISA
Whole blood was collected by cardiac puncture and transferred to EDTA-tubes. Plasma was then isolated within 30 mins by centrifugation for 7.5 mins at 2,500 rpm at 4°C. Isolated plasma was kept at −80°C before analysis for SP (Abcam) according to the manufacturer’s instructions.
Analysis of p38 MAPK by Western blot
Supernatants of whole spinal cord lysates were resolved on a 3–15% gradient gel by polyacrylamide gel electrophoresis (30 μg protein/lane) and immunoblotted with antibodies to phosphorylated p38 MAPK and unphosphorylated total p38 MAPK (all from Cell Signaling Technology) as described previously.4
Laser scanning confocal microscopy for nuclear co-localization of p38 MAPK
Freshly prepared 10 μm spinal cord sections were fixed with 4% paraformaldehyde and blocked with 3% donkey serum. Sections were then permeabilized with 0.01% Triton X-100 (all from Sigma-Aldrich) and then incubated with primary antibodies, anti-phosphorylated p38 MAPK (1:50) (1:100, Cell Signaling Technology). The bound antibodies were detected by incubation with Cy3 conjugated donkey anti-rabbit antibody (Jackson ImmunoResearch) and counterstained with DAPI (Thermo Fisher Scientific) to detect the nulei. Confocal images from the dorsal horn were collected using the Olympus FluoView FV1000 BX2 Upright Confocal at the University Imaging Centers of University of Minnesota. The average percentage of co-expression of phosphorylated p38 MAPK with cell nuclei throughout the spinal cord dorsal horn (six images acquired from randomly selected areas from superficial to the deep dorsal horn) was quantified with Image J.14
Statistical analysis
All data were analyzed using Prism software (v 6.0a, GraphPad Prism Inc., San Diego, CA). Analysis of variance with Bonferroni’s post hoc test was used for comparison between multiple groups as indicate in the figures. A p-value <0.05 was considered significant. All data are presented as mean ± SEM.
Results
There was a gradual decrease in mechanical hyperalgesia starting on day 3 after the co-treatment with EA and netupitant in EA-treated moderate- and non-responders (Moderate/Non-responders + EA + Netupitant); this analgesic effect reached significance on day 9 as compared to untreated controls (Moderate/Non-responders untreated) or treatment with only EA (Moderate/Non-responders + EA) or netupitant (Moderate/Non-responders + Netupitant) and was sustained until day 12, lasted for the period of observation (Figure 1A). Co-treatment gradually attenuated heat, cold, and deep tissue hyperalgesia and reached significance on day 12 (Figure 1B–D). Notably, additional EA treatment in EA-treated poor-responders (Moderate/Non-responders + EA) or administration of netupitant alone (Moderate/Non-responders + Netupitant) did not improve analgesia significantly.
 |
Figure 1 Inhibition of NK1 receptor (NK1R) potentiates EA analgesia. BERK mice pre-screened as moderate- and non-responders to EA treatment were selected and divided into four treatment groups: [i] untreated controls (Moderate/Non-responders untreated), [ii] EA alone (Moderate/Non-responders + EA), [iii] netupitant alone (Moderate/Non-responders + Netupitant), or [iv] EA plus netupitant (Moderate/Non-responders + EA + Netupitant). Four consecutive EA regimens were administered as described in Materials and methods.4 The NK1R inhibitor, netupitant (10 mg/kg) was administered intraperitoneally daily up to day 12. Pain measurements were performed at baseline (BL) and days 3, 6, 9, and 12 before the treatment on each corresponding day (black arrows). (A) Paw withdrawal threshold using von Frey microfilaments (PWT-VF, 1 g) as a measure of cutaneous nociception. (B) Paw withdrawal latency to a heat stimulus (PWL-Heat). (C) Paw withdrawal frequency to a cold stimulus (PWF-Cold, 2 mins). (D) Deep tissue hyperalgesia as measured by grip force (g). * p<0.05 vs BL of matching group; $ p<0.05 vs Mod UT; # p<0.05 vs Mod + EA; € p<0.05 vs Non-UT, † p<0.05 vs Non + EA (two-way ANOVA, Bonferroni). Each value is the mean ± SEM of 6–8 mice. Abbreviations: BL, baseline; EA, electroacupuncture; g, gram; Mod, moderate-responders; Non, non-responders, N, netupitant; PWF-VF, paw withdrawal frequency-von Frey; PWL, paw withdrawal latency; UT, untreated. |
Combined treatment with EA and SB203580 (Moderate/Non-responders + EA + SB) attenuated mechanical (Figure 2A) and heat (Figure 2B) hyperalgesia on day 12, but not cold (Figure 2C) or deep tissue (Figure 2D) hyperalgesia. Similar to the effect of netupitant and EA co-treatment, the analgesic effect of EA and SB203580 co-treatment was sustained and remained progressively greater in attenuating mechanical and heat hyperalgesia with successive treatments. SB203580 treatment alone (Moderate/Non-responders + SB) did not attenuate mechanical or deep tissue hyperalgesia.
