Back to Journals » Cancer Management and Research » Volume 14
Study on Changes in Immune Function After Microwave Ablation of Papillary Thyroid Microcarcinoma
Authors Wu T, Sui GQ, Teng DK, Luo Q, Wang H , Lin YQ
Received 16 January 2022
Accepted for publication 4 September 2022
Published 21 September 2022 Volume 2022:14 Pages 2861—2868
DOI https://doi.org/10.2147/CMAR.S358649
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Ahmet Emre Eşkazan
Ting Wu, Guo-Qing Sui, Deng-Ke Teng, Qiang Luo, Hui Wang, Yuan-Qiang Lin
Department of Ultrasound, China-Japan Union Hospital of Jilin University, Changchun, Jilin, 130000, People’s Republic of China
Correspondence: Hui Wang; Yuan-Qiang Lin, Tel +86-431-84995070 ; +86-15948309530, Email [email protected]; [email protected]
Background: In recent years, papillary thyroid microcarcinoma (PTMC) has been a main cause of the high incidence of thyroid carcinoma. No existing study has reported whether microwave ablation (MWA) affects patients’ immunity. Therefore, this study explored the effects of MWA treatment on the immune functions of patients with PTMC.
Methods: This study included 50 patients diagnosed with PTMC who received MWA treatment under ultrasound guidance at the ultrasound department of our hospital from January 2019 to October 2020. Changes in immune function after MWA treatment in PTMC patients were detected by T lymphocyte subsets and cytokines secreted by T helper cells.
Results: At 1 day and 2 weeks after MWA treatment, the proportions of CD3+, CD4+ and CD4+/CD8+ T lymphocyte subsets and the levels of the cytokines interleukin (IL)-2 and interferon (IFN)-γ in the peripheral blood of the patients were significantly higher than those before MWA treatment (P< 0.05). The levels of CD8+ T lymphocytes, tumour necrosis factor (TNF)-α, IL-4, IL-6, IL-10 and IL-17A were not significantly different from those before MWA treatment (P> 0.05). One month after MWA treatment, the proportions of CD3+, CD4+, CD8+ and CD4+/CD8+ T lymphocytes and the levels of the cytokines IL-2, IL-4, IL-6, IL-10, IFN-γ, TNF-α and IL-17A were not significantly different from those before MWA treatment (P> 0.05).
Conclusion: The immune functions of patients with PTMC are temporarily enhanced after MWA treatment, which has important clinical significance for patients’ anti-PTMC ability.
Keywords: microwave ablation, papillary thyroid microcarcinoma, T lymphocyte, cytokine
In recent years, with the improvement in thyroid ultrasound diagnostic techniques and the widespread application of needle biopsy techniques, the detection rate of papillary thyroid microcarcinoma (PTMC) has increased yearly.1 In China, PTMC is mainly treated with surgical resection. Although surgical resection has a definite effect on PTMC, the removal of most of the thyroid gland causes thyroid dysfunction and necessitates life-long medication use,2 and a permanent neck scar often results from the surgery, affecting patients’ appearance and quality of life.3,4 To avoid these problems, thermal ablation methods, represented by microwave ablation (MWA), have attracted considerable attention. MWA has the advantages of a fast heating speed, short ablation time, large ablation range, low trauma, high safety, and few complications. It has achieved high efficacy in the treatment of benign and malignant tumours of the liver, kidneys and lungs.5,6 In addition, some scholars have noted that MWA can improve immunity and enhance antitumor ability. In recent years, MWA has been increasingly more widely used for the treatment of PTMC. However, no existing study has reported whether MWA affects patients’ immunity or anti-PTMC ability. Therefore, this study examined the proportions of T lymphocyte subsets and cytokine levels before and after MWA treatment of PTMC and studied changes in patients’ immune functions, thereby providing a theoretical basis for studying the immune mechanism of MWA treatment for PTMC.
Materials and Methods
Study Subjects
Fifty patients diagnosed with PTMC who received MWA treatment under ultrasound guidance at the ultrasound department of our hospital from January 2019 to October 2020 were enrolled as the research subjects, including 8 males and 42 females aged 18–60 (41.65±9.92) years old. The average size of the lesions was 83.57±77.23 mm3, and the average ablation time was 21.13±6.43 seconds at an ablation power of 25 W. All of the patients and their families signed informed consent forms, and this study was approved by the ethics committee of our hospital.
