Back to Journals » Patient Preference and Adherence » Volume 18
Structural Equation Modelling to Identify Psychometric Determinants of Medication Adherence in a Survey of Kidney Dialysis Patients
Authors Marshall MR , Curd S, Kennedy J, Khatri D, Lee S, Pireva K, Taule’alo O, Tiavale-Moore P, Wolley MJ, Ma TM, Kam AL, Suh JS, Aspden TJ
Received 12 January 2024
Accepted for publication 26 March 2024
Published 17 April 2024 Volume 2024:18 Pages 855—878
DOI https://doi.org/10.2147/PPA.S454248
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Mark R Marshall,1– 3,* Samantha Curd,4,* Julia Kennedy,4,* Dharni Khatri,4,* Sophia Lee,4,* Krenare Pireva,4,* Olita Taule’alo,4,* Porsche Tiavale-Moore,4,* Martin J Wolley,3,5,6,* Tian M Ma,3,7,* Angela L Kam,3,8,* Jun S Suh,3,* Trudi J Aspden4,*
1School of Medicine, Faculty of Medical and Health Sciences, University of Auckland, Auckland, New Zealand; 2Department of Medicine, Tauranga Hospital, Hauora a Toi Bay of Plenty, Tauranga, New Zealand; 3Department of Renal Medicine, Middlemore Hospital, Counties Manukau Health, Auckland, New Zealand; 4School of Pharmacy, Faculty of Medical and Health Sciences, University of Auckland, Auckland, New Zealand; 5Endocrine Hypertension Research Centre, University of Queensland Frazer Institute, Greenslopes and Princess Alexandra Hospitals, Brisbane, Queensland, Australia; 6Department of Nephrology, Royal Brisbane and Women’s Hospital, Brisbane, Queensland, Australia; 7Institute for Innovation + Improvement, North Shore Hospital, Auckland, New Zealand; 8Barts Health NHS Trust, London, UK
*These authors contributed equally to this work
Correspondence: Mark R Marshall, Tauranga Hospital, Te Whatu Ora Hauora a Toi Bay of Plenty, 29 Cameron Road, Tauranga South, Tauranga, 3112, New Zealand, Tel +6421461766, Email [email protected]
Purpose: Medication non-adherence in dialysis patients is associated with increased mortality and higher healthcare costs. We assessed whether medication adherence is influenced by specific psychometric constructs measuring beliefs about the necessity for medication and concerns about them. We also tested whether medication knowledge, health literacy, and illness perceptions influenced this relationship.
Patients and Methods: This study is based on data from a cross-sectional in-person questionnaire, administered to a random sample of all adult dialysis patients at a teaching hospital. The main outcome was self-assessed medication adherence (8-Item Morisky Medication Adherence Scale). The predictors were: concerns about medications and necessity for medication (Beliefs About Medication Questionnaire); health literacy; medication knowledge (Medication Knowledge Evaluation Tool); cognitive, emotional, and comprehensibility Illness perceptions (Brief Illness Perception Questionnaire). Path analysis was performed using structural equations in both covariance and variance-based models.
Results: Necessity for medication increased (standardized path coefficient [β] 0.30 [95% CI 0.05, 0.54]) and concerns about medication decreased (standardized β − 0.33 [− 0.57, − 0.09]) medication adherence, explaining most of the variance in outcome (r2=0.95). Medication knowledge and cognitive illness perceptions had no effects on medication adherence, either directly or indirectly. Higher health literacy, greater illness comprehension, and a more positive emotional view of their illness had medium-to-large sized effects in increasing medication adherence. These were indirect rather and direct effects mediated by decreases in concerns about medications (standardized β respectively − 0.40 [− 0.63,-0.16], − 0.60 [− 0.85, − 0.34], − 0.33 [− 0.52, − 0.13]).
Conclusion: Interventions that reduce patients’ concerns about their medications are likely to improve adherence, rather than interventions that increase patients’ perceived necessity for medication. Improving patients’ general health literacy and facilitating a better understanding and more positive perception of the illness can probably achieve this. Our study is potentially limited by a lack of generalizability outside of the population and setting in which it was conducted.
Keywords: medication adherence, surveys and questionnaires, latent class analysis, renal dialysis
Introduction
Dialysis patients have the highest medication burden of any disease group, averaging 12–19 prescribed medications per day.1 Non-adherence of dialysis patients with medication, depending on definition, ranges from 3 to 80% in the literature.2–5 Non-adherence in this population is associated with increased mortality and higher healthcare costs.6 It is therefore important to understand and optimize drivers of medication adherence in dialysis patients.
There is a plethora of different reasons for non-adherence amongst such patients, which include sociodemographic issues, healthcare delivery-based factors, economic barriers, polypharmacy, the generally high and sometime overwhelming burden of disease that patients endure, the high prevalence of overt psychiatric disease and anxiety that is evident and perhaps as a consequence of this, as well as other psychosocial factors.4,5,7–13 Clinical studies of interventions to improve medication adherence report varying, but generally temporary and slight effectiveness.14–16 In general, most studies attempting to improve adherence have used utilized generic interventions such as reminder systems, cognitive-educational interventions, behavioral-counselling interventions, with few (if any) identifying and targeting any patient-level psychometric barriers that specifically relate to a given individual’s medication adherence.14,15 This is disappointing since psychosocial variables (eg patients’ beliefs about medication, illness perceptions, personality characteristics), when they have been assessed, have the strongest associations with nonadherence, and by extension are the most promising levers for changing adherence behaviour.17
This study aims to explore whether medication adherence in adult dialysis patients might be causally related to patients’ beliefs about medications. To meet this aim, we assess the relationship between self-assessed adherence and specific psychometric constructs measuring the necessity for medication and the concerns about them. We test whether medication knowledge, patient health literacy levels, and illness perceptions influenced this relationship.
