Back to Journals » Journal of Multidisciplinary Healthcare » Volume 8
Stroke subtype, age, and baseline NIHSS score predict ischemic stroke outcomes at 3 months: a preliminary study from Central Nepal
Authors Shrestha S , Poudel R , Khatiwada D , Thapa L
Received 16 June 2015
Accepted for publication 22 August 2015
Published 1 October 2015 Volume 2015:8 Pages 443—448
DOI https://doi.org/10.2147/JMDH.S90554
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Shakti Shrestha,1 Ramesh Sharma Poudel,2 Dipendra Khatiwada,3 Lekhjung Thapa4
1Department of Pharmacy, Shree Medical and Technical College, 2Department of Pharmacy, 3Department of Community Medicine, 4Department of Neurology, College of Medical Sciences-Teaching Hospital, Chitwan, Nepal
Background: The combined medications practice of using antithrombotic agents and statins with or without antihypertensive agents is common in the treatment of acute ischemic stroke in Nepal. Short-term outcomes of the current practice have been studied. We aim to explore the predictors of ischemic stroke outcomes at 3 months, with the current combined medications practice.
Methods: The study population (N=56) included acute ischemic stroke patients treated at the Neurology Department of the College of Medical Sciences-Teaching Hospital, Chitwan, Nepal, from May 2014 to August 2014 and followed up at 3 months. Death or disability (modified Rankin scale >2) was defined as poor outcomes. Multivariate logistic regression analysis (P<0.10) using potential variables from bivariate analysis (P≤0.20) was adjusted to predict outcomes at 3 months.
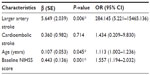
Results: At 3 months, 29 (51.8%) patients were independent, eleven (19.6%) were dependent, while 16 (28.6%) died. Stroke subtype and baseline National Institute of Health Stroke Scale (NIHSS) scores were associated with death/disability (27, 48.2%) at 3 months. Regression analysis showed that large-artery stroke (odds ratio [OR] =284.145, 95% confidence interval [CI] =5.221–15,465.136, P=0.006), age (OR =1.113, 95% CI =1.002–1.236, P=0.045), and baseline NIHSS score (OR =1.557, 95% CI =1.194–2.032, P=0.001) were significant predictors of poor outcome at 3 months.
Conclusion: Stroke subtype, age, and baseline NIHSS score are predictors of ischemic stroke outcomes in Nepalese population treated with the current practice of using combined antithrombotic and statins with or without antihypertensive agents, and these predictors can be used for the improvement of selection of patients for the appropriate treatment.
Keywords: age, Nepal, NIHSS score, ischemic stroke, stroke subtype
Introduction
Stroke is the second leading cause of death1 and a leading cause of serious long-term disability.2 In Nepal, 2.25% of hospital admitted patients were suffering from stroke.3 Intravenous thrombolysis using recombinant tissue plasminogen activator (rt-PA) is one of the potential treatments for acute ischemic stroke, which has to be used within 4.5 hours,4 and several predictors influencing its outcomes have been documented.5 Unfortunately, rt-PA is rarely used in Nepal, with no evidence in literature from this country. Combined medications (antithrombotic agents and statins with or without antihypertensive agents) are common in current practice. Based on this, a study from Nepal has shown admission NIHSS score as a predictor of 7-day mortality in the intensive care unit (ICU), but failed to determine the long-term predictors of outcomes.6 Another study from the same setting including both ICU and inpatients suggests association of NIHSS score and modified Rankin scale (mRS) with outcomes at 3 months in treated patients of acute ischemic stroke, but the outcomes were not predicted using multivariable analysis.7 Our study aims to explore the predictors of ischemic stroke outcomes at 3 months in treated patients with current practice for first time in Nepal.
Methods
Subjects
This was a prospective observational study to assess the predictor of outcomes in acute ischemic stroke patients treated at the Neurology Department of College of Medical Sciences-Teaching Hospital, Chitwan, Nepal, from May 2014 to August 2014. Ethical approval was obtained from the institution, and verbal informed consent was taken from all participants or their relatives for obtaining information from admission to follow-up. All ischemic stroke patients confirmed by computed tomography or magnetic resonance imaging scan, aged >18 years, mRS >2, NIHSS score >0 were included, and those with intracranial hemorrhage were excluded. Patients with mRS >2 were only included since our study required moderate-to-severe stroke patients.
Data collection
Details of demography; vascular risk factors; number of modifiable vascular risk factors; blood pressure on admission; laboratory tests, including random blood sugar on admission; brain imaging, middle cerebral artery (MCA) hypodensity; and time of onset to first dose of antithrombotic agents were recorded at the time of admission using a standardized structured form. Vascular risk factors included a history of previous stroke, coronary artery disease, hyperlipidemia, hypertension, diabetes, atrial fibrillation (AF), status of smoking, and alcohol consumption. Baseline severity of stroke was assessed using the NIHSS score,8 and mRS was used to assess the functional disability at 3 months.9 Ischemic stroke subtype was classified according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria as large-artery atherosclerosis, cardioembolism, small artery occlusion, stroke of other determined cause, and stroke of undetermined cause.10 Treatment information included the use of antithrombotic agents, statins, and antihypertensive agents.
Measurement of outcomes
The primary outcomes were death/disability at 3 months (90±10 days) after onset of stroke symptoms. Death was all-cause case fatality. Disability was defined as mRS >2.11 Patients were followed up by interview/assessment in the neurology outpatient department clinic or by telephone interview. The poor outcome was defined as death or disability (mRS >2), and the good outcome was defined as independent at 3 months (mRS ≤2).
Statistical analysis
Differences between two groups were tested using t-test, Mann–Whitney U test, or χ2 test where appropriate. Variables that were potential in the bivariate analyses (P<0.20) were entered into multivariate logistic regression analysis (P<0.10) to predict the outcome (death/disability) at 3 months. All statistical analyses were performed with IBM SPSS version 20 (IBM Corporation, Armonk, NY, USA).
Results
Out of 56 patients, 35 (62.5%) were males. The mean age was 67.04±13.39 years (minimum 22 years and maximum 92 years). Twenty-six patients (46.4%) had small-vessel stroke followed by 20 (35.7%) with cardioembolic stroke, and ten (17.9%) with large-artery stroke. Of these, 29 (51.8%) had MCA hypodensity ≤33%. Thirty-five (62.5%) patients had hypertension, and 33 (59%) patients were smokers (current and previous). The median (interquartile range) baseline NIHSS score on admission was 13.5 (6–20). Aspirin alone as an antithrombotic agent was used in 36 (64%) patients, while its combination was mostly used with heparin (10, 17.9%). Atorvastatin was the commonest (55, 98.2%) statin used. Also, antihypertensive agents were used in most cases (37, 66.1%) (Table 1).
Out of the total 56 patients, 29 (51.8%) patients were independent, eleven (19.6%) were dependent, and 16 (28.6%) patients died at 3 months. Hence, the total case of death/disability was 27 (48.2%). Bivariate analysis using Pearson χ2 test demonstrated that stroke subtype (P=0.007) was associated with death/disability at 3 months. Similarly, Mann–Whitney U test demonstrated that baseline NIHSS score (P<0.001) was associated with death/disability at 3 months. Of those who had poor outcomes (death/disability) (n=27) at 3 months, 12 (44.4%) had cardioembolic stroke, followed by eight (29.6%) patients with large-artery stroke, and seven (25.9%) with a small-vessel stroke. The median baseline NIHSS score of patients with poor outcome was 20, which was 12 scores more than those who did not have a poor outcome (Table 2).
The association of stroke subtype with baseline NIHSS score on admission was statistically significant at P=0.024. NIHSS on admission was higher in larger-artery (NIHSS =17.0) and cardioembolic (NIHSS =17.5) stroke subtype, but lower (NIHSS =9.0) in the small-vessel subtype.
Multiple logistic regression using the potential variables (sex, stroke subtype, previous stroke, alcohol consumption, age, and baseline NIHSS score) from bivariate analysis (P≤0.20) was adjusted to predict outcomes at 3 months. A model with three statistically significant exploratory variables, which included large-artery stroke subtype, age, and baseline NIHSS score on admission, was obtained to predict poor outcome (death/disability at 3 months).
An increase in age (P=0.045) by 1 year and baseline NIHSS score (P=0.001) by one unit increase the odds of poor outcome by 1.113 and 1.557 times, respectively. The model also demonstrates that patients with large-artery stroke have 284.145 times higher odds of poor outcome than those with small-vessel stroke at P=0.006. Similarly, those with cardioembolic stroke have 1.434 times higher odds of poor outcome than small-vessel stroke, but it is not statistically significant (Table 3).
Discussion
Our study showed that 26 (46.4%) patients had a small-vessel stroke (lacunar), followed by 20 (35.7%) with cardioembolic stroke, and ten (17.9%) with large-artery stroke. A study in a similar setting showed slightly lower incidence of lacunar stroke (40%), but slightly higher incidence of large-artery stroke (36%) and cardioembolic ischemic stroke (19%).6 This study included patients only from ICU, where comparatively severe patients are treated, but we included patients from both ICU and neurology ward. In addition to this, patients with cardioembolic and large-artery stroke had greater severity based on baseline NIHSS score in our study. Lower incidence of cardioembolic stroke has been seen in the People’s Republic of China (7.5%)12 and India (14%).13 This suggests that the Nepalese population are more prone to cardioembolic stroke in comparison to the neighboring countries. This might be due to undetected paroxysmal AF (not detected by routine electroencephalogram), rheumatic heart disease, and untreated atherosclerosis, suggesting an early intervention of these risk factors associated with cardioembolic stroke.
Aspirin as an antithrombotic agent, atorvastatin as an antihyperlipidemic agent, and antihypertensive agents are commonly used as the combined medication for the treatment of ischemic stroke in our study. A similar result was observed in our previous study in which the combined medications practice seemed promising and efficacious in the management of mild-to-moderately severe ischemic stroke.7 In our study, none of the patients was given intravenous rt-PA. Patients often arrive at hospital beyond the recommended time of treatment for intravenous rt-PA. In our study, the median antithrombotic time was 22 hours (minimum 4 hours and maximum 130 hours). There is no evidence of thrombolysis treatment using rt-PA in ischemic stroke patients from Nepal.5
In our study, small-vessel stroke was the most common subtype of stroke, but those with cardioembolic and large-artery stroke had overall poor outcomes at 3 months. The higher odds of large-artery stroke with outcome measurement in regression analysis might be due to small sample size. An observational study done by Hao et al12 also demonstrated cardioembolic stroke as an independent predictor of death or disability at 3 months and small vessel as an independent predictor of good outcomes. A study by Petty et al14 also demonstrated ischemic stroke subtype as a significant predictor of both short-term and long-term outcomes, indicating poor outcomes in cardioembolic and large-vessel stroke. Furthermore, a study by Ferrari et al15 showed large-artery stroke and cardioembolic stroke as independent predictors of early deterioration in patients with a transient ischemic attack or minor ischemic stroke. Higher portion of brain damage has often been observed in large-artery and cardioembolic stroke. Also, the estimated risk of recurrent stroke has been seen higher in large-artery (18.5%) and cardioembolic stroke (5.3%) in comparison to small-vessel stroke (1.4%) at 30 days, contributing to the outcomes of ischemic stroke.14
Our study showed that the mean age of the study population was 67.04±13.39 years. Stroke studies done in Nepalese population by Dewan and Rana6 (67.15±12.58 years) and Maskey et al16 (65.98±10.69 years) have also shown similar results. In contrast, several studies in Nepal have reported lower mean ages.7,17–19 This difference might be due to the difference in the sample size and the fact that patients included in these studies were younger than those included in our study. Age was not statistically significant in bivariate analysis, but the multivariate logistic regression analysis showed age as a good predictor of ischemic stroke outcomes in our study. The predictive analysis suggests that a 1-year increase in age increases the odds of death/disability by 1.113 times. Age has been known to be a clinically relevant variable that has been associated with stroke outcome. Several studies have reported age as a predictor of poor outcomes.20–24 A study also reported age at the time of the event as an important predictor for early recovery of stroke.25
The median (interquartile range) baseline NIHSS score in our study was 13.5 (6–12), and the median baseline NIHSS score in those with large-artery and cardioembolic stroke was similar (17 and 17.5, respectively), whereas for small-vessel stroke, it was 9. The multiple logistic regressions in our study showed baseline NIHSS score as a good predictor of death/disability at 3 months. This demonstrates that an increase in baseline NIHSS score by one unit increases the odds of death/disability by 1.55 times. A study by Jain et al26 showed that one point increase in the stroke scale at baseline increases the likelihood of mortality and worsening of ambulatory function by 2.3 and three times, respectively. Studies showed baseline NIHSS score as a predictor of outcome at discharge following acute hospitalization.27,28 Studies also reported that baseline NIHSS score is also a predictor of 7-day outcomes of ischemic stroke.6,24 Thus, the severity of stroke might have influenced the overall poor outcome favoring cardioembolic and large-artery stroke. Our study was limited to a single center, and there was a variation in the treatment protocol. Despite these limitations, our study explored the predictor of long-term stroke outcomes in the Nepalese setting for the first time.
Conclusion
Stroke subtype, age, and baseline NIHSS score are predictors of ischemic stroke outcomes in the Nepalese population treated with current practice using antithrombotic and statins with or without antihypertensive agents, and these predictors can be used for the improvement of selection of patients for the appropriate treatment. Nepalese are more prone to poor outcome by cardioembolic stroke and large-artery stroke. It is possible to reduce the burden of stroke by early intervention of the risk factors associated with these stroke subtypes such as 24-hour Holter monitoring to rule out any undetected AF by electroencephalogram, and taking appropriate measures to control rheumatic heart disease and atherosclerosis. However, a large-scale multicenter study is needed to establish this preliminary evidence.
Disclosure
The authors report no conflicts of interest in this work.
References
WHO.int. The Top 10 Causes of Death. Geneva, Switzerland: World Health Organization; 2015. Available from: http://www.who.int/media centre/factsheets/fs310/en/. Accessed January 5, 2015. | |
Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics – 2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28–e292. | |
Bhandari GP, Angdembe MR, Dhimal M, Neupane S, Bhusal C. State of non-communicable disease in Nepal. BMC Public Health. 2014;14:23. | |
Prabhakaran S, Ruff I, Bernstein RA. Acute stroke intervention: a systematic review. JAMA. 2015;3139(14):1451–1462. | |
Shrestha S, Poudel RS, Thapa LJ, Khatiwada D. Intravenous thrombolysis and risk factors for ischemic stroke. J Nepal Med Assoc. 2014;52(193):745–750. | |
Dewan KR, Rana PV. A study of seven day mortality in acute ischemic stroke in a teaching hospital in Chitwan. J Nepal Health Res Counc. 2014;12(26):33–38. | |
