Back to Journals » Patient Preference and Adherence » Volume 11
Subcutaneous sumatriptan delivery devices: comparative ease of use and preference among migraineurs
Authors Andre AD, Brand-Schieber E, Ramirez M, Munjal S , Kumar R
Received 19 October 2016
Accepted for publication 20 December 2016
Published 19 January 2017 Volume 2017:11 Pages 121—129
DOI https://doi.org/10.2147/PPA.S125137
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen

Anthony D Andre,1 Elimor Brand-Schieber,2 Margarita Ramirez,1 Sagar Munjal,2 Rajesh Kumar2
1Interface Analysis Associates, Saraftoga, CA, 2Dr Reddy’s Laboratories Inc., Princeton, NJ, USA
Background: Several sumatriptan subcutaneous autoinjector devices for acute treatment of migraine patients are available, each device differs with respect to design and features. Determining device preference and ease of use is important because patients experiencing a migraine attack are often functionally impaired.
Objective: The objective of this human factors study was to compare migraine patients’ device use performance and preferences for three sumatriptan subcutaneous autoinjectors: a disposable two-step device (Zembrace® SymTouch®), a disposable three-step device (Sumavel® DosePro®), and a multistep reloadable device (Imitrex® STATdose®), using simulated injections.
Methods: Each study subject performed two unaided simulated injections with each of three different drug delivery devices, which were presented in counterbalanced order. The participants were then asked to rate the three devices on various subjective measures. The primary end point was overall device preference using a visual analog scale.
Results: A total of 54 subjects participated and each subject performed two simulated injections with each of the three devices. Most subjects preferred the two-step device (88.9%) to the three-step (13.0%) and the reloadable (1.9%). The two-step device had higher mean overall preference ratings (F (2, 159)=56.6, P<0.01) and higher ratings for ease of use, intuitiveness, convenience, portability, and control. The two-step device had a first injection full-dose delivery success rate of 44.4%, higher than both the reloadable (24.1%) and the three-step (3.7%) devices. The number of errors with the two-step device (n=3) was ~90% lower than the three-step (n=49) and reloadable (n=44) devices.
Conclusion: In this human factors study, 54 migraineurs used simulated injections to compare three sumatriptan subcutaneous delivery devices. Zembrace SymTouch, a two-step device, was most preferred compared with Sumavel DosePro and Imitrex STATdose. It also ranked highest for ease of use and various other measures. In this study, migraine patients preferred the autoinjector that they rated as simpler and more intuitive.
Keywords: human factors, autoinjector, sumatriptan, migraine, preference
Introduction
Episodic migraine is a neurologic disease characterized by occasional attacks that involve moderate-to-severe headaches and associated symptoms, such as nausea, photophobia, and phonophobia, the combination of which often leads to functional disability.1,2 In the USA, ~17% of women and 6% of men have migraine, with peak prevalence occurring in both sexes between the ages of 25 and 55 years.3,4 Medications that produce agonist effects on serotonin 5-HT1B and 5-HT1D receptors, collectively known as “triptans”, are the most widely used prescription treatment for the acute management of migraine.5,6 Sumatriptan is the most commonly prescribed triptan, with substantial evidence supporting efficacy in treating migraine headache pain and associated symptoms.7 Subcutaneous (SC) sumatriptan delivery provides the fastest migraine relief,8,9 and patients are likely to benefit from the availability of self-administered drug delivery options that can be used easily and safely.
We recently reported on the validation and approval of a lower-dose sumatriptan, two-step, single-use autoinjector (Zembrace® SymTouch®, formerly DFN-11)10 – the only SC sumatriptan 3 mg autoinjector with a thin-gauge needle that is commercially available in the USA. Until its approval by the US Food and Drug Administration (FDA), migraineurs who were prescribed a 3-mg dose of SC sumatriptan had to manually prepare injections during migraine attacks, an inconvenient process that could lead to inaccurate sumatriptan dosing – particularly with long-term use and the treatment of multiple attacks. Because the safety and efficacy of SC sumatriptan are well established, the choice of a delivery device typically depends on the dose it can provide, even though human factors (ie, patient preference, ease of use, intuitiveness, and ergonomics) may also play an important role in overall satisfaction with acute therapy. Because these factors in the SC delivery of sumatriptan have not previously been evaluated, this study was undertaken to compare migraine patient preference and performance using three SC sumatriptan drug delivery devices across two simulated injections and to identify which device was most preferred, easiest, most intuitive, and least threatening to use.