In this study, we quantify appropriate psychometric constructs in a sample of dialysis patients and perform path analyses of relationships using structural equations.
Materials and Methods
The reader is referred to our previous open-access publication describing the study design and its conduct, including such key issues as sample size calculation.18 This current publication is aligned with the Consensus-Based Checklist for Reporting of Survey Studies, provided in Appendix 1.19
Research Questions
The primary research question: Is medication adherence directly related to beliefs about medications?
The secondary research questions are: Is medication adherence related to health literacy, medication knowledge, or beliefs about illness, and; Is this relationship direct or mediated by medication beliefs?
The hypothesized causal models for our analysis are provided in Figure 1 and are based on relationships identified in previous studies and systematic reviews.17,20–22
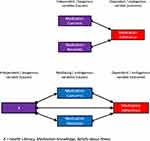 |
Figure 1 Hypothesized causal models for path analysis in this study. |
Setting and Study Design
This study was conducted in a teaching hospital in South Auckland, New Zealand, the sole dialysis provider for a catchment population numbering 509,060 at the time of study. We used a quantitative cross-sectional survey design, involving an in-person questionnaire on a random sample of all people receiving dialysis at the hospital.
Ethical Considerations
The study was performed in accordance with the Declaration of Helsinki. Ethical approval was granted by the University of Auckland Human Participant Ethics Committee (UAHPEC, IORG0007795), protocol/reference number 9660, and the Counties Manukau Health (CMH) Institutional Review Board, protocol/reference number 1504. All participants provided informed consent.
Participants
Eligible people were defined as those aged >16 years undergoing maintenance dialysis for kidney failure. Patients were randomized using Microsoft Excel and approached in list order to participate in the study, with the aim of recruiting 100 participants in total. The sampling frame for the study was stratified by independent versus dependent dialysis modality (50:50). Additionally, as the incidence of dialysis for Māori and Pacific Peoples is much higher than for non-Māori and non-Pacific peoples in New Zealand, we also stratified within each modality strata by ethnicity of NZ Māori versus Pacific Peoples versus “other” (17:17:16)(9). If a patient declined to participate, a new name was sequentially provided from the randomized list and invited to participate in the study until the required number of participants in each stratum was recruited.
Data Collection
The reader is again referred to our previous open-access publication describing the study design and its conduct.18 The article describes in detail the instruments used in the analyses in this article, and the scoring that is used for the development of constructs from the psychometric scales (eg health literacy). The article also provides internal validation that ensures our data and calculations are relevant and legitimate for our sample and population. The survey administered to participants is provided in Appendix 2. The main outcome was self-reported medication adherence, as measured by the Morisky 8-Item Medication Adherence Scale (MMAS-8).23 Other psychometric constructs were assessed from the following survey scales; the Beliefs About Medication Questionnaire (BMQ),24–26 the health literacy screening tool (HLS),27–29 the Medication Knowledge Evaluation Tool (MKET),30–32 and the Brief Illness Perception Questionnaire (BIPQ).33–35 Data collection spanned from 19 July 2013 to 13 Jun 2014.
Data Analysis
We performed path analyses using structural equation modeling (SEM). Survey variables were modelled to fit the following psychometric constructs: Adherence (MMAS-8), Concerns about Medications and Necessity for Medications (BMQ), Health Literacy (HLS), Medication Knowledge (MKET), and Cognitive, Emotional, and Comprehensibility Illness Perceptions (BIPQ).
Path analysis used two different techniques, namely covariance-based structural equation modeling (COV-SEM),36 and variance-based structural equation modeling (VAR-SEM).37 In COV-SEM, multi- and single-item survey variables are incorporated into the model using factor analysis, with subsequent estimation of paths between common factors. In VAR-SEM, survey variables are used to generate weighted composites, and paths estimated between the composites. Technical differences aside, COV-SEM is the appropriate method for theory testing and confirmation, and VAR-SEM for prediction and theory development. In this study, the research objective is both theory testing and development, and both methods were used for convergent validity.
In conducting COV-SEM, we first performed confirmatory factor analysis (CFA), to confirm that survey scales (or subscales) were correctly identifying a single latent variable representing the construct of interest. These measurement models assess strength and direction of relationships between the individual items in the scale and the specified latent variable. We used the following guidance to define goodness-of-COV-SEM fit. Superior model fit was indicated by Root Mean Square Error of Approximation (RMSEA) <0.05, Tucker Lewis Index (TLI) ≥0.95, and Comparative Fit Index (CFI) ≥0.95. Acceptable model fit was indicated by 0.05 ≤ RMSEA ≤ 0.08, and 0.9 ≤ TLI / CFI < 0.95. Mediocre model fit was indicated by 0.08 < RMSEA ≤ 0.1, and 0.85 < TLI / CFI ≤ 0.9. Inadequate model fit was indicated by values outside of these limits.38–41 In the presence of conflicting indices, the RMSEA was prioritized as the most informative fit index. For completeness, we reported the chi-square statistic (χ2) of the current model and the p-value of testing versus a fully saturated one; however, sensitivity to sample size is such that we did not take it into account as a factor for determining model fit in this study.
