Back to Journals » Journal of Inflammation Research » Volume 15
Stressor-Induced Reduction in Cognitive Behavior is Associated with Impaired Colonic Mucus Layer Integrity and is Dependent Upon the LPS-Binding Protein Receptor CD14
Authors Jaggers RM, DiSabato DJ, Loman BR, Kontic D, Spencer KD, Allen JM, Godbout JP, Quan N , Gur TL, Bailey MT
Received 23 August 2021
Accepted for publication 16 February 2022
Published 3 March 2022 Volume 2022:15 Pages 1617—1635
DOI https://doi.org/10.2147/JIR.S332793
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Monika Sharma
Robert M Jaggers,1 Damon J DiSabato,2,3 Brett R Loman,1 Danica Kontic,1 Kyle D Spencer,1,4,5 Jacob M Allen,1 Jonathan P Godbout,2,3 Ning Quan,6 Tamar L Gur,2,7 Michael T Bailey1,2,8
1Center for Microbial Pathogenesis, Abigail Wexner Research Institute at Nationwide Children’s Hospital, Columbus, OH, 43205, USA; 2Institute for Behavioral Medicine Research, Columbus, OH, 43210, USA; 3Department of Neuroscience, The Ohio State University, Columbus, OH, 43210, USA; 4Department of Biomedical Informatics, The Ohio State University, Columbus, OH, USA; 5Graduate Partnership Program, National Center for Advancing Translational Sciences, National Institutes of Health, Bethesda, OH, USA; 6Department of Biomedical Science, Charles E. Schmidt College of Medicine, Florida Atlantic University, Jupiter, FL, 33458, USA; 7Department of Psychiatry, College of Medicine, The Ohio State University, Columbus, OH, 43210, USA; 8Department of Pediatrics, College of Medicine, The Ohio State University, Columbus, OH, 43210, USA
Correspondence: Michael T Bailey, Center for Microbial Pathogenesis, Abigail Wexner Research Institute, W410, Nationwide Children’s Hospital, 700 Children’s Drive, Columbus, OH, 43205, USA, Tel +1 614-722-2764, Email [email protected]
Purpose: Commensal microbes are impacted by stressor exposure and are known contributors to cognitive and social behaviors, but the pathways through which gut microbes influence stressor-induced behavioral changes are mostly unknown. A murine social stressor was used to determine whether host–microbe interactions are necessary for stressor-induced inflammation, including neuroinflammation, that leads to reduced cognitive and social behavior.
Methods: C57BL/6 male mice were exposed to a paired fighting social stressor over a 1 hr period for 6 consecutive days. Y-maze and social interaction behaviors were tested following the last day of the stressor. Serum cytokines and lipopolysaccharide binding protein (LBP) were measured and the number and morphology of hippocampal microglia determined via immunohistochemistry. Intestinal mucous thickness and antimicrobial peptide expression were determined via fluorescent staining and real-time PCR (respectively) and microbial community composition was assessed using 16S rRNA gene amplicon sequencing. To determine whether the microbiota or the LBP receptor (CD14) are necessary for stressor-induced behavioral changes, experiments were performed in mice treated with a broad-spectrum antibiotic cocktail or in CD14−/− mice.
Results: The stressor reduced Y-maze spontaneous alternations, which was accompanied by increased microglia in the hippocampus, increased circulating cytokines (eg, IL-6, TNF-α) and LBP, and reduced intestinal mucus thickness while increasing antimicrobial peptides and cytokines. These stressor-induced changes were largely prevented in mice given broad-spectrum antibiotics and in CD14−/− mice. In contrast, social stressor-induced alterations of social behavior were not microbe-dependent.
Conclusion: Stressor-induced cognitive deficits involve enhanced bacterial interaction with the intestine, leading to low-grade, CD14-dependent, inflammation.
Keywords: social defeat, stress, microbiome, microglia, cytokines, neuroinflammation, mucus barrier
Introduction
The intestinal microbiota are altered by stressor exposure and contribute to stress-induced physiological and behavioral responses through activation of the brain-gut-microbiota axis.1,2 Signals from the intestinal microbiota that are transduced into the central nervous system (CNS) are thought to play a key role in stressor-induced emergence of psychopathology. Several pathways connecting the gut microbiota to the brain and behavior have been suggested, such as signaling through the vagus nerve3–5 and influences on host neurotransmitters and hormones (such as serotonin and oxytocin).6–8 However, little is known about the contribution of the mucosal barrier and subsequent immune activation. We used a murine stressor involving social defeat previously demonstrated to cause cognitive and social deficits through mechanisms dependent upon the IL-1 receptor on hippocampal neurons10 to test whether stressor-induced behavioral change was related to alterations in the intestinal tissue, intestinal microbiota, and subsequent immune activation.
The intestine is lined with a protective layer of mucous that is constitutively produced and secreted by goblet cells and provides a physical barrier that reduces the ability of bacteria to contact the intestinal epithelium.9,10 This is greatly enhanced by the secretion of antimicrobial peptides, such as defensins, lysozyme, and regenerating islet-derived protein 3 (REG3) beta and gamma.11–13 While antimicrobial peptide secretion is constitutive, bacterial penetration of the mucus and contact with the epithelium strongly increases antimicrobial peptides in the intestine. This host response helps to limit the ability of bacteria to translocate from the lumen of the intestine to the interior of the body, but even with these protective responses, bacterial translocation still occurs.14
Exposing laboratory mice to different types of stressful stimuli leads to the translocation of microbes and their products from the lumen of the intestine to the interior of the body.15–18 Stressor-induced inflammatory responses occur in a wide variety of animal models (including chronic unpredictable mild stress, chronic subordinate colony housing, tail shock, and social defeat) as well as in human social stress.16–22 In murine models involving social defeat or chronic unpredictable mild stress, commensal microbes have been linked to the stimulation of low-grade inflammatory responses,15,16,20,23,24 and an increasing number of human studies have suggested that the gut microbiota are involved with stressor-induced inflammation.25–28 Increased inflammatory tone is a common consequence of social stressors,29–31 and increases in inflammatory cytokines, such as IL-1β and IL-6 are well recognized to lead to behavioral changes, such as anxiety-like and depressive-like behaviors32–35 that are associated with enhanced neuronal activity, astrogliosis, and microglial activation.32,36 In murine models of social defeat, microglial activation leads to the recruitment of circulating monocytes into the brain,34,37,38 and some stressor-induced behaviors are dependent upon this monocyte recruitment.34,37,38 This microglial activity and recruitment of peripheral monocytes occurs in a brain region-dependent manner, causing a variety of neuroinflammatory outcomes within stress-relevant brain regions, such as the amygdala, prelimbic cortex, and hippocampus.32,34,38,39 The paradigm utilized in the current study leads to social and cognitive deficits that are dependent upon neuroinflammation specifically within the hippocampus.32 Despite this realization, mechanisms by which the intestine and its microbiota contribute to stressor-induced increases in these inflammatory cytokines, and by extension behavioral responses to stressor exposure, are not completely understood. Studies in human participants have suggested that stressor-induced increases in intestinal permeability contribute to immune activation and behavioral responses.25,40 These responses have been linked to LPS binding protein (LBP), which is released by hepatocytes and intestinal epithelial cells in response to bacterial LPS or to intestinal cytokines.41,42 Serum LPS/LBP complexes bind to CD14 that is constitutively expressed on intestinal epithelial cells, monocytes, macrophages, and neutrophils and leads to cellular activation.43–45 Thus, CD14 may play an essential role in mediating the link between stressor-induced immumodulation, behavioral responses, and the gut microbiome. In this study, mice exposed to social defeat were used to test whether stressor-induced increases in cytokines and microglial activation are affected by disrupting commensal intestinal microbiota. To determine whether LPS/LBP complexes may link the microbiota to stressor-induced inflammation, mice lacking the LPS/LBP receptor (ie, CD14) were also tested.