Methods
Ethical approval
This study was conducted in accordance with the accepted version of the Declaration of Helsinki and/or all relevant federal regulations, as set forth in Parts 50, 56, 312, Subpart C, Subpart D, of Title 21 of the Code of Federal Regulations, and in compliance with Good Clinical Practice guidelines. An Independent Review Board/Independent Ethics Committee (Salus IRB, Austin, TX, USA) reviewed and approved the protocol and the informed consent form.
Subjects
Subjects aged 18–70 years were eligible to participate. They were screened prior to enrollment and were included if they had a medical diagnosis of migraine, had no experience performing any type of self-injection, and had no experience using any injectable migraine treatment. Subjects were excluded if they had no current or recent use of acute migraine treatment (noninjectable) or had not experienced a migraine attack in the previous 12 months.
Study devices
Two-step device
Zembrace SymTouch (Figure 1) is a mechanical, hand-held, single-dose, prefilled, disposable two-step device. It is designed for manual needle insertion, automatic drug delivery, manual needle withdrawal, and automatic needle guarding. The SymTouch autoinjector consists of a prefilled syringe containing 0.5 mL of medication and utilizing a 29-gauge needle with a 5-bevel edge to minimize injection pain and discomfort.11,12 Prior to use, the autoinjector needle is concealed to prevent accidental contact. Once patients remove the cap and depress the autoinjector on the prepared injection site, the autoinjector automatically dispenses the contents of the prefilled syringe using a spring mechanism. Visual indicators in the inspection window (red plunger rod movement) and audible/tactile indicators (clicks) provide feedback on proper use of the autoinjector, including both the start and end of the injection. After the injection, as patients lift the device from the injection site, the needle guard automatically locks over and shields the needle to prevent accidental needle sticks. It can then be disposed of in an approved sharps container.
  | Figure 1 Zembrace® SymTouch® disposable single-use two-step autoinjector. |
Three-step device
Sumavel® DosePro® is a prefilled, single-use, disposable, needle-free system designed for SC delivery of 6-mg sumatriptan into the abdomen or thigh through instantaneous liquid injection (Figure 2). To prepare for injection, patients snap off the gray end cap and flip down the green lever. When ready to inject, they place the device over the prepared injection site. Once pressure is applied downward, a liquid stream of sumatriptan penetrates the skin within 0.1 second. Because the DosePro device contains no sharps or needles, there is no risk of needlestick injuries.
  | Figure 2 Sumavel® DosePro®, the three-step needle-free study device. |
Reloadable device
Imitrex® STATDose®, shown in Figure 3, is a multistep, reloadable autoinjector that delivers SC doses of sumatriptan through the use of 4 or 6 mg cartridges. To use it, patients must place the body of the device into a new cartridge pack and turn it to attach a new syringe. After preparing the injection site, the autoinjector is pressed down on the skin; sumatriptan delivery begins when the button is pressed and ends after 5 seconds. The used device is deposited into a sharps container, and the reusable items are returned to the case for future use.
  | Figure 3 Imitrex® STATdose® System, the multistep, reloadable cartridge-based study device. |
Study conduct
The study was conducted at two sites in California: Interface Analysis Associates in Saratoga and the Crown Plaza Hotel in Sacramento. Room arrangements (ie, a table, chairs, and soft ambient lighting) were similar in both facilities and designed to represent a home-like setting. Subjects attended a single session lasting ~45 minutes. At the beginning of the session, they read a brief introduction containing unbiased content outlining the study purpose and context and then signed an informed consent form. Subjects were not experiencing migraine attacks at the time of study device testing.