 |
Figure 2 Inhibition of p38 MAPK potentiates the analgesic effect of EA. BERK mice pre-screened as moderate- and non-responders to EA treatment were selected and divided into four treatment groups: untreated controls (Moderate/Non-responders untreated), EA alone (Moderate/Non-responders + EA), SB203580 (a p38 MAPK inhibitor) alone (Moderate/Non-responders + SB), or EA plus SB203580 (Moderate/Non-responders untreated + EA + SB). Four consecutive EA regimens were administered as described earlier.4 SB203580 (10 mg/kg, i.p.) was administered daily up to day 12. Pain measurements were performed at BL and days 3, 6, 9, and 12 before the treatment on the corresponding day (black arrows). Hyperalgesia was assessed with (A) PWF-VF, (B) PWL-Heat, (C) PWF-Cold (2 mins), and (D) Grip Force (g). *p<0.05 vs BL of matching group; $ p<0.05 vs Mod UT; € p<0.05 vs Non-UT; † p<0.05 vs Non + EA (two-way ANOVA, Bonferroni). Each value is the mean ± SEM of 6–8 mice. Abbreviations: BL, baseline; EA, electroacupuncture; g, gram; Mod, moderate-responders; Non, non-responders; PWF-VF, paw withdrawal frequency-von Frey; PWL, paw withdrawal latency; SB, SB203580; UT, untreated. |
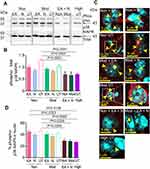
Quantitative Western blot analysis of whole lumbar spinal cords showed that combined treatment with EA and netupitant in both moderate- and non-responders (Non/Moderate-responders + EA + Netupitant) significantly reduced the expression of phospho-p38 MAPK relative to total-p38 MAPK (Figure 3A, B). Nuclear co-localization of phospho-p38 MAPK in the dorsal horn of the spinal cord was highly insightful because nuclear translocation of p38 MAPK is critical in the transcriptional regulation of inflammatory cytokines. We observed a significantly high co-localization of phospho-p38 MAPK immunoreactivity in the nulei of non-responders (red and white dots indicated by yellow arrows in the cyan nuclei) compared to high- responders (Figure 3C, D). Similarly, moderate-responders also showed significantly higher nuclear presence of phospho-p38 MAPK compared to high-responders. Either netupitant or EA alone did not have any effect on nuclear phospho-p38 MAPK in non- or moderate-responders. However, combined treatment with EA and netupitant significantly inhibited p38 MAPK phosphorylation as well as its nuclear translocation. High-responders did not show significant p38 MAPK phosphorylation upon Western blotting (Figure 3A and B) or upon immunofluorescent co-localization in the cytoplasm and/or nuclear translocation (Figure 3C, D). Together these data suggest that the non-responsiveness to EA in non- and moderate-responders may be due to increased spinal p38 MAPK phosphorylation due to higher NK1R activity perhaps due to increased SP levels in these sickle mice.
Discussion
Our study suggests that netupitant may promote EA analgesia via an inhibitory effect on spinal p38 MAPK phosphorylation. SP has been shown to induce the expression of proinflammatory cytokines including IL-6 via activation of p38 MAPK.15 It is likely that increased p38 MAPK phosphorylation induced by higher constitutive levels of SP in moderate- and non-responders compared to high-responders reduces response to EA analgesia. This is in agreement with our observations of reduced spinal p38 MAPK phosphorylation and SP in EA-treated high-responders. Our observations are also in agreement with previous studies demonstrating the attenuation of p38 MAPK phosphorylation by EA in the spinal dorsal horn in complete Freund’s adjuvant-induced inflammatory pain.16
Notably, spinal phospho-p38 MAPK decreased to levels observed in pre-screened high-responders. As p38 MAPK activation is associated with central sensitization, it is likely that netupitant may synergize with EA and abrogate the influence on central sensitization in moderate- and non-responders, thus enhancing the response to EA in these mice.
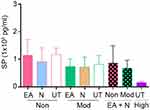
Preclinical data support the analgesic effect of NK1R blockade, but it has not been validated in clinical trials.17 In our BERK mouse model, we did not observe a significant effect of NK1R or p38 MAPK inhibition on analgesic response. However, each inhibitor individually enhanced the response to EA analgesia in moderate- and non-responders, suggesting that perhaps constitutively high SP may blunt response to EA analgesia. This is supported by our observations of much lower SP level in high-responders than in moderate- and non-responders with or without treatment (Figure S1). Thus, spinal SP may predict response to EA analgesia, but spinal SP analysis may not be feasible in patients with SCD. Earlier our laboratory observed that increased SP in the blood is correlative to skin secretagogue, dorsal root ganglion, and spinal cord of BERK mice as compared to control mice.9,10 Therefore, it is likely that circulating SP in patients with SCD may be informative about its presence in the peripheral and central nervous system and may serve as a biomarker to predict response to EA analgesia. Taken together, our data suggest a novel approach to potentiate acupuncture analgesia in SCD without using opioids.