Materials and Methods
Mice
Male C57BL/6 mice or CD14−/− mice (on a C57BL/6 background) between 6 and 8 weeks of age were delivered from Charles River Laboratories, pair housed, and allowed to habituate for 1 week prior to experimentation. Animals received standard laboratory chow and water ad libitum. Procedures were approved by the Animal Care and Use Committees at Ohio State University and the Abigail Wexner Research Institute at Nationwide Children’s Hospital, and conducted in a laboratory accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care according to Guide for the Care and Use of Laboratory Animals: Eighth Edition.46
Experimental Design
Mice were exposed to social defeat each day for 6 consecutive days or left undisturbed as a control. At 14 h following the final cycle of social defeat, social and cognitive behaviors were assessed using a social interaction test followed by a Y-maze test. Immediately following testing on the Y-maze, mice were euthanized via CO2 asphyxiation, and blood collected via cardiac puncture. Brains were collected and formalin fixed. Ileum and colonic tissue, along with their respective luminal contents, were collected and either fixed or frozen in liquid nitrogen.
Antibiotic Administration
Wildtype (WT) mice were gavaged for 3 days prior to and each day of the stressor with 100 µL of an antibiotic cocktail to disrupt the microbiome. The cocktail consisted of ampicillin (1 mg/mL), vancomycin (0.5 mg/mL), neomycin sulfate (1 mg/mL), and metronidazole (1 mg/mL) or sterile H2O as the vehicle treatment as previously reported.20,47,48
Social Stressor
The social stressor was performed as previously described.32 Experimental mice were placed individually into the cages of CD-1 aggressor mice for up to one hour (between 16:00 to 18:00) per night for six consecutive nights. During each cycle, submissive behaviors (upright posture, fleeing, crouching) were observed to ensure that the experimental mice showed signs of defeat. Mice were moved to a new aggressor’s cage if no attack occurred within the first 5 min or if the experimental mouse defeated the aggressor. At the end of the 1 h period, mice were removed and placed back into their home cage, left undisturbed until the following day when the paradigm was repeated. To avoid habituation, different aggressors were used on consecutive nights. The health of the experimental mice was monitored throughout the experiments by looking for wounding, assessing coat condition, and tracking weight. Although handling controls are often used for some models of social defeat,49 control mice were left undisturbed in their home cages in this study as previously described.32 All behavioral and biological measures were obtained 14 h after the last cycle of stressor exposure.
Behavior Testing
The social interaction test was conducted as previously described.32,50 First, a test mouse was placed in an open field box for 5 min with an empty cage. Next, a novel juvenile C57BL/6 mouse was placed in the cage and the test mouse was placed back in the open field box for 5 min. Total distance traveled, time spent in the corner or interacting with the juvenile mouse were recorded. Approximately 1 hr after the social interaction test, mice were placed into the Y-maze for 5 min as previously described.10 Behavior was recorded via an overhead camera and entries into each arm were counted. A spontaneous alternation was defined as entering all three arms before revisiting a previously entered arm. The spontaneous alternations by each mouse were recorded as a percentage of total 3-entry sets.
Real-Time PCR for Assessment of Intestinal Antimicrobial Peptide, Tight Junction, and Cytokine Gene Expression
RNA was isolated from frozen intestinal tissue using PureLink RNA Mini Kit (Thermo Fisher), with cDNA synthesis performed using a reverse transcriptase kit (Thermo Fisher). Power SYBR Green Master Mix with primers (Fwd and Rv, Integrated DNA Technologies) listed in Table 1 were used in PCR reactions. Differences in gene expression were determined by Real-Time PCR (Quantstudio 5, Thermo Fisher) using the delta-delta cycle threshold method with Eef2 used as the housekeeping gene.
 |
Table 1 PCR Primer Sequences |
Serum Cytokine and LPS Binding Protein Analysis
Serum cytokine concentrations were determined using the Meso Scale Diagnostics’ V-Plex Proinflammatory Panel 1 Mouse Kit to simultaneously measure IFN-γ, IL-10, IL-12p70, IL-1β, IL-2, IL-4, IL-5, IL-6, KC/GRO, TNF-α per manufacturer directions (Meso Scale Diagnostics, Rockville, MD). LPS binding protein in the serum was measured using a commercially available ELISA kit per manufacturer’s instructions (Hycult Biotec, Uden, Netherlands).
Analyses of Intestinal Mucous Layer and Bacterial Burden with Fluorescent in-situ Hybridization (FISH) Staining
A section of ileum and mid-colon were collected with intestinal contents left in place and fixed by immersion in Carnoy’s solution (60% methanol, 30% chloroform, 10% acetic acid) for 48–96 h, followed by two washes in methanol for 35 min, ethanol for 30 min, and xylene for 25 min. Cassettes were submerged in melted paraffin at 68°C for 1 h, removed, and kept at RT until sectioning. Blocks were cut into 4 µm sections. Slides were deparaffinized on a 60°C heat block for 10 min followed by an incubation in xylene substitute and 100% ethanol. A solution of hybridization buffer (5M NaCl, 1M Tris-HCl, 20% formamide, 10% SDS, and dH2O) and EUB probe were added to each slide for 12 h in a humid chamber at 49°C. Following the incubation, slides were washed (1M Tris-HCl, 5M NaCl, and dH2O) for 15 min at 49°C. For mucus staining, slides were then incubated with a fluorescein-labeled Ulex Europaeus Agglutinin (UEA-1) antibody (1:500) for 1 h in the dark. After 3 washes, slides were incubated with Prolong Gold anti-fade DAPI mounting media, dried at RT for 24 h, and imaged with a LSM 880 confocal microscope (Zeiss Microscopy). After image capture, inner mucus thickness was analyzed using ImageJ software. All measurements were made by a blinded observer and averaged across 5 randomly chosen crypt cross sections.
Brain Immunohistochemistry and Digital Imaging Analysis
A subset of mice were transcardially perfused with PBS and 4% paraformaldehyde (PFA). Brains were removed and post-fixed in 4% PFA for 24 h and dehydrated in 30% sucrose for 48 h. Fixed tissue was frozen on dry ice and cryosectioned at 30 µm thickness. Sections were stored in cryoprotectant at −20̊C. Brain regions were identified using the Allen Mouse Brain Atlas (Allen Institute). Sections were washed in PBS with 1% BSA, blocked with 5% normal donkey serum, and incubated with primary antibodies: rabbit anti-mouse ΔFosB (1:2000; Abcam; catalog number ab184938), rabbit anti-mouse Iba-1 (1:1000; Wako Chemicals; catalog number 019–19741). Sections were incubated overnight at 4°C, and then washed in PBS and incubated with a fluorochrome-conjugated secondary antibody (Donkey anti-rabbit; AlexaFluor 488; Thermo Fisher). Sections were mounted on charged slides, cover-slipped with Fluoromount (Beckman Coulter, Inc.), and stored at −20°C prior to imaging using an EVOS M7000 imaging system (Thermo Fisher). Iba-1 labeling was analyzed using a digital image analysis in which a threshold for positive staining was determined for each image that included all cell bodies and processes but excluded background staining. Data were processed by densitometric scanning of the threshold targets using ImageJ software in order to determine percent area of labeling in each image. Iba-1 labeling images were taken from the dentate gyrus region on the hippocampus. For ΔFosB, mean fluorescence intensity (MFI) was calculated within the dentate gyrus using ImageJ software. For all IHC, 4 images were taken from 3 different sections per animal.