For the first injection attempt, subjects were given the first assigned device and told that their goal was to deliver the entire dose of medication into the simulated injection site. They received no training or instructions and were told to take whatever time they needed to figure out how to use the device. The initial attempt, which was timed from the moment subjects picked up an assigned device until they activated the injection, was aborted if subjects failed to progress toward an injection after 2 minutes. Before the second attempt, subjects were given a standardized overview of the procedure and written instructions for use, and procedural errors noted on the first attempt were corrected. To ensure subject safety and compliance with study procedures, a moderator was present for all injection attempts; however, no assistance on use of the SC devices was provided during injection attempts.
Subjects used the final production-line version of Zembrace SymTouch and the commercially available Imitrex STATdose and Sumavel DosePro. To simulate acute treatment of migraine, subjects injected into a peeled orange placed in a vessel that was affixed to a lap board. Peeled oranges were used as the model to accommodate the needle-free device; the lap board was then kept on the thighs, a common site of self-injection, which was chosen to eliminate injection site variability.
Measures
Performance, behavioral, and subjective measures were used. To assess performance, subjects’ injection success, failure, and errors were recorded. Injections were considered successful if subjects performed the correct procedure with a study device and used it to administer a full dose of sumatriptan. Attempts could be considered unsuccessful for failure to remove a cap, inject at a proper angle (ie, perpendicular to the site), and failure to activate an injection or administer a full dose. Errors could involve inversion of the autoinjector and/or needlestick injuries. Behavioral measures included indices of excessive effort or frustration, verbal comments made during the study (when applicable), and reactions to the device or instructions for use. Nonoptimal behaviors were defined as an action by the user that did not prevent the user from dosing, but deviated from the correct injection procedure or delayed dosing. Patient-rated measures included ease of use, intuitiveness, efficiency, safety, trustworthiness, convenience, ease of remembering how to use, overall acceptance, and ease of use versus oral formulations.
After the first injection attempt, subjects were asked (Yes/No) whether they believed they had been successful in delivering the sumatriptan dose or had any difficulty with the process, including activating the injection or knowing when the injection was complete. Prior to the second injection attempt, subjects were given a brief overview of the correct injection procedure while being allowed to read written instructions for use, after which they rated the device for intuitiveness (ease of determining correct usage prior to any training or instructions) using a 7-point Likert scale (1= not at all intuitive and 7= very intuitive). Then, using a new study device of the same type, the subject performed the second injection attempt. After the second injection attempt, subjects were asked again about their feelings of success and difficulties with the process, activation, or knowing when the injection was complete. Using a series of 7-point Likert scales (Table 1), subjects then rated preparing, starting, and identifying when injections were complete, as well as overall ease of device use. They also rated the study device for efficiency, safety, trustworthiness, convenience, and ease of remembering proper use. Finally, subjects were asked about device acceptability (accept/reject) and ease of use versus oral formulations (easier/more difficult). This procedure was repeated for the next two types of devices.
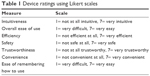  | Table 1 Device ratings using Likert scales |
For the final comparison, subjects were presented with all three devices and using a 0–100 point visual analog scale (VAS [0= most difficult and 100= easiest]) were asked to rate which device was the easiest, most intuitive, most convenient, most portable, and most preferred overall. They next rated which device made them feel most likely they were in control of delivering the medication and which device was the least intimidating. Finally, they were asked (Yes/No) whether any device was worthy of a recommendation to other migraine patients and whether they would refuse any of the devices if prescribed by a health care professional.
Statistical methods
The primary end point analysis was conducted for overall subject preference comparing the three devices using analysis of variance (ANOVA). For secondary end point analyses, the proportion of subjects who successfully performed the injection procedure was analyzed using chi-squared. ANOVA was used to analyze the time to start the injection (from start of handling device to the point of activation) and the rating of all three devices using the 100-point VAS assessment for overall ease of use, intuitiveness, convenience, portability, control, and least intimidating. The 7-point Likert scale ratings for each of the study devices were analyzed using ANOVA on the following measures: ease/difficulty of preparing the device, activating the injection, and knowing when the injection is complete, ease of use, efficiency, trust, convenience, and likelihood of remembering how to use the device in the future. Post hoc comparisons were performed using the Tukey honest significant difference (HSD) test.