For VAR-SEM, we used an open-source implementation of the Partial Least Squares-SEM methodology.42 VAR-SEM does not have well-accepted criteria for goodness-of-model-fit. As a workaround, we assessed model fit by the average R2, the average communality, the average redundancy and the goodness-of-fit (GoF) statistic. The following interpretations are suggested for R2: <0.1 is unacceptable; 0.19–0.25 indicates mediocre fit; 0.33–0.50 indicates adequate fit; 0.67–0.75 indicates superior fit.43 Redundancy statistics attempt to quantify the amount of variance in an outcome variable that is attributable to independent latent variables, and the communality of the average variance extracted. No rule of thumb has been suggested for these parameters, although the higher the redundancy and communality, the higher the predictive power of the model. GoF values greater than 0.7 are considered “very good” within the VAR-SEM community.44
We used Stata 17 software and maximum likelihood methods of estimation. We report standardized parameter estimates, interpreted as the number of standard deviation increases above the mean in the outcome variable for every 1 standard deviation above the mean of the predictor variable. In this study, we defined standardized path coefficients of ≥0.2–0.5 as indicating a medium effect and >0.5 as having a large effect. Statistical significance was determined at an alpha of 0.05.
Results
Patient disposition is presented in Figure S1.
Participant Demographic and Clinical Characteristics
The mean age of participants was 55.2 years (± SD 13.7). Sixty-two identified as male and 38 as female. The median number of medicines prescribed to participants was 8 (IQR 6, 10) and whilst only 3 people lived alone, 66 people managed their own medications alone. Additional participant characteristics are provided in Table 1.
 |
Table 1 Participant Demographic and Clinical Characteristics (n=100) |
Psychometric Scale Characteristics
Psychometric scales are described in two ways. The first is as simple summations, which have limitations since cut-offs compromise validity, reliability, and qualitative classification.45 The second is as factor scores, computed by CFA. The scales for factor scores have no absolute units, but indicate the participants’ relative standing or placement on the latent variable scale.46 The two descriptions are presented visually for each psychometric construct in Figure 2, as histograms constructed so that each bar represents an exact quartile of data (except MKET and some subscales of BIPQ, where they are standard histograms).
Figure 2 Continued. Figure 2 Continued. Figure 2 Continued. Figure 2 Continued. Figure 2 Continued. Figure 2 Continued. Figure 2 Continued. Figure 2 Continued.







Medication Adherence
The distribution of summative scores in Figure 2 suggests that ~50% participants felt themselves to have high medication adherence. The factor scores use all items in the MMAS-8 scale, with each of them having positive and statistically significant loading associated with the latent variable (all p < 0.001). A positive and statistically significant covariance was identified between items C1 and C8 (p = 0.003):
And C3 and C6 (p = 0.004):
Qualitatively, we considered an association between these items to be plausible, and covariance loadings were included in the measurement model. Model fit is very high, allowing for robust interpretation and modelling of adherence as a latent variable. The distribution of the latent variable follows the summative one.
Necessity for Medication
The distribution of summative scores is presented in Figure 2, and suggests that participants strongly believed in the necessity for their medications. The factor scores use all items in the scale. A positive and statistically significant covariance was identified between items DN4 and DN10 (p = 0.001):
And DN3 and DN4 (p < 0.001):
We considered the associations plausible and included the covariance loadings in the measurement model. Model fit is very high. The distribution of the modelled latent variable and summative scores is similar.
Concerns About Medication
The distribution of summative scores in Figure 2 suggests that many people in our sample had concerns about their medication. A necessity–concerns differential can be calculated from the BMQ as the difference between the necessity and the concern scales, with a possible range of −20 to +20. This differential can be interpreted, in a loose and informal sense, as the cost–benefit analysis from each participant’s perspective, for whom costs (concerns) are weighed against their perceived benefits (necessity beliefs).25 The differential can be grouped into 4 categories: accepting, ambivalent, sceptical, and indifferent. None of our participants were indifferent to medication, and only 2 sceptical. The majority of our participants were accepting of medication, although 38 were ambivalent. The factor scores use all items in the BMQ scale, and model fit is very high. The modelled latent variable follows the same distribution as the summative scores
Health Literacy
In the analysis of this scale, the scoring of the first item (F1) is reversed. The distribution of summative scores in Figure 2 suggests that many people in our sample had low health literacy. The factor scores use all items in the scale, with each of them having positive and statistically significant loadings. Model fit was again very high, confirming that many participants in our sample regarded themselves as having low health literacy.
Medication Knowledge
In our previous work with the study data, we showed the MKET scale to be unreliable in our sample (Cronbach’s alpha 0.59 after scale purification). Confirmatory factor analysis verifies that no single latent variable explains the variation of all of the items in the scale. On review of the scale, we decided that there were two different constructs being measured. We assessed items B1, B3, and B4 as representing “hands-on medication knowledge”: what medications are called, when to take them, and how to take them. We assessed items B2, B5, B6 and B7 as representing “conceptual medication knowledge”: why the medication is being prescribed, possible side effects that might arise, actions to be taken in the advent of possible side effects, actions to be taken in advent of a missed dose. These two subscales and resulting constructs were adopted in subsequent modelling.
The distribution of summative scores in Figure 2 suggests that the vast majority of participants in our sample had excellent hands-on medication knowledge, although many had poor conceptual knowledge. The factor scores for both Hands-on Medication Knowledge and Conceptual Medication Knowledge used all items in the scale, with each item having positive and statistically significant loadings associated with the respective latent variable (all p < 0.05). The distribution of factor values is similar to the summative scores, and supports a similar interpretation.