Conclusion
Observations above evince treatable targets to potentiate the analgesic effect of EA in sickle mice with chronic pain. Whereas, netupitant has not been effective in treating pain by itself, the possibility of its potentiation of EA analgesia is highly attractive. These results have translational implications for determining response to EA therapy and targeting the non-responsiveness with FDA approved netupitant.
Acknowledgments
This study is supported by National Institutes of Health (NIH) 1K99AT010012-01 to YW and NIH UO1 HL117664 and Department of Medicine Funds to KG. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors thank Michael Franklin for editorial assistance and English writing. The confocal microscopy images were acquired at the University Image Center in the University of Minnesota.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Finan PH, Carroll CP, Moscou-Jackson G, et al. Daily opioid use fluctuates as a function of pain, catastrophizing, and affect in patients with sickle cell disease: an electronic daily diary analysis. J Pain. 2018;19(1):46–56. doi:10.1016/j.jpain.2017.08.010
2. Lu K, Cheng MC, Ge X, et al. A retrospective review of acupuncture use for the treatment of pain in sickle cell disease patients: descriptive analysis from a single institution. Clin J Pain. 2014;30(9):825–830. doi:10.1097/AJP.0000000000000036
3. Tsai SL, McDaniel D, Taromina K, Lee MT. Acupuncture for sickle cell pain management in a pediatric emergency department, hematology clinic, and inpatient unit. Med Acupunct. 2015;27(6):510–514. doi:10.1089/acu.2015.1146
4. Wang Y, Lei J, Gupta M, et al. Electroacupuncture in conscious free-moving mice reduces pain by ameliorating peripheral and central nociceptive mechanisms. Sci Rep. 2016;6:34493. doi:10.1038/srep34493
5. Cataldo G, Rajput S, Gupta K, Simone DA. Sensitization of nociceptive spinal neurons contributes to pain in a transgenic model of sickle cell disease. Pain. 2015;156(4):722–730. doi:10.1097/j.pain.0000000000000104
6. Kepler CK, Markova DZ, Koerner JD, et al. Substance P receptor antagonist suppresses inflammatory cytokine expression in human disc cells. Spine (Phila Pa 1976). 2015;40(16):1261–1269. doi:10.1097/BRS.0000000000000954
7. Brandow AM, Wandersee NJ, Dasgupta M, et al. Substance P is increased in patients with sickle cell disease and associated with haemolysis and hydroxycarbamide use. Br J Haematol. 2016;175(2):237–245. doi:10.1111/bjh.14300
8. Michaels LA, Ohene-Frempong K, Zhao H, Douglas SD. Serum levels of substance P are elevated in patients with sickle cell disease and increase further during vaso-occlusive crisis. Blood. 1998;92(9):3148–3151.
9. Vincent L, Vang D, Nguyen J, Benson B, Lei J, Gupta K. Cannabinoid receptor-specific mechanisms to alleviate pain in sickle cell anemia via inhibition of mast cell activation and neurogenic inflammation. Haematologica. 2016;101(5):566–577. doi:10.3324/haematol.2015.136523
10. Vincent L, Vang D, Nguyen J, et al. Mast cell activation contributes to sickle cell pathobiology and pain in mice. Blood. 2013;122(11):1853–1862. doi:10.1182/blood-2013-04-498105
11. Paszty C, Brion CM, Manci E, et al. Transgenic knockout mice with exclusively human sickle hemoglobin and sickle cell disease. Science. 1997;278(5339):876–878.
12. Ruzza C, Rizzi A, Malfacini D, et al. In vitro and in vivo pharmacological characterization of pronetupitant, a prodrug of the neurokinin 1 receptor antagonist netupitant. Peptides. 2015;69:26–32. doi:10.1016/j.peptides.2015.03.021
13. Sukhtankar D, Okun A, Chandramouli A, et al. Inhibition of p38-MAPK signaling pathway attenuates breast cancer induced bone pain and disease progression in a murine model of cancer-induced bone pain. Mol Pain. 2011;7:81. doi:10.1186/1744-8069-7-81
14. Bolte S, Cordelieres FP. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc. 2006;224(Pt 3):213–232. doi:10.1111/j.1365-2818.2006.01706.x
15. Fiebich BL, Schleicher S, Butcher RD, Craig A, Lieb K. The neuropeptide substance P activates p38 mitogen-activated protein kinase resulting in IL-6 expression independently from NF-kappa B. J Immunol. 2000;165(10):5606–5611. doi:10.4049/jimmunol.165.10.5606
16. Liang Y, Fang JQ, Du JY, Fang JF. Effect of electroacupuncture on activation of p38MAPK in spinal dorsal horn in rats with complete freund’s adjuvant-induced inflammatory pain. Evid Based Complement Alternat Med. 2012;2012:568273. doi:10.1155/2012/568273
17. Hill R. NK1 (substance P) receptor antagonists–why are they not analgesic in humans? Trends Pharmacol Sci. 2000;21(7):244–246.
Supplementary material
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.