To evaluate the primary end point, a 0.2 effect size was assumed. This meant that, at a 5% (2-sided) level of significance, a sample size of 48 subjects would be needed to provide >80% power to detect the assumed difference between devices. A total sample of 54 subjects was, therefore, selected to counterbalance for any crossover effects.
Results
Subjects
A total of 54 subjects were enrolled in and completed the study. Most subjects were female (70.3%) and Caucasian (44.4%), with a mean age of 37.8 years (range 19–59). The highest level of education attained by most subjects was a high school diploma (59.3%). The most commonly used noninjectable sumatriptan formulation was oral tablets (90.7%). Subject demographics are summarized in Table 2.
  | Table 2 Demographics (N=54) |
Overall device preference
For overall device preference, the primary end point, the vast majority of subjects chose SymTouch, the two-step device, as their first choice (88.9%) compared with DosePro, the three-step device, (13%) and STATdose, the reloadable device (1.9%) presented in Table 3. A post hoc comparison indicated that the mean (SD) overall preference score for SymTouch (95.2 [7.9]) was significantly higher than that of STATdose (43.3 [32.9], P<0.01) and DosePro (64.3 [28.9], P<0.01).
Intuitiveness rating prior to second injection
There was a significant difference in the mean intuitiveness ratings (F (2, 159)=57.9, P<0.01). Post hoc comparison using the Tukey HSD test indicated that the mean (SD) intuitiveness ratings for SymTouch (5.1 [1.6]) were significantly more intuitive than STATdose (3.0 [1.5], P<0.01) and DosePro (2.2 [1.2], P<0.01). The mean ratings for STATdose and DosePro resulted in a rating equivalent to “not intuitive” while SymTouch was “neutral to somewhat intuitive”.
Device disposition ratings after the second unaided injection
Ease of use
Ease of preparing the device: The mean ratings for ease of preparing the devices for injection were significantly different (F (2, 159)=40.5, P<0.01). Post hoc comparisons demonstrated that SymTouch (6.6 [0.7]) was rated significantly higher than STATdose (4.4 [1.8], P<0.01) and DosePro (5.6 [1.4], P<0.01).
Ease of starting the injection
Similarly, there was a significant difference in the mean ratings for ease of starting the injection (F (2, 159)=20.7, P<0.01), and post hoc comparisons showed that the mean (SD) rating for SymTouch (6.8 [0.5]) was significantly higher than STATdose (5.4 [2.1], P<0.01) and DosePro (6.0 [1.2], P<0.01). On this parameter, the mean rating for DosePro was significantly higher than STATdose (P<0.05).
Ease of identifying when the injection was complete
For ease of identifying when the injection was complete, there was a significant difference in the mean ratings across devices (F (2, 159)=40.9, P<0.01). Post hoc comparisons found that SymTouch (6.6 [0.9]) was significantly better than STATdose (M=4.3, SD=1.8, P<0.01), but the difference with DosePro was not significant. In addition, DosePro was rated significantly higher than STATdose (P<0.01).
Ease of use overall
For overall ease of use, SymTouch was rated significantly easier to use than STATdose and DosePro (F (2, 159)=45.1, P<0.01). In post hoc comparisons, SymTouch (6.7 [0.6]) was rated significantly higher than STATdose (4.4 [1.6], P<0.01) and DosePro (5.7 [1.3], P<0.01), and the mean rating for DosePro was significantly higher than for STATdose (P<0.01).
Efficiency
For injection efficiency, there was a significant difference in the mean ratings (F (2, 159)=43.1, P<0.01), and post hoc analyses found that subjects rated SymTouch (6.6 [0.7]) significantly more efficient than STATdose (4.3 [1.8], P<0.01) and DosePro (5.8 [1.3], P<0.01). In addition, DosePro was considered significantly more efficient than STATdose (P<0.01).