Illness Perception
In our previous work on these data, we showed that the cognitive (5 items) and emotional (2 items) subscales of the BIPQ to be unreliable in our sample (Cronbach’s alpha 0.39 and 0.51, respectively). However, after reviewing the items in the subscales, our cumulative clinical experience supported the tenet that a negative cognitive illness perception would be one that is felt to be more severe or threatening, more chronic, and less controllable. Likewise, a negative emotional perception of their illness would be one that causes of great concern, anxiety and low mood. We therefore made the decision to accept the scale unreliability and model the BIPQ subscales as suggested in the original development of the scale.
Factor scores for both Cognitive and Emotional Illness Perception use all items in the subscales, and all have positive loadings although these are statistically significant for only two items (note: scoring of items E3C and E4C reversed as per personal communication E Broadbent 15 Jan 2015). No modification indices could be identified that improved model fit. Despite a priori concern about a lack of reliability from exploratory factor analysis, the final model fit from CFA was acceptable. The final distribution of both summative and factor values is presented Figure 2, and confirms that participants in the sample were split equally between having a negative versus positive cognitive illness perception, and that most had a negative emotional one.
The illness comprehensibility subscale is a single item and thus not amenable to CFA. The final distribution of summative values in Figure 2 confirms that most participants in the sample had a high understanding of their illness.
Main Analysis
Base Model
The base model is presented in Figure 3. Model fit for both SEMs is superior. In the COV-SEM, 95% of the variance of adherence is explained by necessity for medication and concerns about medication. The estimates from both models have high face validity: increased concerns about medication had a medium-sized and statistically significant effect in decreasing medication adherence, while a perception of increased necessity for medication had a similarly sized effect in increasing adherence.
Mediation Analyses
The health literacy mediation model is presented in Figure 4. Of note, model fit is better with COV-SEM than with VAR-SEM, but acceptable without being superior in both. No new modification indices can be identified that were both plausible and improved model fit. In both models, health literacy had no direct effect on medication adherence. However, it had a clear indirect effect – increased health literacy had a medium-sized effect in increasing adherence, mediated by a medium-sized and statistically significant decrease in concerns about medication.
The hands-on and conceptual medication knowledge mediation models are presented in Figures S2 and S3, respectively. Model fit is mediocre with both SEMs. No helpful new modification indices could be identified. Neither hands-on medication or conceptual medication knowledge had direct or indirect effects on medication adherence.
The cognitive perception of illness mediation model is presented in Figure S4. Of note, convergence of the COV-SEM could not be achieved using the base model presented above – additional and different covariances were required between items in the Necessity for Medication subscale before model estimation was successful. Model fit was similar with both SEMs and of borderline acceptability. In both models, cognitive perception of illness had no direct or indirect effect on medication adherence.
The emotional perception of illness mediation model is presented in Figure 5. Model fit for both SEMs was very good. In both models, emotional perception of illness had no direct effect on medication adherence. However, increased emotional distress had an indirect effect in decreasing medication adherence, mediated by a large and statistically significant increase in concerns about medication.
The illness comprehensibility mediation model is presented in Figure 6. Model fit for both SEMs acceptable without being superior. No helpful modification indices could be identified. In both models, illness comprehensibility had no direct effect on medication adherence. However, increased illness comprehension had an indirect effect in increasing medication adherence, mediated by a large and statistically significant decrease in concerns about medication.
Conclusion
Medication non-adherence is a potentially mutable problem, but the question remains – how best to address it? Historically, investigators have trialled co-interventions in multifaceted packages. More recently, better results are being seen with targeted interventions that address specific reasons for non-adherence.47–49 Psychological factors in the context of peoples individual lives are generally acknowledged to be the most powerful determinants of adherence,17,50,51 and it is therefore critical to identify precise and relevant psychometric targets for intervention.
Four major insights arise from this study. First, the adult dialysis patients in our sample had a high perceived necessity for medication, but at the same time considerable concerns about their medications. This is consistent perhaps the only other study of this construct in dialysis patients.52 None of the psychometric constructs in our study had any effect on adherence through increasing perceived necessity for medication. Rather, they acted through reducing perceived concerns. As such, our foremost finding is the pre-eminence of concerns over necessity as a target to improve medication adherence, a somewhat unexpected and unusual finding. Arguably, this could be a local finding that is specific to our patient population. However, the global nature of COVID-19 vaccine hesitancy suggests to us that concerns about medication might be more widespread barrier than appreciated.53 Our finding is also consistent with the only published meta-analysis of the effect of beliefs about medication upon adherence: in that review, concerns had a (directionally only) larger effect on adherence than necessity (z-test between log-odds p = 0.1).54 There are reports of structural analyses by others that are similar to ours but in different populations, and those that have modelled both necessity and concerns together show an effect size that is usually greater for concerns.55–58
The second insight is that improving medication knowledge, in isolation, is unlikely to improve medication adherence. This tenet is consistent with what other literature exist, both in dialysis patients16 and the general population.59 To our knowledge, there have been no studies exploring medication knowledge as a sole intervention, with most addressing medication knowledge in multi-faceted interventions, some of which have been successful.60–63 The contribution of improved medication knowledge to such success is moot. We have found only one other structural analysis of medication knowledge in this literature, although that study did not include assessments from the BMQ, and therefore, is not directly comparable.64 This insight is not meant to indicate that medication knowledge is without merit in efforts to improve medication adherence, since it may have effects on other constructs that might influence the outcome. Our study was not powered to examine more complex structural models that the ones presented, and further studies are merited in this area.
The third insight is that low patient health literacy and illness comprehension levels both have determinantal effects on medication adherence, mediated by increased concerns about medication. This result is consistent with published literature,65,66 suggesting that improving medication adherence might be achieved by clinicians enquiring about and then building health literacy, especially in relation to medications and health conditions. We speculate that supporting people to obtain, understand and use basic health information, will lead to an improved ability to navigate health services, and an improved likelihood of people making decisions that will improve their health outcomes, including those pertaining to their medication. Similarly, patients with a good understanding of their disease and the treatments available to control it are axiomatically more likely to demonstrate higher adherence behaviours.
The last insight is that, as others have also observed, patients with greater emotional dysfunction in response to their disease tend to have poorer medication adherence. This suggests that supporting patients to have a more positive outlook on their illness may decrease distress and promote higher medication adherence,67,68 a tenet supported by a recent clinical trial.51
Our study has a number of strengths. It used a random sample of a regional dialysis population rather than a convenience one and collected a wide range of clinical and psychometric data. The path analysis methods that we used provide structural relationships between multiple psychometric constructs simultaneously, models that are arguably more realistic compared to ones based on simple regression. The study has the standard limitations around sampling frame and generalizability, and our findings may not be applicable to other settings and other populations. Of special note, our study did not include paediatric patients, and important group with distinct medical and psychosocial characteristics who should be studied separately. Notwithstanding, our intention was to identify sensitive psychometric targets for intervention, and having done that we find that many of our conclusions are consistent with the literature. Another limitation of our study is that we could and did not address all psychological constructs that may have affected adherence. Some important omissions that are worthy of further study in this population include assessments of health locus of control, mindfulness, and emotional-stress coping style.69 A final limitation of the study was that we modelled the BMQ as a 10-item scale as originally validated,26 rather than as the modern adaptation (BMQ-S11) that is becoming more prevalent in the literature nowadays.70
An important methodological lesson from our study concerns the scales we used. While technically challenging to handle, they were workable for complex causal models. This should be encouraging to investigators, since structural models are regarded as customary and the best-suited tools for psychological theory development, testing and confirmation. We hope our study encourages others to explore their data using such methods in order to gain richer insights than what can be achieved with standard regression models.
Finally, a comment should be made about the mutability of patient beliefs about medication and interventions for medication adherence that might be potentially effective. Only a handful of relevant articles have been identified by the authors, and some49,71,72 (but not all73–76) showed that both BMQ scores and adherence can both be improved (at least in the short term). The successful interventions in these studies all involved psychological counselling of some sort, based on for example Adherence Therapy, the Health Behaviour Model, or a tailored psychological intervention-based patients’ baseline profiles. Our study demonstrates that beliefs about medication are also clear targets for interventions in the dialysis population and can probably be modified to improve medication adherence if addressed appropriately.
Acknowledgments
We would like to acknowledge the input of the Māori Cultural Resource Unit at Counties Manukau Health, Associate Professor Papaarangi Reid from the University of Auckland, and Susan Reid from Health Literacy NZ for their advice regarding this study.
We thank Dr Morisky for permission to use the MMAS-8. Use of the MMAS-8 is protected by United States copyright laws. A license agreement to use the scale is available from: Donald E. Morisky, ScD, ScM, MSPH, Professor, Department of Community Health Sciences, UCLA School of Public Health, 650 Charles E. Young Drive South, Los Angeles, CA 90095-1772, [email protected].
We thank Dr Horne for permission to use the BMQ. Use of the BMQ is protected by United Kingdom copyright laws. A license agreement to use the scale is available from: Rob Horne, Professor of Behavioural Medicine, Practice & Policy, University College London, BMA House, UCL School of Pharmacy Tavistock Square, London, WC1H 9JP, United Kingdom, [email protected].
We thank Dr Broadbent for permission to use the BIPQ. Use of the BIPQ is protected by New Zealand copyright laws. A license agreement to use the scale is available from: Elizabeth Broadbent, Professor, Psychological Medicine, Faculty of Medical and Health Sciences, The University of Auckland, Private Bag 92019, Auckland 1142, New Zealand, [email protected].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Manley HJ, Garvin CG, Drayer DK, et al. Medication prescribing patterns in ambulatory haemodialysis patients: comparisons of USRDS to a large not-for-profit dialysis provider. Nephrol Dial Transplant. 2004;19(7):1842–1848. doi:10.1093/ndt/gfh280
2. Chiu Y-W, Teitelbaum I, Misra M, de Leon EM, Adzize T, Mehrotra R. Pill burden, adherence, hyperphosphatemia, and quality of life in maintenance dialysis patients. Clin J Am Soc Nephrol. 2009;4(6):1089–1096. doi:10.2215/cjn.00290109
3. Schmid H, Hartmann B, Schiffl H. Adherence to prescribed oral medication in adult patients undergoing chronic hemodialysis: a critical review of the literature. Eur J Med Res. 2009;14(5):185–190. doi:10.1186/2047-783x-14-5-185
4. Seng JJB, Tan JY, Yeam CT, Htay H, Foo WYM. Factors affecting medication adherence among pre-dialysis chronic kidney disease patients: a systematic review and meta-analysis of literature. Int Urol Nephrol. 2020;52(5):903–916. doi:10.1007/s11255-020-02452-8
5. Griva K, Lai AY, Lim HA, Yu Z, Foo MW, Newman SP. Non-adherence in patients on peritoneal dialysis: a systematic review. PLoS One. 2014;9(2):e89001. doi:10.1371/journal.pone.0089001