The inclusion criteria were as follows: (1) papillary carcinoma preoperatively diagnosed by needle cytology or histopathology; (2) a single lesion without extrathyroid invasion or cervical lymph node metastasis; (3) a maximum lesion diameter ≤10 mm; (4) normal thyroid function before MWA treatment and no history of other thyroid diseases (such as acute or chronic thyroiditis); and (5) ineligibility for or refusal of open thyroid surgery. The exclusion criteria were as follows: (1) multiple lesions; (2) extrathyroid invasion, cervical lymph node metastasis or distant metastasis; (3) pregnancy or breastfeeding; (4) other serious systemic or immune-related diseases; (5) severe liver and kidney or cardiopulmonary insufficiency, and (6) severe coagulation dysfunction.
Instruments
(1) The MWA machinery used in this study was an ECO-100C MWA treatment instrument (Nanjing Yigao Microwave System Engineering Co., Ltd.). The ablation needle used was an ECO-100AI3 disposable MWA needle, and the microwave emission frequency for ablation was 2450 MHz. (2) The ultrasound system used in this study was a Mindray Resona 7-colour ultrasound diagnostic instrument with a probe frequency of 7–15 MHz. (3) The ultrasound contrast agent used in this study was 1.8 mg/mL sulfur hexafluoride (Bracco, Italy). The ultrasound contrast agent was dissolved in 5 mL of normal saline and shaken well, and 2.0 mL of the suspension was quickly injected through the patient’s median cubital vein, followed by a bolus of 5 mL of saline. Contrast-enhanced ultrasound was performed before, immediately after and at the follow-up after the MWA treatment. (4) An FC 500MCL flow cytometer (Beckman Coulter, USA) was used to determine the proportions of CD3+, CD4+, CD8+ and CD4+/CD8+ T lymphocytes in the peripheral blood. A BD FACSAria II flow cytometer (BD, USA) was used to detect cytokine levels in the peripheral blood.
Methods
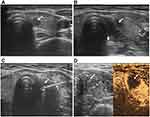
(1) Preoperative preparation: All of the patients underwent a routine ultrasound examination to determine the tumour size, shape, number, location, echo, peripheral blood flow distribution and adjacent vital organs and the presence of suspicious lymph nodes (Figure 1A). The supine position was used to allow full exposure of the neck. All patients underwent ECG monitoring, oxygen inhalation, and intravenous line placement. Routine disinfection and draping were performed before MWA, and 1% lidocaine hydrochloride was percutaneously injected for local infiltration anaesthesia.
(2) Liquid insulation zone: For lesions close to important organs or tissues, such as the carotid artery, trachea, oesophagus or recurrent laryngeal nerve, an insulation solution was repeatedly injected before ablation treatment. A 20-mL syringe was used to inject saline into the space around the tissue for insulation, forming a liquid insulation zone at least 5 mm in width7 (Figure 1B).
(3) Skin opening: A needle equipped with a 20-mL syringe was used to open the skin at the puncture point to create a channel for insertion of the microwave antenna through the skin.
(4) MWA: The MWA instrument was turned on, and cold circulation was checked. After the microwave antenna was inserted into the target area through the skin opening, multisection fixation ablation mode was used in combination with expanded ablation at an ablation power of 25 W. To achieve radical treatment, the ablation range should be expanded by at least 2 mm into the normal thyroid tissue beyond the tumour (Figure 1C).
(5) Immediately after MWA treatment, contrast-enhanced ultrasound was performed to determine the ablation scope, and whether the filling defect area completely covered and exceeded the range of the tumour before MWA treatment was evaluated. If the ablation range was not satisfactory, ablation was repeated (Figure 1D).
(6) After MWA treatment, to prevent bleeding, an ice pack was placed on the ablation area for 30 minutes.
Detection Indicators
Venous blood samples were collected from the patients before MWA treatment and 1 day, 2 weeks and 1 month after MWA treatment to detect the proportions of T lymphocyte subsets (CD3+, CD4+, CD8+ and CD4+/CD8+) and cytokine levels (interleukin (IL)-2, interferon (IFN)-γ, tumour necrosis factor (TNF)-α, IL-4, IL-6, IL-10 and IL-17A) in the peripheral blood.
Statistical Analysis
SPSS 26.0 software was used for data processing. Normally distributed measurement data are presented as the means±standard deviations, and nonnormally distributed data are presented as the medians and interquartile ranges. Intragroup comparisons were performed by a paired t-test or rank-sum tests of related samples. P<0.05 indicated a statistically significant difference.
Results
Complications
During MWA treatment, 1 of the 50 patients developed hoarseness and recovered completely within 1 hour after MWA treatment, and on the next day, laryngoscopy showed that the movement ranges of both vocal folds were normal. Two of the 50 patients developed radial pain in the area behind the ear on the same side as the ablation, which resolved within 30 minutes after MWA treatment.
Effectiveness of MWA Treatment
Contrast-enhanced ultrasound performed 1 day after MWA showed that the lesion area was completely covered by the ablation range and was free of contrast agent filling. The volume of the ablation area gradually decreased at 2 weeks and 1 month after MWA.
Changes in T Lymphocyte Subsets and Cytokines
Changes in T lymphocyte subsets in the peripheral blood of the patients before and after MWA treatment: One day and 2 weeks after MWA treatment, the proportions of CD3+, CD4+ and CD4+/CD8+ T lymphocytes in the peripheral blood of the patients were significantly higher than those before MWA treatment (P<0.05), while the proportion of CD8+ T lymphocytes was not significantly different from that before MWA treatment (P>0.05). One month after MWA treatment, the proportions of CD3+, CD4+, CD8+ and CD4+/CD8+ T lymphocytes in the peripheral blood were not significantly different from those before MWA treatment (P>0.05) (Table 1). The changes in T lymphocyte subsets in the peripheral blood of the patients before and after MWA treatments are shown in Figure 2A.
 |
Changes in cytokine levels in the peripheral blood of the patients before and after MWA treatment: One day and 2 weeks after MWA treatment, the levels of IL-2 and IFN-γ secreted by T helper 1 (Th1) cells were significantly higher than those before MWA treatment (P<0.05), while the TNF-α concentration was not significantly different from that before MWA treatment (P>0.05). Moreover, the levels of IL-4, IL-6 and IL-10 secreted by T helper 2 (Th2) cells were not significantly different from those measured before MWA treatment (P>0.05), and the level of IL-17A secreted by T-helper 17 (Th17) cells was not significantly different from that measured before MWA treatment (P>0.05).
One month after MWA treatment, the levels of IL-2, IL-4, IL-6, IL-10, IFN-γ, TNF-α and IL-17A were not significantly different from those measured before MWA treatment (P>0.05) (Table 2). The changes in the cytokine levels in the peripheral blood of the patients before and after MWA treatment are shown in Figure 2B.
 |
Table 2 Changes in Cytokine Levels Before and After MWA Treatment (n=50, Pg/Ml) |
Discussion
With the flourishing development of ablation techniques, MWA has gradually become a novel method for the treatment of thyroid diseases. The efficacy of MWA for the treatment of benign thyroid nodules and recurrent thyroid cancer has been widely recognized.8,9 The use of MWA for PTMC is becoming standardized and is being extensively applied. The expert group in China has issued a number of consecutive expert consensuses10,11 with the aims of affirming the efficacy of MWA for the treatment of PTMC, standardizing the indications and contraindications of MWA for PTMC and promoting the application of MWA for PTMC treatment. Previous studies have reported changes in the immune capacity after MWA treatment for liver cancer, showing that the proportions of CD4+ and CD4+/CD8+ T lymphocytes in the peripheral blood increased after ablation, suggesting that MWA treatment for primary liver cancer not only inactivates tumour cells but also increases the number of immune cells available to exert antitumor functions. Some scholars have studied changes in serum Th1- and Th2-type cytokines in patients with liver cancer after radiofrequency ablation12 and found that Th1-type cytokines (IL-2 and TNF-α) increased in peripheral blood after ablation, while Th2-type cytokines (IL −4 and IL-10) decreased, suggesting that radiofrequency ablation can improve immune functions and enhance antitumor abilities. However, no report on changes in immune functions after MWA for PTMC are available. Therefore, this study analysed changes in T lymphocyte subsets (CD3+, CD4+, CD8+ and CD4+/CD8+) and cytokine concentrations (IL-2, IL-4, IL-6, IL-10, TNF-α, IFN-γ and IL-17A) to explore the effects of MWA on immune function in PTMC patients.
No serious complications occurred after MWA treatment in the 50 patients included in this study. The range of the contrast-enhanced ultrasound completely covered the lesion on the first day after MWA treatment, and the volume of the ablation area gradually decreased at 2 weeks and 1 month after MWA treatment, indicating that MWA is a safe and effective treatment method.
T lymphocyte subsets (CD3+, CD4+, CD8+ and CD4+/CD8+) are the main indicators of cellular immune functions, all of which play an important role in immune regulation by playing an antiviral role and regulating the functions of the immune system.13 This study found that 1 day and 2 weeks after MWA treatment, the proportions of CD3+, CD4+ and CD4+/CD8+ T lymphocytes in the patients’ peripheral blood increased significantly, suggesting enhancement of the patients’ immune functions, which is consistent with the results of domestic and international scholars who have studied the use of MWA for liver cancer.14 The change in the number of CD3+ T lymphocytes can be used to evaluate the overall immune functional status of tumour patients. An increase in the number of CD3+ T lymphocytes indicates an increase in the total number of mature T lymphocytes and an enhanced biological effect. This study found that the number of CD3+ T lymphocytes in the peripheral blood of the patients increased 1 day and 2 weeks after MWA treatment, indicating that MWA treatment has a positive regulatory effect on T lymphocytes. CD4+ T lymphocytes are the most important hub cells in the regulation of immune responses. These cells can stimulate the expression of cytokines by a variety of immune cells and Th cells and mainly play an important role in antitumor immunity. In this study, the CD4+ T lymphocyte count in the peripheral blood of the patients at 1 day and 2 weeks after MWA treatment increased significantly, indicating that MWA promotes the secretion of immune cells. CD8+ T lymphocytes have a specific immunosuppressive effect as part of the immune response and play a negative regulatory role by releasing inhibitory cytokines. The results of this study showed no significant changes in CD8+ T lymphocytes before and after MWA treatment, which may indicate that MWA treatment does not act by regulating CD8+ T lymphocytes. The proportion of CD4+/CD8+ T lymphocytes is a sensitive indicator of immune function disorders of the human body and reflects the overall status of immune function. The results of this study showed that the proportion of CD4+/CD8+ T lymphocytes in the peripheral blood increased significantly 1 day and 2 weeks after MWA treatment, indicating that the overall immune function of the body was enhanced. The various changes in the T lymphocyte subsets indicate that MWA treatment not only inactivates tumour cells but also increases the number of immune cells released by the body.14
We also studied major changes in cytokines secreted by Th1 cells (IL-2, INF-γ and TNF-α), Th2 cells (IL-4, IL-6 and IL-10) and Th17 cells (IL-17A). In this study, IL-2 and IFN-γ levels increased at 1 day and 2 weeks after MWA treatment, indicating that the body’s antitumor immune effect increased and that the immune ability increased in a short period after MWA. However, the levels of these factors at 1 month after MWA were similar to the preoperative levels; a possible explanation is that as the inactivated tumour tissue is gradually absorbed by the body, the secretion of various cytokines gradually reaches a dynamic balance.
The results of this study showed that the proportions of CD3+, CD4+ and CD4+/CD8+ T lymphocytes in the peripheral blood of patients after MWA were significantly increased and that the levels of IL-2 and INF-γ after MWA were significantly higher than those before MWA, indicating that MWA for PTMC is conducive to short-term improvement in patients’ immune function.
At present, the mechanism of immune regulation after MWA treatment of PTMC is still not clear. According to a similar study,15 the possible regulatory mechanisms are as follows:
(1) Reduction in the tumour burden: MWA employs microwave needle thermoablation to cause coagulation and necrosis of tumour cells to completely inactivate them, thereby reducing the tumour burden, decreasing the secretion of immunosuppressive factors and relieving the immunosuppressive effect of cancer cells on the body. (2) Thermal effect and membrane structure theory: cancer cells undergo degeneration and necrosis after exposure to the heating stimulus of the MWA needle and remain in the body as antigens. The antigenic tumour determinants on the surfaces of cancer cells are fully exposed, which increases the antigenicity of the tumour cells and facilities antibody binding to the tumour cells. When these antigens are taken up by antigen-presenting cells, they induce T lymphocytes to exert immune responses, thereby improving the body’s immune functions. (3) Immune responses of cytokines and humoural factors: A previous study showed that thermal therapy can increase the activity of lymphocytes and enhance the body’s immunity by increasing the secretion of cytokines. Tumour cells reduce IL-2 levels in the body by releasing sIL-2R to competitively bind to the IL-2 secreted by Th1 cells, thus exerting an inhibitory effect on the immune system. (4) Inflammatory response and immune stimulation: pathological examinations of tumour tissue after heat treatment revealed a large number of lymphocytes surrounding the necrotic foci, suggesting that the inflammatory response can eliminate tumour cells,16 and through this mechanism, IFN-γ promotes the expression of inflammatory factors to exert an immune effect. (5) Immune response of heat shock protein: MWA mainly converts microwave energy into heat energy, which then kills cancer cells as the temperature increases. When cancer cells are heated, the body is believed17 to undergo a stress response and can synthesize a stress protein, heat shock protein (HSP), which protects the body. After T lymphocytes recognize heat shock proteins, they participate in cellular immunity to initiate a specific immune response.
The limitations of this study are that only changes in various T lymphocyte subsets and cytokines at 1 day, 2 weeks and 1 month after MWA were compared with those before MWA, and no long-term follow-up values were analysed. Therefore, our next step is to further explore the long-term effects of MWA on the immune function of the body. Additionally, this study had a small sample size, and future studies require a large sample size for in-depth research.
An inclusion criterion in this study was the absence of cervical lymph node metastasis. However, neck lymph node dissection is still a hot topic in surgery.18 The main objective of this study was to evaluate changes in T lymphocyte subsets and cytokines in patients’ peripheral blood. In future research, we will select more meaningful markers for analysis, including changes in markers such as calcitonin or carcinoembryonic antigen before and after MWA treatment. During our research, we found that many patients with thyroid cancer have Hashimoto’s thyroiditis, and some scholars have reported that Hashimoto’s thyroiditis has a good prognostic effect on papillary thyroid carcinoma.19 Therefore, the influence of Hashimoto’s thyroiditis on the immune function of PTMC patients after ablation is also an important topic.
In summary, MWA for PTMC is safe and effective. The CD3+, CD4+ and CD4+/CD8+ T lymphocytes and the cytokines IL-2 and IFN-γ in the peripheral blood of the patients increased at 1 day and 2 weeks after MWA treatment, indicating that patients’ immune functions are enhanced in the short term, which has important clinical significance for anti-PTMC activity.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of China-Japan Union Hospital of Jilin University (protocol code 2019082112 and date of approval: 21 August 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Author Contributions
All authors made significant contributions to the work reported, whether the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; provided final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was funded by Changchun Kedi Economic & Trade Co., Ltd. (grant number 3R219D393430) and the Finance Department of Jilin Province (grant number 2019SCZ027).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Ito Y, Oda H, Miyauchi A. Insights and clinical questions about the active surveillance of low-risk papillary thyroid microcarcinomas [Review]. Endocr J. 2016;63(4):323–328. doi:10.1507/endocrj.EJ15-0637
2. Chahardahmasumi E, Salehidoost R, Amini M, et al. Assessment of the early and late complication after thyroidectomy. Adv Biomed Res. 2019;8:14. doi:10.4103/abr.abr_3_19
3. Gardner IH, Doherty GM, Mcaneny D. Intraoperative nerve monitoring during thyroid surgery. Curr Opin Endocrinol Diabetes Obes. 2016;23(5):394–399. doi:10.1097/MED.0000000000000283
4. Goldfarb M, Casillas J. Thyroid cancer-specific quality of life and health-related quality of life in young adult thyroid cancer survivors. Thyroid. 2016;26(7):923–932. doi:10.1089/thy.2015.0589
5. Izzo F, Granata V, Grassi R, et al. Radiofrequency ablation and microwave ablation in liver tumors: an update. Oncologist. 2019;24(10):e990–e1005. doi:10.1634/theoncologist.2018-0337
6. Yong C, Mott SL, Laroia S, et al. Outcomes of microwave ablation for small renal masses: a single center experience. J Endourol. 2020;34(11):1134–1140. doi:10.1089/end.2020.0348
7. Xiaoyin T, Ping L, Dan C, et al. Risk assessment and hydrodissection technique for radiofrequency ablation of thyroid benign nodules. J Cancer. 2018;9(17):3058–3066. doi:10.7150/jca.26060
8. Cheng Z, Che Y, Yu S, et al. US-guided percutaneous radiofrequency versus microwave ablation for benign thyroid nodules: a prospective multicenter study. Sci Rep. 2017;7(1):9554. doi:10.1038/s41598-017-09930-7
9. Zheng BW, Wang JF, Ju JX, et al. Efficacy and safety of cooled and uncooled microwave ablation for the treatment of benign thyroid nodules: a systematic review and meta-analysis. Endocrine. 2018;62(2):307–317. doi:10.1007/s12020-018-1693-2
10. Xu D, Ge M, Yang A, et al. Expert consensus workshop report: guidelines for thermal ablation of thyroid tumors (2019 edition). J Cancer Res Ther. 2020;16(5):960–966. doi:10.4103/jcrt.JCRT_558_19
11. Ye X, Fan W, Wang H, et al. Expert consensus workshop report: guidelines for thermal ablation of primary and metastatic lung tumors (2018 edition). J Cancer Res Ther. 2018;14(4):730–744. doi:10.4103/jcrt.JCRT_221_18
12. Ji L, Gu J, Chen L, et al. Changes of Th1/Th2 cytokines in patients with primary hepatocellular carcinoma after ultrasound-guided ablation. Int J Clin Exp Pathol. 2017;10(8):8715–8720.
13. Alkozai EM, Nijsten MW, De Jong KP, et al. Immediate postoperative low platelet count is associated with delayed liver function recovery after partial liver resection. Ann Surg. 2010;251(2):300–306. doi:10.1097/SLA.0b013e3181b76557
14. Leuchte K, Staib E, Thelen M, et al. Microwave ablation enhances tumor-specific immune response in patients with hepatocellular carcinoma. Cancer Immunol Immunother. 2021;70(4):893–907. doi:10.1007/s00262-020-02734-1
15. Bastianpillai C, Petrides N, Shah T, et al. Harnessing the immunomodulatory effect of thermal and non-thermal ablative therapies for cancer treatment. Tumour Biol. 2015;36(12):9137–9146. doi:10.1007/s13277-015-4126-3
16. Mogensen TH. IRF and STAT transcription factors - from basic biology to roles in infection, protective immunity, and primary immunodeficiencies. Front Immunol. 2018;9:3047. doi:10.3389/fimmu.2018.03047
17. Taha EA, Ono K, Eguchi T. Roles of extracellular HSPs as biomarkers in immune surveillance and immune evasion. Int J Mol Sci. 2019;20(18):4588. doi:10.3390/ijms20184588
18. Gambardella C, Patrone R, Di Capua F, et al. The role of prophylactic central compartment lymph node dissection in elderly patients with differentiated thyroid cancer: a multicentric study. BMC Surg. 2019;18(Suppl 1):110. doi:10.1186/s12893-018-0433-0
19. Marotta V, Sciammarella C, Chiofalo MG, et al. Hashimoto’s thyroiditis predicts outcome in intrathyroidal papillary thyroid cancer. Endocr Relat Cancer. 2017;24(9):485–493. doi:10.1530/ERC-17-0085
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.