Convenience
The three devices differed significantly in the mean ratings for convenience of use during an attack (F (2, 159)=34.6, P<0.01). In post hoc comparisons, SymTouch (6.5 [0.9]) was shown to be significantly more convenient than STATdose (4.1 [1.9], P<0.01) and DosePro (5.6 [1.5], P<0.01), and DosePro was rated significantly higher than STATdose (P<0.01).
Safe use
For the question how safe or unsafe the migraine patient felt in using the device, there was a significant difference in the mean ratings (F (2, 159)=19.3, P<0.01). Post hoc comparisons indicated that the mean safety rating for SymTouch (6.5 [0.9]) was significantly higher than STATdose (4.8 [1.9], P<0.01) and DosePro (5.6 [1.5], P<0.01). In addition, the DosePro mean safety rating was significantly safer than STATdose (P<0.05).
Trustworthiness
For trustworthiness, there was a significant difference in mean ratings (F (2, 159)=20.5, P<0.01). Post hoc analyses indicated that subjects rated SymTouch (6.4 [0.9]) significantly higher than STATdose (4.7 [1.7], P<0.01) and DosePro (5.5 [1.5], P<0.01) and that DosePro was rated significantly higher than STATdose (P<0.05).
Device acceptance
SymTouch received the highest user acceptance rating of the three devices (SymTouch: 94.4%, DosePro: 70.4% and STATdose: 44.4%). Subject feedback related to the use of the needle-free device DosePro included reactions to the sound, number of steps, and concern about handling the device during a migraine. Feedback for the cartridge-based device included reactions to the number of steps and parts required to use the device during a migraine.
Remembering correct usage
The devices differed significantly for ease of remembering how to use them correctly (F (2, 159)=47.5, P<0.01). Post hoc comparisons indicated remembering how to use SymTouch (6.5 [0.7]) was significantly easier than STATdose (3.8 [1.8], P<0.01) and DosePro (5.5 [1.5], P<0.01), and the mean rating for DosePro was significantly higher than that for STATdose (P<0.01).
Comparison with oral formulations
SymTouch had the highest percentage of users who stated it was easier than taking oral tablets (SymTouch: 35.2%, DosePro: 20.4%, and STATdose: 9.3%), and more subjects said that SymTouch was about as easy to use as oral tablets (SymTouch: 48.1%, DosePro: 33.3%, and STATdose: 9.3%). STATdose users were most likely to state that the device was more difficult to use than taking oral tablets (STATdose: 81.5%, DosePro: 46.3%, and SymTouch: 16.7%).
Final comparisons between the three devices
The results of the final comparisons between the three devices are shown in Table 3 and are described below.
Easiest to use
When asked to rank and rate the devices for overall ease of use, there was a significant difference in the mean ease of use ratings (F (2, 159)=68.8, P<0.01). Post hoc comparisons indicated that SymTouch (93.2 [10.6]) was significantly easier to use than STATdose (43.9 [26.8], P<0.01) and DosePro (60.7 [25.6], P<0.01) and that DosePro was significantly easier to use than STATdose (P<0.01).
Most intuitive
When asked to rank and rate the devices in terms of intuitiveness, there was a significant difference in the mean intuitive ratings (F (2, 159)=55.3, P<0.01). Post hoc comparisons indicated that the mean intuitiveness score for SymTouch (88.74 [14.3]) was significantly more intuitive than STATdose (45.4 [26.2], P<0.01) and DosePro (50 [27.6], P<0.01). There was no significant difference in intuitiveness between DosePro and STATdose.
Convenience
In terms of which device was considered most convenient to use during a migraine, the mean convenience ratings differed significantly (F (2, 159)=68.6, P<0.01). Post hoc comparisons showed that SymTouch (95.2 [7.9]) was significantly more convenient than STATdose (45.2 [30.7], P<0.01) and DosePro (67.4 [21.8], P<0.01) and that DosePro was rated as significantly more convenient than STATdose (P<0.01).