6. Weinhandl ED, Arneson TJ, St Peter WL. Clinical outcomes associated with receipt of integrated pharmacy services by hemodialysis patients: a quality improvement report. Am J Kidney Dis. 2013;62(3):557–567. doi:10.1053/j.ajkd.2013.02.360
7. Oliveira J, Sousa H, Bártolo A, Figueiredo D, Ribeiro O. Illness perception and treatment adherence in haemodialysis: a systematic review. Psychol Health Med. 2023;28(7):1641–1655. doi:10.1080/13548506.2022.2099559
8. Ghimire S, Castelino RL, Lioufas NM, Peterson GM, Zaidi ST, Chilcot J. Nonadherence to medication therapy in haemodialysis patients: a systematic review. PLoS One. 2015;10(12):e0144119. doi:10.1371/journal.pone.0144119
9. Sousa H, Ribeiro O, Paúl C, et al. Social support and treatment adherence in patients with end-stage renal disease: a systematic review. Semin Dial. 2019;32(6):562–574. doi:10.1111/sdi.12831
10. Dodd R, Palagyi A, Guild L, Jha V, Jan S. The impact of out-of-pocket costs on treatment commencement and adherence in chronic kidney disease: a systematic review. Health Policy Plan. 2018;33(9):1047–1054. doi:10.1093/heapol/czy081
11. Huang CW, Wee PH, Low LL, et al. Prevalence and risk factors for elevated anxiety symptoms and anxiety disorders in chronic kidney disease: a systematic review and meta-analysis. Gen Hosp Psychiatry. 2021;69:27–40. doi:10.1016/j.genhosppsych.2020.12.003
12. Lowe KM, Cruz JB, Jones KM. Complications in patients with chronic kidney disease. Crit Care Nurs Clin North Am. 2022;34(4):395–407. doi:10.1016/j.cnc.2022.07.005
13. Fissell RB, Karaboyas A, Bieber BA, et al. Phosphate binder pill burden, patient-reported non-adherence, and mineral bone disorder markers: findings from the DOPPS. Hemodial Int. 2016;20(1):38–49. doi:10.1111/hdi.12315
14. Matteson ML, Russell C. Interventions to improve hemodialysis adherence: a systematic review of randomized-controlled trials. Hemodial Int. 2010;14(4):370–382. doi:10.1111/j.1542-4758.2010.00462.x
15. Milazi M, Bonner A, Douglas C. Effectiveness of educational or behavioral interventions on adherence to phosphate control in adults receiving hemodialysis: a systematic review. JBI Database System Rev Implement Rep. 2017;15(4):971–1010. doi:10.11124/jbisrir-2017-003360
16. Murali KM, Mullan J, Roodenrys S, Hassan HC, Lambert K, Lonergan M. Strategies to improve dietary, fluid, dialysis or medication adherence in patients with end stage kidney disease on dialysis: a systematic review and meta-analysis of randomized intervention trials. PLoS One. 2019;14(1):e0211479. doi:10.1371/journal.pone.0211479
17. Karamanidou C, Clatworthy J, Weinman J, Horne R. A systematic review of the prevalence and determinants of nonadherence to phosphate binding medication in patients with end-stage renal disease. BMC Nephrol. 2008;9(1):2. doi:10.1186/1471-2369-9-2
18. Aspden T, Wolley MJ, Ma TM, et al. Understanding barriers to optimal medication management for those requiring long-term dialysis: rationale and design for an observational study, and a quantitative description of study variables and data. BMC Nephrol. 2015;16(1):102. doi:10.1186/s12882-015-0097-2
19. Sharma A, Minh Duc NT, Luu Lam Thang T, et al. A Consensus-Based Checklist for Reporting of Survey Studies (CROSS). J Gen Intern Med. 2021;36(10):3179–3187. doi:10.1007/s11606-021-06737-1
20. Crayton E, Fahey M, Ashworth M, Besser SJ, Weinman J, Wright AJ. Psychological determinants of medication adherence in stroke survivors: a systematic review of observational studies. Ann Behav Med. 2017;51(6):833–845. doi:10.1007/s12160-017-9906-0
21. Selinger CP, Robinson A, Leong RW. Clinical impact and drivers of non-adherence to maintenance medication for inflammatory bowel disease. Expert Opin Drug Saf. 2011;10(6):863–870. doi:10.1517/14740338.2011.583915
22. Bhattarai B, Walpola R, Mey A, Anoopkumar-Dukie S, Khan S. Barriers and strategies for improving medication adherence among people living with COPD: a systematic review. Respir Care. 2020;65(11):1738–1750. doi:10.4187/respcare.07355
23. Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. doi:10.1097/00005650-198601000-00007
24. Horne R. Patients’ beliefs about treatment: the hidden determinant of treatment outcome? J Psychosom Res. 1999;47(6):491–495. doi:10.1016/S0022-3999(99)00058-6
25. Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47(6):555–567. doi:10.1016/S0022-3999(99)00057-4
26. Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health. 1999;14(1):1–24. doi:10.1080/08870449908407311
27. Wallace LS, Rogers ES, Roskos SE, Holiday DB, Weiss BD. Brief report: screening items to identify patients with limited health literacy skills. J Gen Intern Med. 2006;21(8):874–877. doi:10.1111/j.1525-1497.2006.00532.x
28. Chew LD, Griffin JM, Partin MR, et al. Validation of screening questions for limited health literacy in a large VA outpatient population. J Gen Intern Med. 2008;23(5):561–566. doi:10.1007/s11606-008-0520-5
29. Marshall MR, Rajah E, Wolley MJ, Reid S, Aspden T. SP723validation of A 3-item health literacy screener in a multiethnic New Zealand Dialysis Population. Nephrol Dial Transplant. 2015;30(suppl_3):iii617–iii617. doi:10.1093/ndt/gfv200.42
30. Okuyan B, Sancar M, Izzettin FV. Assessment of medication knowledge and adherence among patients under oral chronic medication treatment in community pharmacy settings. Pharmacoepidemiol Drug Saf. 2013;22(2):209–214. doi:10.1002/pds.3275
31. Okuyan B, Sancar M, Izzettin FV, Morisky DE. Erratum to and corrections on the article entitled “Assessment of medication knowledge and adherence among patients under oral chronic medication treatment in community pharmacy settings”. Pharmacoepidemiol Drug Saf. 2013;22(2):218–220. doi:10.1002/pds.3394
32. McPherson ML, Smith SW, Powers A, Zuckerman IH. Association between diabetes patients’ knowledge about medications and their blood glucose control. Res Social Adm Pharm. 2008;4(1):37–45. doi:10.1016/j.sapharm.2007.01.002
33. Broadbent E, Petrie KJ, Main J, Weinman J. The brief illness perception questionnaire. J Psychosom Res. 2006;60(6):631–637. doi:10.1016/j.jpsychores.2005.10.020
34. Weinman J, Petrie KJ, Moss-morris R, Horne R. The illness perception questionnaire: a new method for assessing the cognitive representation of illness. Psychol Health. 1996;11(3):431–445. doi:10.1080/08870449608400270
35. Moss-Morris R, Petrie KJ. Discriminating between chronic fatigue syndrome and depression: a cognitive analysis. Psychological Medicine. 2001;31(3):469. doi:10.1017/S0033291701003610
36. Jöreskog KG. A general approach to confirmatory maximum likelihood factor analysis. Psychometrika. 1969;34(2):183–202. doi:10.1007/BF02289343
37. Wold H. 11 - Path models with latent variables: the NIPALS approach**NIPALS = Nonlinear Iterative Partial Least Squares. In: Blalock HM, Aganbegian A, Borodkin FM, Boudon R, Capecchi V, editors. Quantitative Sociology. Academic Press; 1975:307–357.
38. Browne MW. Alternative ways of assessing model fit. Test Struct Equation Models. 1993;21(2):136–162.
39. Jöreskog KG, Sörbom D. LISREL 8: Structural Equation Modeling with the SIMPLIS Command Language. Scientific Software International; 1993.
40. Bentler PM, Bonett DG. Significance tests and goodness of fit in the analysis of covariance structures. Psychol Bull. 1980;88(3):588. doi:10.1037/0033-2909.88.3.588
41. Lt H, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equat Model. 1999;6(1):1–55. doi:10.1080/10705519909540118
42. Venturini S, Mehmetoglu M. Plssem: a stata package for structural equation modeling with partial least squares. Journal of Statistical Software. 2019;88(8):1–35. doi:10.18637/jss.v088.i08
43. Hair JF, Ringle CM, Sarstedt M. Partial least squares structural equation modeling: rigorous applications, better results and higher acceptance. Long Range Planning. 2013;46(1–2):1–12. doi:10.1016/j.lrp.2013.01.001
44. Sanchez G. PLS Path Modeling with R. Trowchez Editions; 2013.
45. McNeish D, Wolf MG. Thinking twice about sum scores. Behavior Research Methods. 2020;52(6):2287–2305. doi:10.3758/s13428-020-01398-0
46. Distefano C, Zhu M, Mindrila D. Understanding and using factor scores: considerations for the applied researcher. Pract Assess Res Eval. 2008;14(1):20.
47. Krousel-Wood MA, Muntner P, Islam T, Morisky DE, Webber LS. Barriers to and determinants of medication adherence in hypertension management: perspective of the cohort study of medication adherence among older adults. Med Clin North Am. 2009;93(3):753–769. doi:10.1016/j.mcna.2009.02.007
48. Bender B, Milgrom H, Apter A. Adherence intervention research: what have we learned and what do we do next? J Allergy Clin Immunol. 2003;112(3):489–494. doi:10.1016/s0091-6749(03)01718-4
49. Nguyen T-M-U, Caze AL, Cottrell N. Validated adherence scales used in a measurement-guided medication management approach to target and tailor a medication adherence intervention: a randomised controlled trial. BMJ Open. 2016;6(11):e013375. doi:10.1136/bmjopen-2016-013375
50. McHorney CA, Zhang NJ, Stump T, Zhao X. Structural equation modeling of the proximal-distal continuum of adherence drivers. Patient Prefer Adherence. 2012;6:789–804. doi:10.2147/ppa.S36535
51. Sabouri F, Rambod M, Khademian Z. The effect of positive thinking training on hope and adherence to treatment in hemodialysis patients: a randomized controlled trial. BMC Psychol. 2023;11(1):6. doi:10.1186/s40359-023-01036-2
52. Horne R, Sumner S, Jubraj B, Weinman J, Frost S. Haemodialysis patients’ beliefs about treatment: implications for adherence to medication and fluid-diet restrictions. Int J Pharm Pract. 2001;9(3):169–175. doi:10.1111/j.2042-7174.2001.tb01045.x
53. Daly M, Jones A, Robinson E. Public trust and willingness to vaccinate against COVID-19 in the US from October 14, 2020, to March 29, 2021. JAMA. 2021;325(23):2397–2399. doi:10.1001/jama.2021.8246
54. Horne R, Chapman SC, Parham R, Freemantle N, Forbes A, Cooper V. Understanding patients’ adherence-related beliefs about medicines prescribed for long-term conditions: a meta-analytic review of the Necessity-Concerns Framework. PLoS One. 2013;8(12):e80633. doi:10.1371/journal.pone.0080633
55. Chapman S, Dale P, Svedsater H, et al. Modelling the effect of beliefs about asthma medication and treatment intrusiveness on adherence and preference for once-daily vs. twice-daily medication. NPJ Prim Care Respir Med. 2017;27(1):61. doi:10.1038/s41533-017-0061-7
56. Lemay J, Waheedi M, Al-Sharqawi S, Bayoud T. Medication adherence in chronic illness: do beliefs about medications play a role? Patient Prefer Adherence. 2018;12:1687–1698. doi:10.2147/ppa.S169236
57. Beck EM, Cavelti M, Kvrgic S, Kleim B, Vauth R. Are we addressing the ‘right stuff’ to enhance adherence in schizophrenia? Understanding the role of insight and attitudes towards medication. Schizophr Res. 2011;132(1):42–49. doi:10.1016/j.schres.2011.07.019
58. Peyre M, Gauchet A, Roustit M, Leclercq P, Epaulard O. Influence of the first consultation on adherence to antiretroviral therapy for HIV-infected patients. Open AIDS J. 2016;10(1):182–189. doi:10.2174/1874613601610010182
59. Sabate E. Adherence to long-term therapies: evidence for action. Report of WHO Adherence to Longterm Therapies Project. Geneva Switzerland: World Health Organization; 2003.
60. Ashcroft K, Kim E, Elefant E, Benson C, Carter JA. Meta-analysis of caregiver-directed psychosocial interventions for schizophrenia. Community Ment Health J. 2018;54(7):983–991. doi:10.1007/s10597-018-0289-x
61. Fiqri AM, Sjattar EL, Irwan AM. Cognitive Behavioral Therapy for self-care behaviors with type 2 diabetes mellitus patients: a systematic review. Diabetes Metab Syndr. 2022;16(7):102538. doi:10.1016/j.dsx.2022.102538
62. Li IH, Hsieh WL, Liu WI. A systematic review and meta-analysis of the effectiveness of adherence therapy and its treatment duration in patients with schizophrenia spectrum disorders. Patient Prefer Adherence. 2023;17:769–780. doi:10.2147/ppa.S401650
63. Santo K, Kirkendall S, Laba TL, et al. Interventions to improve medication adherence in coronary disease patients: a systematic review and meta-analysis of randomised controlled trials. Eur J Prev Cardiol. 2016;23(10):1065–1076. doi:10.1177/2047487316638501
64. Seo MA, Min SK. Development of a structural model explaining medication compliance of persons with schizophrenia. Yonsei Med J. 2005;46(3):331–340. doi:10.3349/ymj.2005.46.3.331
65. Hyvert S, Yailian AL, Haesebaert J, et al. Association between health literacy and medication adherence in chronic diseases: a recent systematic review. Int J Clin Pharm. 2023;45(1):38–51. doi:10.1007/s11096-022-01470-z
66. Schönfeld MS, Pfisterer-Heise S, Bergelt C. Self-reported health literacy and medication adherence in older adults: a systematic review. BMJ Open. 2021;11(12):e056307. doi:10.1136/bmjopen-2021-056307
67. Watson PW, Garety PA, Weinman J, et al. Emotional dysfunction in schizophrenia spectrum psychosis: the role of illness perceptions. Psychol Med. 2006;36(6):761–770. doi:10.1017/s0033291706007458
68. Morrison VL, Holmes EAF, Parveen S, et al. Predictors of self-reported adherence to antihypertensive medicines: a multinational, cross-sectional survey. Value Health. 2015;18(2):206–216. doi:10.1016/j.jval.2014.12.013
69. Bąk-Sosnowska M, Gruszczyńska M, Wyszomirska J, Daniel-Sielańczyk A. The influence of selected psychological factors on medication adherence in patients with chronic diseases. Healthcare. 2022;10(3):426. doi:10.3390/healthcare10030426
70. Jónsdóttir H, Friis S, Horne R, Pettersen KI, Reikvam A, Andreassen OA. Beliefs about medications: measurement and relationship to adherence in patients with severe mental disorders. Acta Psychiatr Scand. 2009;119(1):78–84. doi:10.1111/j.1600-0447.2008.01279.x
71. Daley DJ, Deane KH, Gray RJ, et al. Adherence therapy improves medication adherence and quality of life in people with Parkinson’s disease: a randomised controlled trial. Int J Clin Pract. 2014;68(8):963–971. doi:10.1111/ijcp.12439
72. Bender BG, Apter A, Bogen DK, et al. Test of an interactive voice response intervention to improve adherence to controller medications in adults with asthma. J Am Board Fam Med. 2010;23(2):159–165. doi:10.3122/jabfm.2010.02.090112
73. Pouls BPH, Bekker CL, Gundogan F, et al. Gaming for Adherence to Medication using Ehealth in Rheumatoid arthritis (GAMER) study: a randomised controlled trial. RMD Open. 2022;8(2):e002616. doi:10.1136/rmdopen-2022-002616
74. Hermansson-Borrebaeck R, Andersson U, Jakobsson U, Midlöv P. Beliefs about medications when treating hypertension in primary health care: results from ”PERson-centredness in hypertension management using information Technology (PERHIT)”. Blood Press. 2023;32(1):2226736. doi:10.1080/08037051.2023.2226736
75. Crawshaw J, Weinman J, McRobbie D, Auyeung V. Initial evaluation of a brief pharmacy-led intervention to modify beliefs about medicines and facilitate adherence among patients hospitalised with acute coronary syndrome. Eur J Hosp Pharm. 2022;29(1):18–25. doi:10.1136/ejhpharm-2019-002041
76. Hjemås BJ, Bøvre K, Mathiesen L, Lindstrøm JC, Bjerknes K. Interventional study to improve adherence to phosphate binder treatment in dialysis patients. BMC Nephrol. 2019;20(1):178. doi:10.1186/s12882-019-1334-x
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.