Poudel RS, Thapa LJ, Shrestha S, et al. Efficacy of combined antithrombotic, statin and antihypertensive agents in acute ischemic stroke. J Nepal Med Assoc. 2015;53(197):5–11. | |
Clark WM, Hourihane JM. Clinical stroke scales. In: Herndon RM, editor. Hand Book of Neurological Rating Scale. New York, NY: Demos Vermande; 1997:161–186. | |
Rankin J. Cerebral vascular accidents in patients over the age of 60. I: general considerations. Scott Med J. 1957;2:127–136. | |
Adams HP Jr, Bendixen BH, Kappelle LJ, Gordon DL, Marsh EE. Classification of subtype of acute ischemic stroke: definition for use in multicenter clinical trial, TOAST. Trial of Org 10172 Acute Stroke treatment. Stroke. 1993;24(1):35–41. | |
van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19(5):604–607. | |
Hao Z, Liu M, Wang D, Wu B, Tao W, Chang X. Etiologic subtype predicts outcome in mild stroke: prospective data from hospital stroke registry. BMC Neurol. 2013;13:154. | |
Dash D, Bhashin A, Pandit AK, et al. Risk factors and etiologies of ischemic strokes in young patients: a tertiary hospital study in north India. J Stroke. 2014;16(3):173–177. | |
Petty GW, Brown RD Jr, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Ischemic stroke subtypes: a population-based study of functional outcome, survival, and recurrence. Stroke. 2000;31(5):1062–1068. | |
Ferrari J, Knoflach M, Kiechl S, et al. Early clinical worsening in patients with TIA or minor stroke: the Austrian stroke unit registry. Neurology. 2010;74(2):136–141. | |
Maskey A, Parajuli M, Kholi SC. A study of risk factors of stroke in patients admitted in Manipal Teaching Hospital, Pokhara. Kathmandu Univ Med J. 2011;9(36):244–247. | |
Naik M, Rauniyar RK, Sharma UK, Dwivedi S, Karki DB, Samuel JR. Clinico-radiological profile of stroke in eastern Nepal: a computed tomographic study. Kathmandu Univ Med J. 2006;4(2):161–166. | |
Devkota KC, Thapamagar SB, Malla S. Retrospective analysis of stroke and its risk factors at Nepal Medical College Teaching Hospital. Nepal Med Coll J. 2006;8(4):269–275. | |
Pathak V, Kanth R, Pant H. Stroke: a case series study in Nepal Medical College Teaching Hospital. Nepal Med Coll J. 2006;8(3):180–181. | |
Prencipe M, Culasso F, Rasura M, et al. Long-term prognosis after a minor stroke: 10-year mortality and major stroke recurrence rates in a hospital-based cohort. Stroke. 1998;29(1):126–132. | |
Selvarajah JR, Smith CJ, Hulme S, Georgiou RF, Vail A, Tyrrell PJ. Prognosis in patients with transient ischaemic attack (TIA) and minor stroke attending TIA services in the North West of England: the NORTHSTAR Study. J Neurol Neurosurg Psychiatry. 2008;79(1):38–43. | |
van Wijk I, Kappelle LJ, van Gijn J, et al. Long-term survival and vascular event risk after transient ischaemic attack or minor ischaemic stroke: a cohort study. Lancet. 2005;365(9477):2098–2104. | |
Kim JT, Kim HJ, Yoo SH, et al. MRI findings may predict early neurologic deterioration in acute minor stroke or transient ischemic attack due to intracranial atherosclerosis. Eur Neurol. 2010;64(2):95–100. | |
Coutts SB, Hill MD, Campos CR, et al. Recurrent events in transient ischemic attack and minor stroke: what events are happening and to which patients? Stroke. 2008;39(9):2461–2466. | |
Ahmed R, Zuberi BF, Afsar S. Stroke scale score and early prediction of outcome after stroke. J Coll Physicians Surg Pak. 2004;14(5):267–269. | |
Jain A, Houten DV, Sheikh L. Retrospective study on National Institute of Health Stroke Scale as a predictor of patients recovery after stroke. J Cardiovasc Nurs. Epub October 16, 2014. Available from: http://journals.lww.com/jcnjournal/Abstract/publishahead/Retrospective_Study_on_National_Institutes_of.99666.aspx. Accessed June 1, 2015. | |
Kennuir CL, Hammer M, Jovin T, Reddy V, Wechsler L, Jadhav A. Predictors of outcome in patients presenting with Acute Ischemic Stroke and Mild Stroke Scale Scores. J Stroke Cerebrovasc Dis. 2015;24(7):1685–1689. | |
Tseng MC, Chang KC. Stroke severity and early recovery after first-ever ischemic stroke: results of a hospital-based study in Taiwan. Health Policy. 2006;79(1):73–78. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.