Additional preference dimensions
SymTouch was rated as the most portable device (SymTouch: 75.9%, STATdose: 24.1%, and DosePro: 0%). It was also the device that gave migraine patients the feeling of being most in control of delivering the medication (SymTouch: 77.8%, DosePro: 16.7% and STATdose: 5.6%) and least intimidating (SymTouch: 92.6%, DosePro: 3.7% and STATdose: 3.7%). Almost all subjects (96.3%) stated they would recommend SymTouch to other migraine patients. DosePro would be recommended less than half of the time (40.7%) and STATdose less than a quarter of the time (24.1%). When asked about which devices they reject, no subject (0%) stated they would not use the SymTouch autoinjector, some said they would not use the DosePro (25.9%) and nearly half said they would not use the STATdose (44.4%).
Performance end points
Success rate, time to injection and errors
As shown in Table 4, migraine patients were more successful in preparing and administering the full dose with SymTouch. For the first injection, which occurred prior to any instruction or training, SymTouch was found to have a statistically significant higher success rate (44.4%) relative to STATdose (24.1%) and finally, DosePro (3.7%; P<0.05). For the second injection, which occurred immediately after instruction and training, the differences in success rates (SymTouch, 100%; DosePro, 90.7% and STATdose, 72.2%) were not statistically significant.
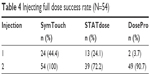  | Table 4 Injecting full dose success rate (N=54) |
Mean time to start the first injection (Table 5) was significantly different among the three devices (F, (2, 77)=56.3, P<0.01). Post hoc comparison indicated that the mean time to start the injection for SymTouch was significantly faster than STATdose (30.56 [19.67] vs 73.9 [31.8] seconds, P<0.01). DosePro was omitted from this analysis as only three subjects successfully completed the preparation for injection within the 2-minute preset time limit (only two followed with a completed injection as well). Post hoc analysis of the second injection indicated that the mean time to start the injection for SymTouch was 7.7 (3.7) seconds, still significantly faster than STATdose (40.6 [32.9] seconds, P<0.01) and DosePro (16.8 [7.1] seconds, P<0.05).
  | Table 5 Mean time to start injection in seconds |
Comparison of nonoptimal behaviors
For the first injection, the greatest number of nonoptimal behaviors was committed using the DosePro device, with a total of 108, whereas STATdose had 95 nonoptimal behaviors, and SymTouch had 76 nonoptimal behaviors. For the second injection, STATdose produced the greatest number of nonoptimal behaviors (n=8), followed by 1 nonoptimal behavior by SymTouch and 0 nonoptimal behaviors committed while using DosePro.
Use errors
DosePro had the highest number of use errors (n=49) of the three devices that resulted in no dose being delivered during the first injection. These errors were due to subjects injecting with the cap on, releasing the green lever before removing the cap, activating the device with the cap on, and being unable to start the injection due to tampering. STATdose had the second highest number of errors (n=44) that failed to deliver a full dose. Errors were related to loading or unloading the syringe, injecting both cartridges, not compressing and pressing the injection button at the same time to start the injection, premature activation, tampering with the syringe cartridges, and attempting to use the case to inject without preparing the device. SymTouch had the fewest number of errors (n=3), which included subjects inverting the device and turning the red cap while still affixed and resting on the injection site.
For the second injection, SymTouch resulted in no additional errors. DosePro resulted in three events where there was a failure to activate due to the way the user prepared the device, which we attribute to having been tampered with and did not activate. STATdose resulted in 13 additional use errors (9 related to not compressing and pressing button to start the injection, 2 related to prematurely activating the injection, and 2 related to depositing the used syringe into the pen holder).
Discussion
The results of this study showed that subjects preferred the Zembrace SymTouch autoinjector and rated it most highly on a number of performance measures. In addition, among study subjects with no prior self-injection experience and given no instructions for use or verbal instruction on how to use the device, the SymTouch device had the highest injection success rate for the first simulated injection (44.4%) compared with the other two autoinjectors tested (Imitrex STATdose, 24.1%; Sumavel DosePro, 3.7%). Immediately prior to the second simulated injection attempt, subjects were instructed on the device use and earlier performance errors were corrected, which minimized differences in the rate of successful injection the second time. The first simulated injection attempt may better reflect real-world experience of episodic migraine patients who may experience intervals of weeks to months between attacks. A device that is intuitive and simple to use, with instructions for use that are easy to remember, may be an advantage for an episodic migraine sufferer.
A similar result was observed when the time to start the injection was measured. For the first injection, only SymTouch and the cartridge-based device were compared (30.6 vs 74 seconds, respectively; P<0.01) because only two subjects were able to properly prepare the needle-free device for injection within the allotted time. For the second injection, SymTouch had the fastest time to start the injection (7.7 seconds), significantly less (P<0.01) than the cartridge-based device (40.6 seconds) and less than half the time, but insignificantly so, compared with the needle-free device (16.8 seconds; P<0.05).
The simulated injections in this study were performed by migraine patients between migraine attacks, thus the effect of migraine pain and other symptoms on device use was not evaluated. However, migraine sufferers have been shown to be able to reliably evaluate their abilities during migraine between attacks. Migraine attacks are frequently accompanied by light sensitivity, such that patients seek a dim-light environment, and cognition and coordination may be impaired during a migraine attack.2,13 Thus, it is reasonable to speculate that the simplicity and intuitiveness of an autoinjector device will be more relevant to successful use during a migraine attack. For migraine patients who are cognitively impaired during an attack and are light sensitive and need the lights off, the ability to see and handle the various device parts in a certain order may be a limitation for multiparts and multistep devices. On the basis of the results of this study, the two-step SymTouch may be the best positioned among the three tested devices for successful performance during a migraine attack. This concept is also supported by subjects rating SymTouch highest for anticipated convenience of use during a migraine and ease of remembering how to use the device in future attacks.
Most subjects chose SymTouch as their preferred device (88.9%) because of convenience, ease of use, and fewest number of steps. Because it has been shown that success of acute migraine treatment may be related to treating early in the course of an attack, a device that is simple and easy to use may facilitate early treatment and improve treatment adherence.
Overall, among injection-naive migraine patients performing simulated injections, the SymTouch autoinjector device had the highest rate of successful use, was most preferred by the majority of patients, and was rated most highly on all measures of preference and performance compared with the other two tested devices. However, ease of use and other subjective measures can be influenced by subjects’ debriefing after the first simulated injection or other unintended biases and lead to deviations from the truth. As a result, it is unknown the effect that biases had on these study results. The study, comprised of 54 subjects, was designed to eliminate as much systematic bias as possible.
Given the limited time that a health care professional has to spend with migraine patients14 and the recommendation that migraine patients administer treatment early to relieve pain and associated symptoms and improve functioning, an SC device that is intuitive, simple to use, and quick to prepare for injection may provide clinical benefits to prescribers and patients. On the basis of the results of this study, SymTouch appears to have these qualities.
These ratings showed that migraine patients found SymTouch to be about the same or easier as taking a pill. For DosePro, their experience was about the same or more difficult and for STATdose their experience more difficult than taking a pill. Patients cited the number of steps as the reason they rated the needle-free and cartridge-based devices as more difficult.
This study has several limitations. It was conducted in a controlled laboratory environment using simulated injections. As a result, we were unable to compare needle with needle-free sumatriptan delivery or assess the effects of needle gauge on injection site pain and reactions – factors that may affect patient preference for SC sumatriptan delivery devices in clinical practice. In addition, as the study devices were tested interictally (ie, while subjects were not experiencing migraine attacks), these results may not be fully generalizable to migraine patients in uncontrolled settings. Furthermore, it is important to note that this study evaluated first time users of all three autoinjectors and may not reflect long term use experience. It is viable that with experience and long term use of these products, users’ injection success rate and preference outcome may be different.
Conclusion
The findings of this comparison study between three sumatriptan SC delivery devices indicate that migraine patients had the greatest frequency of success and were fastest in preparing and administering the full dose of sumatriptan when using the Zembrace SymTouch autoinjector. Compared with Initrex STATdose and Sumavel DosePro, SymTouch was preferred by subjects and ranked first on all subjective factors obtained, including overall ease of use, intuitiveness, convenience, portability, control, and least intimidating.
Acknowledgments
The study was conducted by Interface Analysis Associates, Saratoga, CA, USA, with funding from Dr Reddy’s Laboratories, Ltd. Jennifer Kingsburg served as a participating investigator, collected and analyzed data, and provided substantial contributions to the study. We thank Dr Alix Bennett for critical review of the manuscript and many helpful comments and editorial contributions.
Disclosure
ADA is Principal at Interface Analysis Associates. MR is employed by Interface Analysis Associates. EBS, SM, and RK are employees of Dr Reddy’s Laboratories. Zembrace® SymTouch® is produced by Dr Reddy’s and is marketed by Promius Pharma, LLC, a subsidiary of Dr Reddy’s. Sumavel® is a registered trademark of Endo International plc or one of its affiliates. DosePro® is a registered trademark of Zogenix, Inc. Imitrex® STATdose System®, and Imitrex® STATdose Pen® are registered trademarks of the GlaxoSmithKline group of companies. DRL Publication No 790. The authors report no other conflicts of interest in this work.
References
Naegel S, Obermann M. Topiramate in the prevention and treatment of migraine: efficacy, safety and patient preference. Neuropsychiatr Dis Treat. 2010;6:17–28. | ||
Gil-Gouveia R, Oliveira AG, Martins IP. Subjective cognitive symptoms during a migraine attack: a prospective study of a clinic-based sample. Pain Physician. 2016;19(1):E137–E150. | ||
Lipton RB, Bigal ME, Diamond M, et al; AMPP Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343–349. | ||
Smitherman TA, Burch R, Sheikh H, Loder E. The prevalence, impact, and treatment of migraine and severe headaches in the United States: a review of statistics from national surveillance studies. Headache. 2013;53:427–436. | ||
Gilmore B, Michael M. Treatment of acute migraine headache. Am Fam Physician. 2011;83(3):271–280. | ||
Rizzoli PB. Acute and preventive treatment of migraine. Continuum (Minneap Minn). 2012;18:764–782. | ||
Gooriah R, Nimeri R, Ahmed F. Evidence-based treatments for adults with migraine. Pain Res Treat. 2015;2015:629382. | ||
Adelman JU, Lewit EJ. Comparative aspects of triptans in treating migraine. Clin Cornerstone. 2001;4(3):53–64. | ||
Loder E. Triptan therapy in migraine. N Engl J Med. 2010;363(1):63–70. | ||
Brand-Schieber E, Munjal S, Kumar R, Andre AD, Valladao W, Ramirez M. Human factors validation study of 3 mg sumatriptan autoinjector, for migraine patients. Med Devices (Auckl). 2016;9:131–137. | ||
Hirsch L, Gibney M, Berube J, Manocchio J. Impact of a modified needle tip geometry on penetration force as well as acceptability, preference, and perceived pain in subjects with diabetes. J Diabetes Sci Technol. 2012;6(2):328–335. | ||
Jaber A, Bozzato GB, Vedrine L, Prais WA, Berube J, Laurent PE. A novel needle for subcutaneous injection of interferon beta-1a: effect on pain in volunteers and satisfaction in patients with multiple sclerosis. BMC Neurol. 2008;8:38. | ||
Gil-Gouveia R, Oliveira AG, Martins IP. The impact of cognitive symptoms on migraine attack-related disability. Cephalalgia. 2016;36(5):422–430. | ||
Gottschalk A, Flocke SA. Time spent in face-to-face patient care and work outside the examination room. Ann Fam Med. 2005;3(6):488–493. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