16S rRNA Gene Amplicon Sequencing
Intestinal contents were sent to The Environmental Sample Preparation and Sequencing Facility at Argonne National Laboratory for DNA extraction, library preparation, and high-throughput sequencing. Paired-end (250 nt forward and reverse) sequences of the V4 hypervariable region of the 16S rRNA gene (515F-806R) were generated on Illumina MiSeq. Quantitative Insights into Microbial Ecology 2 (QIIME2) was utilized for amplicon processing, quality control with DADA2, downstream taxonomic assignment using the SILVAv132 database, and diversity analyses.51,52 All samples (ileum and colon contents) were rarefied to 18,000 reads/sample (all samples included) for diversity and differential abundance analyses.
Statistical Analyses
A two-factor analysis of variance was used to assess differences in organ weight, behavioral responses, immunofluorescence/fluorescent in situ hybridization staining, gene expression, bacterial alpha diversity, and protein levels using the group (stressor vs control) and treatment (vehicle vs antibiotics) as between subjects variables. Protected t-tests (using Bonferroni correction) were utilized for pairwise comparisons when significant group x treatment interactions were identified. If group x treatment interactions were not statistically significant, only the main effects were reported. In follow-up studies utilizing CD14−/− mice, Students t-test was used to assess differences in behavioral responses, immunofluorescence/flourescent in situ hybridization staining, gene expression, and protein levels. ADONIS PERMANOVA including all factors and interactions was used to test bacterial beta diversity. Differential abundance of bacterial taxa was determined by Mann–Whitney test. In all cases, p < 0.05 was considered statistically significant.
Results
Social Stressor Effects on Cognitive Behavior in Vehicle-Treated and Antibiotic-Treated Mice
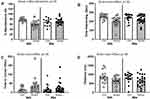
The social defeat stressor reduced working memory in the Y-maze, which was ameliorated by treatment with antibiotics (stressor x antibiotic interaction, F(1, 56) = 10.41, p < 0.01; Figure 1A). In general, stressor-exposure resulted in a lower percentage of spontaneous alternations, but this reduction was not evident in mice treated with antibiotics (Figure 1A). Stressor-exposure also reduced social behavior, as indicated by a reduction in the duration of time interacting with a juvenile conspecific (main effect of stressor, F(1, 73) = 12.01, p < 0.01; Figure 1B) with a concomitant increase in time spent in the corner (main effect of stressor, F(1, 73) = 4.55, p < 0.05; Figure 1C). These main effects of stressor-exposure were observed in animals treated with antibiotics or water as a vehicle control (Figure 1B and C), suggesting that social behavior was not affected by antibiotics in this model. These effects were accompanied by an overall reduction in the distance mice traveled during the social preference task (main effect of stressor, F(1, 73) = 7.01, p < 0.05; Figure 1D).
Hippocampal Neuronal and Microglial Restructuring in Mice Exposed to the Stressor with or Without Antibiotics
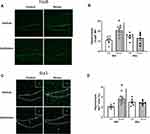
Neuronal activity and microglial restructuring have been shown to be increased in the dentate gyrus of the hippocampus of mice exposed to social defeat.32 Thus, we determined whether neuronal activity (ΔFosB) and microglial restructuring (Iba-1 staining area) were affected by stress and antibiotics. Hippocampal ΔFosB expression was increased in mice exposed to the social stressor in a microbe-dependent manner (stressor x antibiotic interaction, F(1, 20) = 7.08, p < 0.05; Figure 2A and B). Vehicle-treated mice exposed to the stressor had higher ΔFosB expression in hippocampus compared to mice that were not exposed to the stressor (Figure 2B). However, ΔFosB expression was similar in antibiotic-treated mice exposed to the stressor or left unstressed as a control (Figure 2A and B). Similarly, the percent area of Iba1+ staining was increased in the hippocampus of vehicle-treated, but not antibiotic-treated mice exposed to the social stressor (stressor x antibiotic interaction, F(1, 20) = 5.93, p < 0.05; Figure 2C and D). Higher magnification images (Figure 2C) showed microglia with smaller cell bodies in the control mice (consistent with resting microglia), but larger cell bodies and thicker processes in microglia in mice exposed to the stressor (consistent with reactive microglia). Microglia with larger cell bodies were not evident in stressor-exposed mice given antibiotics (consistent with the % area of Iba1+ staining).
Microbiome Composition in Stressor-Exposed and Antibiotic-Treated Mice
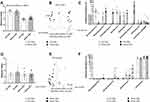
To determine whether stressor-induced changes in hippocampal ΔFosB, as well as stressor-induced behavioral changes, were related to changes in the gut microbiome, we used 16S rRNA gene sequencing to characterize the ileal and the colonic bacteriome. As expected, antibiotic treatment reduced alpha diversity in the colon, as assessed by Shannon’s diversity index (main effect of antibiotics, F(1, 32) = 184.40, p < 0.0001, Figure 3A) as well as observed ASV’s, Faith’s phylogenetic diversity, and Pielou’s evenness (data not shown). The stressor did not affect measures of alpha diversity but did affect beta diversity (Bray-Curtis distances main effect of stressor, F = 5.62, p < 0.005, Figure 3B). The effects of the stressor on microbiome composition were not prevented by antibiotics, but antibiotics had an orthogonal effect on microbiome beta diversity (Bray-Curtis distances, main effect of antibiotics, F = 22.66, p < 0.001, Figure 3B). The relative abundances of colonic bacterial genera are shown in Supplemental Table 1 and the relative abundance of taxa that were affected by stressor exposure are found in Figure 3C. Specifically, stressor exposure led to significant increases in unclassified genera within the families Muribaculaceae (U = 238.00, p < 0.001) and Rikenellaceae (U = 227.50, p < 0.001), as well as increases in the genera Muribaculum (U = 203.00, p < 0.01), Odoribacter (U = 203.00, p < 0.01), and Streptococcus (U = 203.50, p < 0.01). There was also an increase in Enterobacteriaceae (U = 203.00, p < 0.01), but this was primarily evident in the stressor-exposed mice treated with antibiotics. In addition to this increase, mice exposed to the social stressor had reductions in Clostridiales vadin BB60 (U = 74.50, p < 0.05) (Figure 3C).
Neither the stressor (F(1, 32) = 1.16, n.s.) nor antibiotics (F(1, 32) = 1.81, n.s.) affected alpha diversity in the ileum as assessed by Shannon’s diversity index (Figure 3D) or observed ASV’s, Faith’s phylogenetic diversity, and Pielou’s evenness (not shown). However, both the stressor (F = 2.31, p < 0.05) and antibiotics (F = 6.35, p < 0.001) affected beta diversity (Figure 3E). Taxa relative abundances in the ileum are found in Supplemental Table 2 and taxa that were affected by stress are found in Figure 3F. As with the colon, ileal relative abundances of multiple taxa, including genera within the families Rikenellaceae (U = 168.00, p < 0.05) and Muribaculaceae (U = 177.00, p < 0.05) were increased in stressor exposed mice. In addition, stressor exposure increased Lactobacillus (U = 182.00, p < 0.05) and Streptococcus (U = 198.50, p < 0.01) in the ileum (Figure 3F). Even though stressor exposure had multiple effects on bacterial composition and bacterial relative abundances, these bacterial measures did not statistically correlate with stressor-induced behavior (data not shown). Thus, we assessed whether bacterial interactions with the intestinal epithelium, rather than bacterial composition itself, might explain why antibiotics prevented stressor-induced changes in cognitive behavior as well as hippocampal FosB and Iba1 staining.
Intestinal Mucus Thickness and Cytokines in Stressor-Exposed Mice
Intestinal mucous and the tight junctions between intestinal epithelial cells play essential roles in preventing bacteria from contacting the lumen of the intestine and translocating across the epithelial barrier. Thus, we assessed the expression of tight junction genes, mucous thickness and antimicrobial peptides in the ileum and colon. Although neither the stressor nor antibiotics affected tight junction gene expression in the ileum (Table 2), stressor and antibiotic-exposure, increased claudin-2 and zona occludens gene expression in the colon (stressor x antibiotics interaction, F (1, 32) = 6.18, p < 0.05; Table 2). Stressor-exposure did not affect claudin-1 gene expression, but antibiotics increased claudin-1 (main effect of antibiotics, F (1, 32) = 5.41, p < 0.05) and tended to increase zona occludens (main effect of antibiotics, F (1, 32) = 4.11, p = 0.07).
 |
Table 2 Tight Junction Gene Expression |
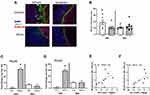
In addition to assessing tight junction genes, intestinal mucus thickness was also assessed. The mucous layer in the ileum was diffuse in all animals (as is typical in healthy animals), making it difficult to measure thickness. However, mucous thickness in the colon was reduced by stressor exposure (stress x antibiotic interaction, F (1, 32) = 4.60, p < 0.05, Figure 4A and B). Although no main effects of antibiotics were observed, treatment with antibiotics reduced mucous thickness in non-stressed control mice (as indicated by the stressor x antibiotic interaction, F(1, 32) = 4.60, p < 0.05; Figure 4B). Bacterial contact with the epithelium, such as when mucus barrier integrity is compromised, results in an antimicrobial response. Thus, antimicrobial peptides were assessed. Both Reg3b and Reg3g gene expression were increased in the ileum (Supplemental Figure 1) and the colon of mice exposed to the stressor (Figure 4C and D), but this increase did not occur in mice that were treated with antibiotics leading to significant stress x antibiotic interactions (Reg3b (F(1, 32) = 12.80, p < 0.01; Reg3g (F(1, 32)= 5.47, p < 0.05). Interestingly, in stressor-exposed mice, the relative abundance of Muribaculaceae in the colon was correlated with Reg3b (r = 0.52, p < 0.05) and Reg3g (r = 0.73, p < 0.05), gene expression in the colon (Figure 4E and F). While Reg3b and Reg3g gene expression were strongly increased by stress, the effects of the stressor on other inflammatory markers were less pronounced. Lyz1 gene expression was unaffected by stress in the colon, but there was a stress x antibiotic interaction in the ileum (F(1, 25) = 7.53, p < 0.05). Likewise, there was a stress x antibiotic interaction for Tnfa (F(1, 25) = 12.75, p < 0.001), Nos2 (F(1, 25) = 11.39, p < 0.01), Il22 (F(1, 25) = 17.03, p < 0.05), and Saa1 (F(1, 25) = 10.99, p < 0.05) in the ileum (Figure 5A). For these genes, stressor exposure increased their expression, but only in the vehicle-treated mice (ie, antibiotics prevented the stressor-induced increases resulting in the interaction) (Figure 5A). Stressor exposure also increased Il6 (main effect of stress, F(1, 25) = 4.11, p < 0.05), whereas antibiotics reduced Il1b (main effect of antibiotics, F(1, 25) = 4.31, p < 0.05) (Figure 5A). In the colon, there were stress x antibiotic interactions for Il6 (F(1, 25) = 7.97, p < 0.05), Tnfa (F(1, 25) = 9.22, p < 0.05), Nos2 (F(1, 25) = 5.68, p < 0.05), Il10 (F(1, 25) = 4.46, p < 0.05), and Il22 (F(1, 25) = 4.56, p < 0.05), such that stressor exposure increased the expression of these genes in the colons of vehicle-treated but not antibiotic-treated mice (Figure 5B). In addition, antibiotics reduced Il1b (main effect of antibiotics (F(1, 25) = 11.14, p < 0.01), whereas stress increased Saa1 (main effect of stress (F(1, 25) = 4.10, p = 0.05) (Figure 5B).
Inflammatory Cytokines in Stressor-Exposed Mice with or Without Antibiotics
Bacterial contact with the epithelium can lead to the expression of LBP and inflammatory cytokines.43 Thus, we assessed inflammatory mediators in the serum. Mice exposed to the social stressor had higher levels of LBP (main effect of stress F(1, 32) = 5.54, p < 0.05), IL-6 (main effect of stress F(1, 32) = 9.92, p < 0.01), and IL-1β (main effect of stress F(1, 32) = 9.92, p < 0.05) than did non-stressor control mice, whereas the administration of antibiotics reduced LBP (F(1, 32) = 9.33, p < 0.01), IL-6 (F(1, 32) = 7.92, p < 0.05), and IL-1β (F(1, 32) = 7.03, p < 0.05) (main effects of antibiotics; Figure 6A–C). Exposure to the stressor also increased TNFα (F(1, 32) = 29.36, p < 0.001) and IL-10 (F(1, 32) = 61.25, p < 0.0001) in the blood, but these were main effects of the stressor that were evident in vehicle and antibiotic treated mice (Figure 6D and E). LBP levels in the serum were inversely correlated with spontaneous alternations in the Y-maze (r=−.36, p < 0.05; Figure 6F), but cytokine levels were not correlated with behavior in the Y-maze (data not shown). Thus, we focused further analyses on LBP-related signaling.
Mucus Thickness, Peripheral Cytokines, and Cognitive Behavior in CD14−/− Mice Exposed to the Stressor
LPS binds to LBP; LPS-LBP complexes, in turn, bind to CD14 found either in its soluble form or on the surface of leukocytes and intestinal epithelial cells, which induces cytokine production. To determine whether CD14 was required for the social stressor-induced increases in cytokines and behavioral responses, CD14−/− mice were exposed to the stressor (or left undisturbed as a control). Exposing CD14−/− mice to the stressor did not change mucus thickness (colon: t(9) = 0.31, Figure 7A and B), Reg3b (colon: t(16) = 0.28, n.s., Figure 7C; ileum: t(16) = 0.54, n.s., Supplemental Figure 1) or Reg3g (colon: t(16) = 0.89, n.s., Figure 7D; ileum: t(16) = 0.29, n.s., Supplemental Figure 1). The levels of LBP (t(16) = 1.11, n.s), IL-6 (t(16) = 1.64, n.s.), and IL-1β (t(16) = 0.82, n.s.) were similar in Stress and Ctrl CD14-/- mice (7e-g), but TNF-α (t(16) = 2.98, p < 0.05) and IL-10 (t(16) = 3.56, p < 0.01) were increased in stressor-exposed CD14−/− mice (Figure 7H and I). Importantly, exposure to the stressor had no effect on the number of spontaneous alternations (t(16) = 0.59, n.s., Figure 8A), FosB (t(16) = 1.09, n.s.) or Iba1+ (t(16) = 1.31, n.s.) staining in the hippocampus (Figure 8B) of mice lacking the LBP receptor (ie, CD14−/− mice).
Discussion
We used a murine social stressor to determine whether host–microbe interactions are necessary for stressor-induced changes in cognitive and social behaviors. Exposure to the stressor impaired social interactions and performance on the Y-maze. Stressor-induced changes in cognitive, but not social, behavior could be prevented by antibiotics, demonstrating that cognitive behavior is dependent on an intact microbiome. Although it is not yet known why cognitive, but not social, behaviors are microbiome-dependent, we found that stressor exposure disrupted the intestinal barrier. The intestinal barrier includes the epithelial barrier, which is largely dependent upon epithelial tight junctions53 and the mucus barrier.54,55 In our study, stressor exposure reduced mucus thickness in the intestine while increasing LBP levels in circulation. The stressor-induced increases in LBP were correlated with cognitive, but not social, behavior. Antibiotics prevented the stressor-induced increases in LBP and mice lacking CD14, which is the receptor for LBP, did not show stressor-induced changes in cognitive behavior. Together, these data indicate that CD14 is a key link between exposure to social stressors, gut microbes, and cognitive behavior.
Psychosocial stressors lead to immune activation in both humans and laboratory animals, which contributes to the behavioral sequelae of stressful stimuli such as reductions in cognitive ability56 and reduced sociability.57–59 Social defeat models are widely used in the neurosciences36,60–62 and, more recently, microbiome research.63–65 And, while these models can involve wounding that contribute to immune and behavioral sequelae,66–68 the current stressor was developed to limit defeat-associated wounds. Interactions between the aggressors and subordinates were carefully monitored during the social defeat, and none of the mice in this study were found to have wounds that penetrated the skin and punctured underlying tissues. Because there was no evidence of overt immune activation due to wounding (such as redness or purulence), we focused on intestinal bacteria as potential contributors to stressor-induced immunomodulation. Our findings are consistent with an increasing number of studies that have linked stressor-induced immune activation to the gut microbiota.23,69,70 Low-grade inflammation correlates with changes in the composition of the gut microbiota,20,71 and the administration of broad-spectrum antibiotics, as well as the use of germ-free mice, show the importance of gut microbes for stressor-induced immune activation and priming.47,70,72 Interestingly, we have observed changes in microbial composition, as well as increases in bacterial translocation, in mice exposed to a prolonged restraint stressor.15 However, this type of chronic, physical restraint leads to suppressed inflammatory responses rather than enhanced responses.73–78 Although the reasons that more acute, social stressors enhance immune activity, whereas more chronic stressors suppress immune activity even though both affect the microbiome and the gut barrier are not completely clear, it is possible that glucocorticoids are involved. Prolonged restraint leads to chronic elevations in corticosterone,79 which has well-known anti-inflammatory properties,80 whereas social defeat leads to elevations in corticosterone that are shorter in duration.81 Moreover, social defeat leads to glucocorticoid insensitivity,82,83 suggesting that chronic elevations in corticosterone after restraint suppresses inflammatory responses (and affects hippocampal neurons involved in cognitive processes), whereas glucocorticoid insensitivity after social defeat is conducive to enhanced inflammatory processes. These inflammatory processes occur throughout the body, including the brain, and gut microbes have been implicated in stressor-induced neuroinflammation.23,84 Our behavioral tests demonstrated that cognitive behavior was diminished by the social stressor in a microbe-dependent manner.
In previous studies, we have found that stressor exposure reduces bacterial alpha diversity in the cecum,20,85 but not in the colon,86,87 with significant differences in bacterial relative abundances in both niches. Our current findings are consistent with these previous studies, showing a lack of stressor effect on bacterial alpha diversity in the colon, but significant differences in beta diversity and in the relative abundances of specific bacterial taxa. Stressor exposure increased the relative abundances of unclassified genera of bacteria in the families Muribaculaceae and Rikenellaceae, as well as bacteria in the genera Muribaculum and Odoribacter. The microbiome encodes for multiple carbohydrate enzymes that are capable of degrading a broad range of carbohydrates, including those found in mucous.88,89 Interestingly, Rikenellaceae, and more prominently Muribaculaceae, are mucus monosaccharide foragers.90 Thus, it is tempting to speculate that an expansion of taxa capable of degrading mucus and utilizing its monosaccharides was responsible for the observed stressor-induced decrease in mucus thickness. However, this study was not designed to assess the functional capacity of the microbiome, which would require shotgun, metagenomic sequencing or functional assays to determine whether stressor-induced changes in the microbiome led to changes in the mucus (or vice versa, since mucus composition is known to affect the microbiome).91–93 Increased abundance in Rikenellaceae has also been observed in subordinate mice, and fecal transplants from subordinate mice into germ-free recipients can recapitulate many behaviors associated with subordination, including depressive-like behavior and social avoidance.94 Thus, it was reasonable to test whether bacterial relative abundances were related to behavior. However, in our analyses, the relative abundance of specific bacterial types did not directly correlate with any behavioral differences. Instead, Muribaculaceae relative abundance was positively correlated with Reg3b and Reg3g gene expression, suggesting that stressor-induced increases in these antimicrobial peptides are driven by Muribaculaceae that are capable of breaking down mucus. Because the bacteria were statistically correlated with antimicrobial peptide expression, but microbiome composition was not statistically correlated with serum cytokine levels, microglia measures, or behavioral responses, we began to question whether bacterial contact with the epithelium, rather than overall changes in microbiome composition, was an important mediator between the gut microbiota and behavioral responses to stressor exposure.
Reduced mucus thickness, such as that observed during stressor exposure, can allow bacteria to stimulate the intestinal epithelium to produce antimicrobial peptides, like Reg3b and Reg3g,95,96 which were strongly increased in stressor-exposed mice and correlated with Muribaculum relative abundance in the colon. This stressor-induced increase, along with increases in inflammatory cytokines, was attenuated by the administration of antibiotics. Interestingly, antibiotics disrupted mucus thickness, consistent with previous reports,97,98 but they did not increase Reg3b or Reg3g. Because antibiotics significantly reduce bacteria in the intestine, the results emphasize that the combination of a reduction in mucus, along with the presence of bacteria, leads to increases in antimicrobial peptides and cytokines in the intestine. Stressor-induced increases in antimicrobial peptides were not strongly correlated with behavior or with circulating cytokines, but it is known that LBP is also increased when bacteria contact the intestinal epithelium.99 Because LBP induces inflammatory responses and has been related to behavior,25,26 we assessed whether LBP may link gut microbes to stressor-induced behavioral responses.
Commensal bacteria are able to translocate from the intestine during stressor exposure.15,16 The translocation of bacteria or bacterial LPS, as well as bacterial contact with the epithelium, leads to the expression of LBP,99 which is an acute phase protein released by the liver as well as by intestinal epithelial cells.100 CD14 is constitutively expressed on monocytes, macrophages, and neutrophils where it binds to LPS-LBP complexes to activate TLR4101 and in some cases TLR2.102 Although the relationships between individual gut bacteria and CD14 are not completely known, bacterial taxa that were the most strongly increased by the social stressor (eg, Muribaculaceae unclassified, Muribaculum, Rikenellaeae) are Gram-negative. The Toll-like receptor for Gram-negative bacterial LPS, namely TLR4, has been shown to play an essential role in stressor-induced immune enhancement,21,82 suggesting possible links between these bacteria and stressor-induced immune enhancement. Given the interactions between CD14 and TLR4, and the importance of TLR4 for stressor-induced immunopotentiation, it was not surprising that the stressor did not increase IL-6 or TNF-α in CD14−/− mice. However, it was surprising that reductions in colonic mucus thickness, and subsequent increases in antimicrobial peptides and LBP itself did not occur in CD14−/− mice. While these effects may be due to loss of CD14 signaling in innate immune cells, CD14 is also expressed on intestinal epithelial cells where it is involved with cytokine production103 and disruption of tight junctions and epithelial permeability.104 Indeed, Gram-negative LPS strongly affects goblet cells, Paneth cells, and enterocytes (the cellular sources of mucus, antimicrobial peptides, and LBP). Although further investigation is needed to delineate differential effects of immune and epithelial CD14+ cell subsets on the intestine, our results are the first to show an essential role of CD14 for stressor-induced disruption of colonic mucus and increase of serum LBP. It should be noted that studies involving CD14−/− were conducted separately from studies using wildtype mice. Although a limitation of the study, we have found the social stress paradigm to be highly reproducible and CD14−/− mice exposed to stress still had stressor-induced increases in TNF-α and IL-10, suggesting that the stressor was capable of inducing physiological changes in CD14−/−.
Elevated serum LBP has been linked to a myriad of diseases and conditions in human studies, such as Parkinson’s105 and Alzheimer’s diseases.106 And while stressors (such as marital distress) have been shown to increase LBP, and increased serum LBP has been linked to emotional disorders, such as major depressive disorder,25,107 no studies have linked LBP to cognitive behavior. In addition, few studies have provided evidence that stressor-induced increases in LBP are dependent upon an intact microbiota supporting a paradigm wherein the stress response leads to LBP and subsequent CD14 activation through microbiota-dependent mechanisms.
In murine models of social defeat, microglial activation leads to the recruitment of inflammatory monocytes into the brain that influence behavioral responses through cytokine production.34,108–112 Interestingly, social defeat-induced cognitive deficits, as assessed on the Y maze, have been shown to be dependent upon IL-1-induced activation of neurons in the hippocampus.32 In the current study, the social defeat stressor increased microglial restructuring (based on Iba1 staining percent area) and neuronal activity (based on ΔFosB staining) in the hippocampus. Treatment with antibiotics prevented microglial restructuring, neuronal activity, and cognitive deficits in stressor-exposed mice suggesting that stressor-induced reductions in cognition involve the gut microbiota. Interestingly, social behavior was reduced in stressor-exposed mice regardless of whether the mice were treated with antibiotics. This is consistent with previous results showing that cognitive, but not social behavior, is dependent upon inflammation-induced neuronal activity in the hippocampus. It should be noted that metronidazole, which was used in the antibiotic cocktail, can be absorbed and enter the brain to have neurological side effects in humans. However, the mechanisms by which this occurs are hypothesized to be related to neuroinflammation.113 In mice given the antibiotic cocktail, there was no evidence of neuroinflammation. Rather, mice given antibiotics had a reduction in microglial restructuring after stressor exposure. When considered together, the data suggest that differences in cognitive behavior were microbiome-dependent and not due to nonspecific effects of metronidazole. The specificity of the behavioral dependence of cognitive, but not social, behavior on the presence of microbes highlights the complexity of the relationship between gut microbes and the brain. Recent studies demonstrate a key role for microglia in the medial prefrontal cortex (mPFC) and TLR2/4 in mediating alterations in social behavior following the social stressor.59 Our work now suggests hippocampal neurobiological underpinnings for the gut-brain-axis in cognitive behavior. Future work should focus on dissecting a specific role for gut microbiome changes and alterations in microglial restructuring in the mPFC versus the hippocampus.
Conclusion
This study highlights the importance of dynamic host–microbe interactions at the intestinal mucosal interface for behavioral sequelae of stressor exposure. Exposure to social stressors leads to reduced intestinal mucus thickness that allows commensal bacteria to contact the epithelium. These bacteria increase the expression of antimicrobial peptides (in the intestine) and of inflammatory cytokines and LBP in circulation. The increase in LBP is a key component of these stressor-induced effects, because mice lacking the LBP receptor (ie, CD14) do not show increases in inflammatory cytokines. When considering that mice treated with antibiotics do not show a stressor-induced increase in LBP (or change in behavior), along with findings that CD14−/− mice do not show stressor-induced behavioral change, we propose that bacterial penetration of intestinal mucus leading to LBP production is necessary for social stressor-induced cognitive deficits.
Acknowledgments
This work was supported by NIH R21MH108167 and R33MH108167 (to MB), NIH T32 fellowship (DE014320 to BL and JA), and in part by the Intramural/Extramural research program of the NCATS, NIH (ZIC TR000410-03). The authors gratefully acknowledge Andrew M. Bailey for help with manuscript preparation.
Disclosure
DJD, RMJ, BRL, DK, KDS, JMA, NQ, JPG, and TLG do not have any conflicts of interest to declare. MTB owns stock options in Scioto Biosciences, which is not related to the current project.
References
1. Rea K, Dinan TG, Cryan JF. Gut microbiota: a perspective for psychiatrists. Neuropsychobiology. 2020;79(1):50–62. doi:10.1159/000504495
2. Foster JA, Rinaman L, Cryan JF. Stress & the gut-brain axis: regulation by the microbiome. Neurobiol Stress. 2017;7:124–136. doi:10.1016/j.ynstr.2017.03.001
3. O’Leary OF, Ogbonnaya ES, Felice D, et al. The vagus nerve modulates BDNF expression and neurogenesis in the hippocampus. Eur Neuropsychopharmacol. 2018;28(2):307–316. doi:10.1016/j.euroneuro.2017.12.004
4. Bercik P, Park AJ, Sinclair D, et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol Motil. 2011;23(12):1132–1139. doi:10.1111/j.1365-2982.2011.01796.x
5. Fulling C, Dinan TG, Cryan JF. Gut microbe to brain signaling: what happens in vagus. Neuron. 2019;101(6):998–1002. doi:10.1016/j.neuron.2019.02.008
6. Gheorghe CE, Martin JA, Manriquez FV, Dinan TG, Cryan JF, Clarke G. Focus on the essentials: tryptophan metabolism and the microbiome-gut-brain axis. Curr Opin Pharmacol. 2019;48:137–145. doi:10.1016/j.coph.2019.08.004
7. Sarkar A, Harty S, Johnson KV, et al. The role of the microbiome in the neurobiology of social behaviour. Biol Rev Camb Philos Soc. 2020;95(5):1131–1166. doi:10.1111/brv.12603
8. Sgritta M, Dooling SW, Buffington SA, et al. Mechanisms underlying microbial-mediated changes in social behavior in mouse models of autism spectrum disorder. Neuron. 2019;101(2):246–259 e246. doi:10.1016/j.neuron.2018.11.018
9. Ermund A, Schutte A, Johansson ME, Gustafsson JK, Hansson GC. Studies of mucus in mouse stomach, small intestine, and colon. I. Gastrointestinal mucus layers have different properties depending on location as well as over the Peyer’s patches. Am J Physiol Gastrointest Liver Physiol. 2013;305(5):G341–347. doi:10.1152/ajpgi.00046.2013
10. Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci USA. 2008;105(39):15064–15069. doi:10.1073/pnas.0803124105
11. Bevins CL, Salzman NH. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol. 2011;9(5):356–368. doi:10.1038/nrmicro2546
12. Meyer-Hoffert U, Hornef MW, Henriques-Normark B, et al. Secreted enteric antimicrobial activity localises to the mucus surface layer. Gut. 2008;57(6):764–771. doi:10.1136/gut.2007.141481
13. Vaishnava S, Yamamoto M, Severson KM, et al. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science. 2011;334(6053):255–258. doi:10.1126/science.1209791
14. Bailey MT. Psychological stress, immunity, and the effects on indigenous microflora. Adv Exp Med Biol. 2016;874:225–246.
15. Bailey MT, Engler H, Sheridan JF. Stress induces the translocation of cutaneous and gastrointestinal microflora to secondary lymphoid organs of C57BL/6 mice. J Neuroimmunol. 2006;171(1–2):29–37. doi:10.1016/j.jneuroim.2005.09.008
16. Lafuse WP, Gearinger R, Fisher S, Nealer C, Mackos AR, Bailey MT. Exposure to a social stressor induces translocation of commensal lactobacilli to the spleen and priming of the innate immune system. J Immunol. 2017;198(6):2383–2393. doi:10.4049/jimmunol.1601269
17. Fleshner M. Stress-evoked sterile inflammation, danger associated molecular patterns (DAMPs), microbial associated molecular patterns (MAMPs) and the inflammasome. Brain Behav Immun. 2013;27(1):1–7. doi:10.1016/j.bbi.2012.08.012
18. Maslanik T, Tannura K, Mahaffey L, et al. Commensal bacteria and MAMPs are necessary for stress-induced increases in IL-1β and IL-18 but not IL-6, IL-10 or MCP-1. PLoS One. 2012;7(12):e50636. doi:10.1371/journal.pone.0050636
19. Schmidt D, Reber SO, Botteron C, et al. Chronic psychosocial stress promotes systemic immune activation and the development of inflammatory Th cell responses. Brain Behav Immun. 2010;24(7):1097–1104. doi:10.1016/j.bbi.2010.04.014
20. Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav Immun. 2011;25(3):397–407. doi:10.1016/j.bbi.2010.10.023
21. Bailey MT, Engler H, Powell ND, Padgett DA, Sheridan JF. Repeated social defeat increases the bactericidal activity of splenic macrophages through a Toll-like receptor-dependent pathway. Am J Physiol Regul Integr Comp Physiol. 2007;293(3):R1180–1190. doi:10.1152/ajpregu.00307.2007
22. Yang HL, Li MM, Zhou MF, et al. Links between gut dysbiosis and neurotransmitter disturbance in chronic restraint stress-induced depressive behaviours: the role of inflammation. Inflammation. 2021;44(6):2448–2462. doi:10.1007/s10753-021-01514-y
23. Li N, Wang Q, Wang Y, et al. Fecal microbiota transplantation from chronic unpredictable mild stress mice donors affects anxiety-like and depression-like behavior in recipient mice via the gut microbiota-inflammation-brain axis. Stress. 2019;22(5):592–602. doi:10.1080/10253890.2019.1617267
24. Lv WJ, Wu XL, Chen WQ, et al. The gut microbiome modulates the changes in liver metabolism and in inflammatory processes in the brain of chronic unpredictable mild stress rats. Oxid Med Cell Longev. 2019;2019:7902874. doi:10.1155/2019/7902874
25. Kiecolt-Glaser JK, Wilson SJ, Bailey ML, et al. Marital distress, depression, and a leaky gut: translocation of bacterial endotoxin as a pathway to inflammation. Psychoneuroendocrinology. 2018;98:52–60. doi:10.1016/j.psyneuen.2018.08.007
26. Kiecolt-Glaser JK, Wilson SJ, Shrout MR, et al. The gut reaction to couples’ relationship troubles: a route to gut dysbiosis through changes in depressive symptoms. Psychoneuroendocrinology. 2021;125:105132. doi:10.1016/j.psyneuen.2021.105132
27. Madison A, Kiecolt-Glaser JK. Stress, depression, diet, and the gut microbiota: human-bacteria interactions at the core of psychoneuroimmunology and nutrition. Curr Opin Behav Sci. 2019;28:105–110. doi:10.1016/j.cobeha.2019.01.011
28. Huang CJ, Stewart JK, Shibata Y, Slusher AL, Acevedo EO. Lipopolysaccharide-binding protein and leptin are associated with stress-induced interleukin-6 cytokine expression ex vivo in obesity. Psychophysiology. 2015;52(5):687–694. doi:10.1111/psyp.12387
29. Powell ND, Sloan EK, Bailey MT, et al. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via beta-adrenergic induction of myelopoiesis. Proc Natl Acad Sci USA. 2013;110(41):16574–16579. doi:10.1073/pnas.1310655110
30. Murray DR, Haselton MG, Fales M, Cole SW. Subjective social status and inflammatory gene expression. Health Psychol. 2019;38(2):182–186. doi:10.1037/hea0000705
31. Ross KM, Cole SW, Carroll JE, Dunkel Schetter C. Elevated pro-inflammatory gene expression in the third trimester of pregnancy in mothers who experienced stressful life events. Brain Behav Immun. 2019;76:97–103. doi:10.1016/j.bbi.2018.11.009
32. DiSabato DJ, Nemeth DP, Liu X, et al. Interleukin-1 receptor on hippocampal neurons drives social withdrawal and cognitive deficits after chronic social stress. Mol Psychiatry. 2020;26:4770–4782. doi:10.1038/s41380-020-0788-3
33. Liu X, Nemeth DP, McKim DB, et al. Cell-type-specific interleukin 1 receptor 1 signaling in the brain regulates distinct neuroimmune activities. Immunity. 2019;50(3):764–766. doi:10.1016/j.immuni.2019.02.012
34. McKim DB, Weber MD, Niraula A, et al. Microglial recruitment of IL-1beta-producing monocytes to brain endothelium causes stress-induced anxiety. Mol Psychiatry. 2018;23(6):1421–1431. doi:10.1038/mp.2017.64
35. Niraula A, Witcher KG, Sheridan JF, Godbout JP. Interleukin-6 induced by social stress promotes a unique transcriptional signature in the monocytes that facilitate anxiety. Biol Psychiatry. 2019;85(8):679–689. doi:10.1016/j.biopsych.2018.09.030
36. Weber MD, Godbout JP, Sheridan JF. Repeated social defeat, neuroinflammation, and behavior: monocytes carry the signal. Neuropsychopharmacology. 2017;42(1):46–61. doi:10.1038/npp.2016.102
37. McKim DB, Patterson JM, Wohleb ES, et al. Sympathetic release of splenic monocytes promotes recurring anxiety following repeated social defeat. Biol Psychiatry. 2016;79(10):803–813. doi:10.1016/j.biopsych.2015.07.010
38. Wohleb ES, Powell ND, Godbout JP, Sheridan JF. Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J Neurosci. 2013;33(34):13820–13833. doi:10.1523/JNEUROSCI.1671-13.2013
39. Sawicki CM, McKim DB, Wohleb ES, et al. Social defeat promotes a reactive endothelium in a brain region-dependent manner with increased expression of key adhesion molecules, selectins and chemokines associated with the recruitment of myeloid cells to the brain. Neuroscience. 2015;302:151–164. doi:10.1016/j.neuroscience.2014.10.004
40. Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16(1):22–34. doi:10.1038/nri.2015.5
41. Wan Y, Freeswick PD, Khemlani LS, et al. Role of lipopolysaccharide (LPS), interleukin-1, interleukin-6, tumor necrosis factor, and dexamethasone in regulation of LPS-binding protein expression in normal hepatocytes and hepatocytes from LPS-treated rats. Infect Immun. 1995;63(7):2435–2442. doi:10.1128/iai.63.7.2435-2442.1995
42. Wolk K, Witte E, Hoffmann U, et al. IL-22 induces lipopolysaccharide-binding protein in hepatocytes: a potential systemic role of IL-22 in Crohn’s disease. J Immunol. 2007;178(9):5973–5981. doi:10.4049/jimmunol.178.9.5973
43. Stehle JR, Leng X, Kitzman DW, Nicklas BJ, Kritchevsky SB, High KP. Lipopolysaccharide-binding protein, a surrogate marker of microbial translocation, is associated with physical function in healthy older adults. J Gerontol a Biol Sci Med Sci. 2012;67(11):1212–1218. doi:10.1093/gerona/gls178
44. Ulevitch RJ, Tobias PS. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–457. doi:10.1146/annurev.iy.13.040195.002253
45. Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249(4975):1431–1433. doi:10.1126/science.1698311
46. National Research Council. Guide for the Care and Use of Laboratory Animals. Washington (DC): National Research Council; 2011.
47. Allen RG, Lafuse WP, Galley JD, Ali MM, Ahmer BM, Bailey MT. The intestinal microbiota are necessary for stressor-induced enhancement of splenic macrophage microbicidal activity. Brain Behav Immun. 2012;26(3):371–382. doi:10.1016/j.bbi.2011.11.002
48. Ochoa-Reparaz J, Mielcarz DW, Ditrio LE, et al. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J Immunol. 2009;183(10):6041–6050. doi:10.4049/jimmunol.0900747
49. Golden SA, Covington HE, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 2011;6(8):1183–1191. doi:10.1038/nprot.2011.361
50. McKim DB, Niraula A, Tarr AJ, Wohleb ES, Sheridan JF, Godbout JP. Neuroinflammatory dynamics underlie memory impairments after repeated social defeat. J Neurosci. 2016;36(9):2590–2604. doi:10.1523/JNEUROSCI.2394-15.2016
51. Bolyen E, Rideout JR, Dillon MR, et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. 2019;37(8):852–857. doi:10.1038/s41587-019-0209-9
52. Quast C, Pruesse E, Yilmaz P, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41(Databaseissue):D590–596. doi:10.1093/nar/gks1219
53. Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci. 2015;9:392. doi:10.3389/fncel.2015.00392
54. Johansson ME, Ambort D, Pelaseyed T, et al. Composition and functional role of the mucus layers in the intestine. Cell Mol Life Sci. 2011;68(22):3635–3641. doi:10.1007/s00018-011-0822-3
55. Johansson ME, Hansson GC. Keeping bacteria at a distance. Science. 2011;334(6053):182–183. doi:10.1126/science.1213909
56. Koskinen MK, van Mourik Y, Smit AB, Riga D, Spijker S. From stress to depression: development of extracellular matrix-dependent cognitive impairment following social stress. Sci Rep. 2020;10(1):17308. doi:10.1038/s41598-020-73173-2
57. Eagle AL, Manning CE, Williams ES, et al. Circuit-specific hippocampal DeltaFosB underlies resilience to stress-induced social avoidance. Nat Commun. 2020;11(1):4484. doi:10.1038/s41467-020-17825-x
58. Muir J, Tse YC, Iyer ES, et al. Ventral hippocampal afferents to nucleus accumbens encode both latent vulnerability and stress-induced susceptibility. Biol Psychiatry. 2020;88(11):843–854. doi:10.1016/j.biopsych.2020.05.021
59. Nie X, Kitaoka S, Tanaka K, et al. The innate immune receptors TLR2/4 mediate repeated social defeat stress-induced social avoidance through prefrontal microglial activation. Neuron. 2018;99(3):464–479 e467. doi:10.1016/j.neuron.2018.06.035
60. Bath KG, Russo SJ, Pleil KE, Wohleb ES, Duman RS, Radley JJ. Circuit and synaptic mechanisms of repeated stress: perspectives from differing contexts, duration, and development. Neurobiol Stress. 2017;7:137–151. doi:10.1016/j.ynstr.2017.05.001
61. Takahashi A, Chung JR, Zhang S, et al. Establishment of a repeated social defeat stress model in female mice. Sci Rep. 2017;7(1):12838. doi:10.1038/s41598-017-12811-8
62. Yin W, Gallagher NR, Sawicki CM, McKim DB, Godbout JP, Sheridan JF. Repeated social defeat in female mice induces anxiety-like behavior associated with enhanced myelopoiesis and increased monocyte accumulation in the brain. Brain Behav Immun. 2019;78:131–142. doi:10.1016/j.bbi.2019.01.015
63. Galley JD, Bailey MT. Impact of stressor exposure on the interplay between commensal microbiota and host inflammation. Gut Microbes. 2014;5(3):390–396. doi:10.4161/gmic.28683
64. Kosuge A, Kunisawa K, Arai S, et al. Heat-sterilized Bifidobacterium breve prevents depression-like behavior and interleukin-1beta expression in mice exposed to chronic social defeat stress. Brain Behav Immun. 2021;96:200–211. doi:10.1016/j.bbi.2021.05.028
65. Wang S, Ishima T, Qu Y, et al. Ingestion of Faecalibaculum rodentium causes depression-like phenotypes in resilient Ephx2 knock-out mice: a role of brain-gut-microbiota axis via the subdiaphragmatic vagus nerve. J Affect Disord. 2021;292:565–573. doi:10.1016/j.jad.2021.06.006
66. Bailey MT, Avitsur R, Engler H, Padgett DA, Sheridan JF. Physical defeat reduces the sensitivity of murine splenocytes to the suppressive effects of corticosterone. Brain Behav Immun. 2004;18(5):416–424. doi:10.1016/j.bbi.2003.09.012
67. Engler H, Bailey MT, Engler A, Sheridan JF. Effects of repeated social stress on leukocyte distribution in bone marrow, peripheral blood and spleen. J Neuroimmunol. 2004;148(1–2):106–115. doi:10.1016/j.jneuroim.2003.11.011
68. Foertsch S, Reber SO. The role of physical trauma in social stress-induced immune activation. Neurosci Biobehav Rev. 2020;113:169–178. doi:10.1016/j.neubiorev.2020.02.025
69. Wei L, Li Y, Tang W, et al. Chronic unpredictable mild stress in rats induces colonic inflammation. Front Physiol. 2019;10:1228. doi:10.3389/fphys.2019.01228
70. van de Wouw M, Lyte JM, Boehme M, et al. The role of the microbiota in acute stress-induced myeloid immune cell trafficking. Brain Behav Immun. 2020;84:209–217. doi:10.1016/j.bbi.2019.12.003
71. Bharwani A, Mian MF, Foster JA, Surette MG, