Back to Journals » Clinical, Cosmetic and Investigational Dentistry » Volume 14
Study of Alveolar Bone Remodeling Using Deciduous Tooth Stem Cells and Hydroxyapatite by Vascular Endothelial Growth Factor Enhancement and Inhibition of Matrix Metalloproteinase-8 Expression in vivo
Authors Saskianti T , Nugraha AP, Prahasanti C, Ernawati DS, Tanimoto K, Riawan W, Kanawa M, Kawamoto T , Fujimoto K
Received 16 December 2021
Accepted for publication 11 March 2022
Published 24 March 2022 Volume 2022:14 Pages 71—78
DOI https://doi.org/10.2147/CCIDE.S354153
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Christopher E. Okunseri
Tania Saskianti,1 Alexander Patera Nugraha,2 Chiquita Prahasanti,3 Diah Savitri Ernawati,4 Kotaro Tanimoto,5 Wibi Riawan,6 Masami Kanawa,7 Takeshi Kawamoto,8,9 Katsumi Fujimoto8,10
1Department of Pediatric Dentistry, Faculty of Dental Medicine, Universitas Airlangga, Surabaya, Indonesia; 2Department of Orthodontics, Faculty of Dental Medicine, Universitas Airlangga, Surabaya, Indonesia; 3Department of Periodontology, Faculty of Dental Medicine, Universitas Airlangga, Surabaya, Indonesia; 4Department of Oral Medicine, Faculty of Dental Medicine, Universitas Airlangga, Surabaya, Indonesia; 5Department of Orthodontics and Craniofacial Developmental Biology, Graduate School of Biomedical & Health Sciences, Hiroshima University, Hiroshima, Japan; 6Biomolecular Biochemistry, Faculty of Medicine, Brawijaya University, Malang, Indonesia; 7Natural Science Center for Basic Research and Development, Hiroshima University, Hiroshima, Japan; 8Department of Dental and Medical Biochemistry, Graduate School of Biomedical & Health Sciences, Hiroshima University, Hiroshima, Japan; 9Writing Center, Hiroshima University, Higashi-Hiroshima, Japan; 10Department of Molecular Biology and Biochemistry, Graduate School of Biomedical & Health Sciences, Hiroshima University, Hiroshima, Japan
Correspondence: Tania Saskianti, Department of Pediatric Dentistry, Faculty of Dental Medicine, Universitas Airlangga, Jalan Prof. Dr. Moestopo No. 47, Surabaya, 60132, Indonesia, Tel +62 81 232014445, Email [email protected]
Background: Periodontitis progression is characterized by alveolar bone loss, and its prevention is a major clinical problem in periodontal disease management. Matrix metalloproteinase-8 (MMP-8) has been shown to adequately monitor the treatment of chronic periodontitis patients as gingival crevicular fluid MMP-8s were positively associated with the severity of periodontal disease. Moreover, modulating the vascular endothelial growth factor (VEGF) levels in bones could be a good way to improve bone regeneration and cure periodontitis as VEGF promotes endothelial cell proliferation, proteolytic enzyme release, chemotaxis, and migration; all of which are required for angiogenesis.
Purpose: The aim of this study was to determine the effect of hydroxyapatite incorporated with stem cells from exfoliated deciduous teeth (SHED) in Wistar rats’ initial alveolar bone remodeling based on the findings of MMP-8 and VEGF expressions.
Methods: A hydroxyapatite scaffold (HAS) in conjunction with SHED was transplanted into animal models with alveolar mandibular defects. A total of 10 Wistar rats (Rattus norvegicus) were divided into two groups: HAS and HAS + SHED. Immunohistochemistry staining was performed after 7 days to facilitate the examination of MMP-8 and VEGF expressions.
Results: The independent t-test found significant downregulation of MMP-8 and upregulation VEGF expressions in groups transplanted with HAS in conjunction with SHED compared with the HAS group (p < 0.05).
Conclusion: The combination of SHED with HAS on alveolar bone defects may contribute to initial alveolar bone remodeling as evident through the assessments of MMP-8 and VEGF expressions.
Keywords: angiogenesis, medicine, osteogenesis, scaffold, tissue engineering
Introduction
Periodontal disease is an infectious and inflammatory condition that damages the teeth’s supporting structures through bone resorption and periodontal tissue loss caused by acute (sometimes violent) or chronic inflammation.1 Periodontal disease may result in edentulism and has been linked to severe systemic disorders, including atherosclerosis, cardiovascular disease, diabetes, and rheumatoid arthritis.2–5 This may have a direct impact on afflicted individuals’ general health, social life, and nutritional status, endangering their entire quality of life.6–9 The global prevalence of periodontal disease is believed to be around 11%, which is the sixth most common human disease with a significant public health burden worldwide.10 As a response, it is critical to provide a timely and effective therapy for periodontal disease.
The ultimate goal of periodontal therapy is to slow the progression of periodontitis and enhance periodontal tissue regeneration.11 Scaling and root planing, as well as periodontal surgery for periodontal tissue rebuilding, are the major treatments for periodontal tissue inflammation.12 However, the clinical outcomes in patients with periodontal disease are not completely satisfactory because the destroyed tissue is not regenerated.13 The desired therapeutic outcome is a proper regeneration of alveolar bone, root cementum, and periodontal ligaments in the previously damaged periodontium.14 As a result, various therapeutic options have been proposed, including stem cell–based tissue engineering and regenerative therapy.15–17
Among mesenchymal stem cells (MSCs) from dental tissue, human exfoliated deciduous tooth cells (SHEDs) are prominent.18 Dental stem cells were initially isolated from the dental pulp of permanent teeth (DPSC) and then from the dental pulp of deciduous teeth (SHED).19 Miura et al were the first to successfully employ SHED in vivo in conjunction with a scaffold for bone tissue building applications. Other research showed that SHED and human DPSC transplantation in the calvaria of immunodeficient mice resulted in nearly the same quantity of new bone formation as human bone marrow MSC transplantation.20 As they originate from a more immature subpopulation than permanent teeth, SHED have a higher proliferation rate and differentiation potential since they can differentiate into neural cells, adipocytes, osteoblasts, and odontoblasts.19 In addition, SHED are capable of spontaneously producing large volumes of bone in vivo.19,21 Because of the ease of availability, SHED are excellent source of stem cells.
In addition to the source of the stem cell, other aspects are critical for successful tissue engineering, such as the biomaterial to be selected as a scaffold and the correct linkage between them.22 To regenerate the bone tissue defect, the selected biomaterial must allow cells to migrate, proliferate, and differentiate into bone cells, but local angiogenesis is also required to provide the necessary nutrients and environmental factors for correct bone tissue development.23 Hydroxyapatite (HA) is a frequently used biomaterial for constructing a scaffold. When utilized as a bone graft, HA, a key mineral component of human hard tissue that is widely used clinically to repair alveolar bone defects, is a bioactive material that also exhibits osseointegration, osteoconduction, and osteogenesis characteristics.19,24 However, little research exists on the initial alveolar bone-remodeling ability of HA as a scaffold material used as therapy along with the use of SHED as an osteoinductive substance in alveolar bone defects.
Matrix metalloproteinase-8 (MMP-8) and vascular endothelial growth factor (VEGF) are involved in regenerative therapy with transplanted SHED in alveolar bone defects. In this study, SHED was combined with a hydroxyapatite scaffold (HAS) and transplanted onto rat models with alveolar bone defects to demonstrate the potential effects of these incorporated materials on initial bone remodeling by evaluating MMP-8 and VEGF expressions. Because of its high level of expression from neutrophils, MMP-8 plays a role in initiating collagen degradation in the extracellular matrix during embryogenesis, bone healing, and bone regeneration, as well as reflecting the inflammatory response in the first wound repair stage.25–29 Moreover, angiogenesis is controlled by a number of growth factors, most notably VEGF, which is produced by inflammatory and stromal cells that are recruited to the site of the bone injury to promote blood vessel formation. Because of its primary ability to stimulate neovascularization, VEGF is of special importance in bone regeneration.30–32 Thus, the aim of this study is to investigate the effect of both HA with SHED on MMP-8 and VEGF expression in the alveolar defects of Wistar rats (Rattus norvegicus).
Materials and Methods
Ethical Approval
The Universitas Airlangga, Faculty of Dental Medicine ethics committee granted ethical approval for both human sampling and animal experiments (171/HRECC.FODM/VIII/2017).
Study Design
This was an experimental laboratory study with a posttest-only control group design. The sample size was calculated using the minimal sample size formula. The sample count was five experimental animals in each group (N=10, n=5). Each group’s sample was selected at random by assigning a tag number to each experimental animal and selected blindly.
Cell Culture
The SHED was collected from deciduous teeth that met the following criteria: #83 and #73 deciduous teeth that were free of cavities, had no root resorption confirmed by apical radiography, and had a vital and intact pulp. Healthy deciduous teeth were taken from a healthy 9-year-old male child who was undergoing orthodontic treatment at the Universitas Airlangga Dental Hospital, Surabaya, Indonesia. Patient confidentiality was protected, and a signed informed consent from the patient’s parents was acquired.
The SHED was isolated using the same protocol as previously described.33 The stemness of the SHED was confirmed by cluster of differentiation (CD) 105 (+) and CD 45 (-). The medium was changed every four days to remove the detached cell from the culture plate, and the cells were maintained for four passages. To remove debris, the cells were washed with a phosphate buffer saline. To separate the cells and transfer them to a larger culture plate, trypsin-EDTA 0.05% was used. The SHED cells in the four passages were prepared for the next step of the investigation after they attained 70–80% confluence.33–35 A 20-mL suspension of the SHED at passage four to five with a density of 106 cells was seeded into HAS (bio hydrox hydroxyapatite, Biomaterial Center Dr. Soetomo Tissue Bank) before being placed in a 24-well tissue culture plate and prepared for the experimental group. The dose was determined using the data from a prior in vivo investigation, which reported 106 cells per sample.
Alveolar Bone-Defective Animal Model Preparation
Ten healthy, three-month-old male Wistar rats (R. norvegicus) of approximately 150–250 grams body weight were obtained from the Research Center of the Faculty of Dental Medicine, Universitas Airlangga, Surabaya, Indonesia. Five samples were randomly allocated to one of two groups: HAS and HAS + SHED.
To minimize animal suffering, all experimental procedures involving animals were carried out in accordance with the National Institute of Health’s Guide for the Care and Use of Laboratory Animals.35 Because the animal models originated from different places, they were acclimatized for a week at a temperature of 21–23°C with controlled humidity (50 ± 5%) in a 12-hour artificial light cycle (8 am to 8 pm) to let them adjust to the environment. All of the rats were placed in polycarbonate cages sized 0.90 m × 0.60 m × 0.60 m. Furthermore, all animal models were fed a regular pellet diet and given free access to water, and the husk was replaced every three days. Food consumption and fecal parameters of all animal models were routinely inspected and observed.36 Following the induction of an alveolar bone defect by extracting the rat’s mandibular incisor, samples from the HAS and HAS + SHED groups were transplanted into the affected area. A 5.0 suture monofilament was utilized to initiate the interrupted suture’s repair of the incision following transplantation.37
All animal models were euthanized seven days after the transplantation to analyze early alveolar bone remodeling. Euthanasia was performed via an overdosed rodent anesthesia, an intravenous injection of 100 mg/kg BW (Pentobarbital, PubChem, USA). This method of euthanasia was selected to alleviate any pain caused by the euthanization process. The affected alveolar bone samples were collected for histological investigation following the animal trial. Using sterile sharp surgical scissors (metzenbaum scissors fine tips, no cat. 3565, Medesy, Maniago, Italy) and a tweezer (Tweezer de bakey mini, no cat. 1007/10-TO, Medesy, Maniago, Italy), the animal model’s head was cut from the back, exposing the anterior mandible and allowing the afflicted alveolar bone sample to be obtained. All of the animals were examined for any signs of general toxicity, such as edema and their body weight was assessed using a digital scale (ZB22-P, Zieis®, USA). A single blind observer performed all of the measurements. Finally, the affected tissue was removed and fixed in a 10% neutral buffer formalin solution.
Tissue Embedding, Sectioning, and Processing
The sample was decalcified and submerged in 10% EDTA (Ajax Finechem, Thermo Fisher Scientific, Taren Point, Australia; cat no. 17,892). The samples were then processed overnight (Leica TP1020, USA) before being embedded in molten paraffin wax (Leica HistoCore Arcadia H - Heated Paraffin Embedding Station, USA). A 5 m rotary microtome (RM2235, Leica, USA) was used to cut the sections. Flattened paraffin ribbons were collected onto polysine microscope slides (Thermo Scientific) and dried at 60°C for 16 hours (Sakura Heater, Tokyo, Japan).38
Immunohistochemistry Staining
A 3.3’-diaminobenzidine stain kit (DAB; cat no. D7304-1SET, Sigma Aldrich, US) was used for immunohistochemistry staining. This study used a 1:500 dilution of VEGF antibody monoclonal (AbMo; cat. no sc-7269) and MMP-8 (cat. no sc-514803; Santa Cruz BiotechnologyTM, US). Using a Nikon H600L light microscope (Japan) at 400× magnification, two observers manually counted and examined the number of VEGF expressions in the periodontal tissue in five fields of view. Each marker was also magnified by 1000× for context (Nikon, Japan).38
Statistical Analysis
To analyze the data in this study, the Statistical Package for Social Science (SPSS) 20.0 version (IBM corporation, Illinois, Chicago, United States) software was utilized. A t-test (p < 0.05) was used to compare the significant differences in VEGF and MMP-8 expressions across the groups.
Results
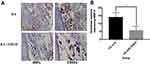
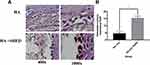
To examine whether SHED + HAS affected MMP-8 and VEGF expression after transplantation, immunohistochemical staining was performed on day 7. The number of MMP-8–expressing cells in the HA + SHED group was significantly lower than those in the HAS group (p < 0.05; see Figure 1, Table 1). Meanwhile, the number of VEGF-positive cells in the HA + SHED group was significantly higher than those in the control group (see Figure 2, Table 1).
 |
Table 1 The Expression of MMP-8 and VEGF in Osteoblast in the Periodontal Tissue Afflicted Area of Wistar Rats (R. norvegicus); n=5 |
Discussion
Periodontitis progression is characterized by alveolar bone loss. A range of treatment techniques have been proposed, including bone grafts, directed tissue regeneration, root conditioning, enamel matrix derivatives, and a combination of the above procedures. Among these attempts, an unequivocal success of these treatments has not been found. Novel technologies based on tissue engineering (using stem cells and scaffolding) may emerge as possible therapies.1
In this study, the animal experiment was done in seven days to analyze the early markers of alveolar bone remodeling via the expressions of VEGF and MMP-8. This experimental work supports the idea that SHED seeded in HAS could decrease the number of biomarker expressions for detecting alveolar bone destruction (such as MMP-8 expression) in bone defects after seven days when compared with the HAS group. Due to their role in the pathological breakdown of the extracellular matrix (ECM) within periodontal tissues, several pieces of evidence show that the active MMP-8 (collagenase-2) derived from neutrophils is the most critical mechanism in the tissue destruction associated with periodontal disease. Pathogens in dental plaque can trigger host cells to increase MMP-8 release, which is one of the indirect causes of tissue damage that occurs in periodontitis.39,40 A high level of MMP-8 in the HAS group could be explained by an increased immune response to the presence of the scaffold as a foreign object. A significant decrease in MMP-8 expression was noted in the HAS + SHED group compared with the HAS group (p < 0.05). This result supports the theory that the SHED as an MSCs lineage may play a role in supporting the immunomodulation towards an inflammatory response suppression. Similar findings by Mauney et al and Rahyussalim et al showed that when MSCs were induced for osteogenic differentiation, their expressions of MMP-1 and MMP-8 decreased. MMP-8, a collagenase that degrades collagen, was regulated to ensure the greatest possible ECM environment and structural formation after osteogenic differentiation.27,41
Ceramic scaffolds such as HAS offer the greatest promise for stem cell–based bone engineering due to its high cell adhesion and proliferation,42,43 and is also essential in promoting SHED proliferation and differentiation.35 Furthermore, using HA as a biodegradable scaffold provides skeletal support for osteogenic cell development during the early stages of bone repair. When SHED was seeded in an HA scaffold, the VEGF angiogenesis markers expressing cells significantly increased compared with those in the HAS group (p < 0.05). This could be explained by the fact that HA is a porous bioceramic that permits the formation of capillaries and other blood vessels. Due to their ease of vascularization and high oxygen permeability, the pores of an HA scaffold aid in osteogenesis.44,45 SHED, in addition to its ability to differentiate into osteoblasts, may also differentiate into vascular endothelial cells.46 Angiogenesis and osteogenesis are very strongly linked. Angiogenesis is required to sustain and maintain bone formation and maintenance. Blood vessels also serve as a network of communication for bones and surrounding tissues.47,48 Cetinkaya et al showed that VEGF expression was greatly elevated throughout the healing stage of periodontal disease.49 Further, the study showed that VEGF expression was more connected with the non-inflammatory component of the enlarged tissue than with the inflammatory component as there was a clear positive association between the number of blood vessels and VEGF expression only in the healing group. These findings could suggest a relationship between VEGF production and vascularization in the resolution of inflammation and the spontaneous healing of periodontal tissues.
The VEGF expressed by osteoblasts is important in supporting bone regeneration during inflammation and maintaining bone hemostasis. VEGF plays crucial roles in some phases of the bone-remodeling process. A previous study showed that VEGF depletion in osteoblasts inhibits the bone-remodeling process. Macrophages, as inflammatory cells, require VEGF to promote their migration during inflammation phase. Adequate VEGF levels or expressions are necessary in maintaining angiogenesis and osteogenesis in the bone-defective area.50 In the alveolar bone-defective area, the microenvironment was hypoxic. In addition, VEGF and stem cell migration was regulated by the condition of hypoxia. The vascularization supports bone development and the proliferation of osteoblast cells.51
SHED showed a prominent ability to differentiate into osteogenic and odontogenic lineage in vitro.33 Regenerative therapy using SHED and HAS can regenerate alveolar-defective animal models by increasing VEGF expression and decreasing MMP-8 expression. Compared with DPSCs, SHED showed both a higher capacity to increase osteoblast markers related to osteoblastic differentiation and expressed higher levels of alkaline phosphatase (ALP), Col I and osteocalcin (OCN) compared with DPSCs.52 The stemness and multipotency of SHED was maintained by some growth factor, such as basic fibroblast growth factor and VEGF.53
The limitations of this study were that the observations and evaluations were performed seven days post transplantation of SHED seeded in HAS on the animal model, and only an immunohistochemical examination was performed. Further studies are necessary to evaluate the changes in the alveolar bone and periodontal tissue post transplantation of SHED seeded in HAS in the alveolar bone defect in animal models. With a longer observation time, further studies using other methods, such as quantitative reverse transcription polymerase chain reaction (RT-qPCR) or the Western blot analysis, could be conducted to estimate the expression of bone molecular markers. Future studies are also required to confirm the effective dose of the selected biomaterials when they are ready to be applied in clinical human studies.
Conclusion
The expression of VEGF increases significantly with treatment of SHED seeded in HAS, whereas MMP-8 expression in the alveolar bone decreases in SHED seeded in HAS, as observed immunohistochemically.
Acknowledgments
Research reported in this publication was supported by International Collaboration Research Grant 2021 (No: 792/UN3.15/PT/2021) from Universitas Airlangga.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Irani FC, Wassall RR, Preshaw PM. Impact of periodontal status on oral health-related quality of life in patients with and without type 2 diabetes. J Dent. 2015;43(5):506–511. doi:10.1016/j.jdent.2015.03.001
2. Ide M, Linden GJ. Periodontitis, cardiovascular disease and pregnancy outcome–focal infection revisited? Br Dent J. 2014;217(8):467–474. doi:10.1038/sj.bdj.2014.903
3. Lalla E, Papapanou PN. Diabetes mellitus and periodontitis: a tale of two common interrelated diseases. Nat Rev Endocrinol. 2011;7(12):738–748. doi:10.1038/nrendo.2011.106
4. Araújo VM, Melo IM, Lima V. Relationship between periodontitis and rheumatoid arthritis: review of the literature. Mediators Inflamm. 2015;2015:259074. doi:10.1155/2015/259074
5. Loos BG. Systemic effects of periodontitis. Int J Dent Hyg. 2006;4(Suppl 1):34–52. doi:10.1111/j.1601-5037.2006.00200.x
6. Chapple IL. Time to take periodontitis seriously. BMJ. 2014;348:g2645. doi:10.1136/bmj.g2645
7. Chapple IL, Van der Weijden F, Doerfer C, et al. Primary prevention of periodontitis: managing gingivitis. J Clin Periodontol. 2015;42(Suppl 16):S71–S76. doi:10.1111/jcpe.12366
8. Petersen PE, Ogawa H. The global burden of periodontal disease: towards integration with chronic disease prevention and control. Periodontol 2000. 2012;60(1):15–39. doi:10.1111/j.1600-0757.2011.00425.x
9. Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366(9499):1809–1820. doi:10.1016/S0140-6736(05)67728-8
10. Kassebaum NJ, Bernabé E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. Global burden of severe periodontitis in 1990–2010: a systematic review and meta-regression. J Dent Res. 2014;93(11):1045–1053. doi:10.1177/0022034514552491
11. Karring T, Nyman S, Gottlow J, Laurell L. Development of the biological concept of guided tissue regeneration–animal and human studies. Periodontol 2000. 1993;1(1):26–35. doi:10.1111/j.1600-0757.1993.tb00204.x
12. Nyman S, Lindhe J, Karring T, Rylander H. New attachment following surgical treatment of human periodontal disease. J Clin Periodontol. 1982;9(4):290–296. doi:10.1111/j.1600-051x.1982.tb02095.x
13. Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Primers. 2017;3(1):17038. doi:10.1038/nrdp.2017.38
14. Gottlow J, Nyman S, Karring T, Lindhe J. New attachment formation as the result of controlled tissue regeneration. J Clin Periodontol. 1984;11(8):494–503. doi:10.1111/j.1600-051x.1984.tb00901.x
15. Sanz AR, Carrión FS, Chaparro AP. Mesenchymal stem cells from the oral cavity and their potential value in tissue engineering. Periodontol 2000. 2015;67(1):251–267. doi:10.1111/prd.12070
16. Sallum EA, Ribeiro FV, Ruiz KS, Sallum AW. Experimental and clinical studies on regenerative periodontal therapy. Periodontol 2000. 2019;79(1):22–55. doi:10.1111/prd.12246
17. Ouchi T, Nakagawa T. Mesenchymal stem cell-based tissue regeneration therapies for periodontitis. Regen Ther. 2020;14:72–78. doi:10.1016/j.reth.2019.12.011
18. Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97(25):13625–13630. doi:10.1073/pnas.240309797
19. Miura M, Gronthos S, Zhao M, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA. 2003;100(10):5807–5812. doi:10.1073/pnas.0937635100
20. Nakajima K, Kunimatsu R, Ando K, et al. Comparison of the bone regeneration ability between stem cells from human exfoliated deciduous teeth, human dental pulp stem cells and human bone marrow mesenchymal stem cells. Biochem Biophys Res Commun. 2018;497(3):876–882. doi:10.1016/j.bbrc.2018.02.156
21. Arthur A, Shi S, Zannettino AC, Fujii N, Gronthos S, Koblar SA. Implanted adult human dental pulp stem cells induce endogenous axon guidance. Stem Cells. 2009;27(9):2229–2237. doi:10.1002/stem.138
22. Langer R, Vacanti JP. Tissue engineering. Science. 1993;260(5110):920–926. doi:10.1126/science.8493529
23. Kaigler D, Pagni G, Park CH, Tarle SA, Bartel RL, Giannobile WV. Angiogenic and osteogenic potential of bone repair cells for craniofacial regeneration. Tissue Eng Part A. 2010;16(9):2809–2820. doi:10.1089/ten.tea.2010.0079
24. Kunimatsu R, Nakajima K, Awada T, et al. Comparative characterization of stem cells from human exfoliated deciduous teeth, dental pulp, and bone marrow-derived mesenchymal stem cells. Biochem Biophys Res Commun. 2018;501(1):193–198. doi:10.1016/j.bbrc.2018.04.213
25. Hardy E, Fernandez-Patron C. Destroy to rebuild: the connection between bone tissue remodeling and matrix metalloproteinases. Front Physiol. 2020;11:47. doi:10.3389/fphys.2020.00047
26. Almalki SG, Agrawal DK. Effects of matrix metalloproteinases on the fate of mesenchymal stem cells. Stem Cell Res Ther. 2016;7(1):129. doi:10.1186/s13287-016-0393-1
27. Mauney J, Volloch V. Adult human bone marrow stromal cells regulate expression of their MMPs and TIMPs in differentiation type-specific manner. Matrix Biol. 2010;29(1):3–8. doi:10.1016/j.matbio.2009.09.003
28. Al-Majid A, Alassiri S, Rathnayake N, Tervahartiala T, Gieselmann DR, Sorsa T. Matrix metalloproteinase-8 as an inflammatory and prevention biomarker in periodontal and peri-implant diseases. Int J Dent. 2018;2018:7891323. doi:10.1155/2018/7891323
29. An F, Du J, Cao Y, et al. MMP8 polymorphism is associated with susceptibility to osteonecrosis of the femoral head in a Chinese Han population. Oncotarget. 2017;8(13):21561–21566. doi:10.18632/oncotarget.15371
30. Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56(4):549–580. doi:10.1124/pr.56.4.3
31. Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423(6937):332–336. doi:10.1038/nature01657
32. Gupta R, Tongers J, Losordo DW. Human studies of angiogenic gene therapy. Circ Res. 2009;105(8):724–736. doi:10.1161/CIRCRESAHA.109.200386
33. Saskianti T, Nugraha AP, Prahasanti C, Ernawati DS, Suardita K, Riawan W. Immunohistochemical analysis of stem cells from human exfoliated deciduous teeth seeded in carbonate apatite scaffold for the alveolar bone defect in Wistar rats (Rattus norvegicus). F1000Res. 2020;9:1164. doi:10.12688/f1000research.25009.2
34. Saskianti T, Yuliartanti W, Ernawati DS, Prahasanti C, Suardita K. BMP4 expression following stem cells from human exfoliated deciduous and carbonate apatite transplantation on Rattus norvegicus. J Krishna Inst Medical Sci. 2018;7(2):56–61.
35. Saskianti T, Ramadhani R, Budipramana ES, Pradopo S, Suardita K. Potential proliferation of stem cell from human exfoliated deciduous teeth (SHED) in carbonate apatite and hydroxyapatite scaffold. J Int Dent Med Res. 2017;10(2):350.
36. Nugraha AP, Narmada IB, Ernawati DS, et al. In vitro bone sialoprotein-I expression in combined gingival stromal cells and platelet rich fibrin during osteogenic differentiation. Trop J Pharmaceut Res. 2018;17(12):2341–2345. doi:10.4314/tjpr.v17i12.4
37. Khoswanto C. A new technique for research on wound healing through extraction of mandibular lower incisors in Wistar Rats. Eur J Dent. 2019;13(2):235–237. doi:10.1055/s-0039-1694312
38. Savi FM, Brierly GI, Baldwin J, Theodoropoulos C, Woodruff MA. Comparison of different decalcification methods using rat mandibles as a model. J Histochem Cytochem. 2017;65(12):705–722. doi:10.1369/0022155417733708
39. Franco C, Patricia HR, Timo S, Claudia B, Marcela H. Matrix metalloproteinases as regulators of periodontal inflammation. Int J Mol Sci. 2017;18(2):440. doi:10.3390/ijms18020440
40. Preshaw PM. Host modulation therapy with anti-inflammatory agents. Periodontol 2000. 2018;76(1):131–149. doi:10.1111/prd.12148
41. Rahyussalim AJ, Sahputra RE. The effect of mesenchymal stem cell-enriched scaffolds on MMP-8 and TGF-β levels of vertebrae postlaminoplasty in rabbit model. Stem Cells Cloning. 2021;14:27–37. doi:10.2147/SCCAA.S314107
42. Jiménez NT, Carlos Munévar J, González JM, Infante C, Lara SJP. In vitro response of dental pulp stem cells in 3D scaffolds: a regenerative bone material. Heliyon. 2018;4(9):e00775. doi:10.1016/j.heliyon.2018.e00775
43. Motamedian SR, Tabatabaei FS, Akhlaghi F, Torshabi M, Gholamin P, Khojasteh A. Response of dental pulp stem cells to synthetic, allograft, and xenograft bone scaffolds. Int J Periodontics Restorative Dent. 2017;37(1):49–59. doi:10.11607/prd.2121
44. Burg KJ, Porter S, Kellam JF. Biomaterial developments for bone tissue engineering. Biomaterials. 2000;21(23):2347–2359. doi:10.1016/s0142-9612(00)00102-2
45. Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26(27):5474–5491. doi:10.1016/j.biomaterials.2005.02.002
46. d’Aquino R, Graziano A, Sampaolesi M, et al. Human postnatal dental pulp cells co-differentiate into osteoblasts and endotheliocytes: a pivotal synergy leading to adult bone tissue formation. Cell Death Differ. 2007;14(6):1162–1171. doi:10.1038/sj.cdd.4402121
47. Kanczler JM, Oreffo RO. Osteogenesis and angiogenesis: the potential for engineering bone. Eur Cell Mater. 2008;15:100–114. doi:10.22203/ecm.v015a08
48. Liu J, Kerns DG. Mechanisms of guided bone regeneration: a review. Open Dent J. 2014;8(1):56–65. doi:10.2174/1874210601408010056
49. Cetinkaya BO, Keles GC, Ayas B, Sakallioglu EE, Acikgoz G. The expression of vascular endothelial growth factor in a rat model at destruction and healing stages of periodontal disease. J Periodontol. 2007;78(6):1129–1135. doi:10.1902/jop.2007.060397
50. Hu K, Olsen BR. Osteoblast-derived VEGF regulates osteoblast differentiation and bone formation during bone repair. J Clin Invest. 2016;126(2):509–526. doi:10.1172/JCI82585
51. Liu Y, Olsen BR. Distinct VEGF functions during bone development and homeostasis. Arch Immunol Ther Exp (Warsz). 2014;62(5):363–368. doi:10.1007/s00005-014-0285-y
52. Ching HS, Luddin N, Rahman IA, Ponnuraj KT. Expression of odontogenic and osteogenic markers in DPSCs and SHED: a review. Curr Stem Cell Res Ther. 2017;12(1):71–79. doi:10.2174/1574888x11666160815095733
53. Nowwarote N, Sukarawan W, Pavasant P, Foster BL, Osathanon T. Basic fibroblast growth factor regulates phosphate/pyrophosphate regulatory genes in stem cells isolated from human exfoliated deciduous teeth. Stem Cell Res Ther. 2018;9(1):345. doi:10.1186/s13287-018-1093-9
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.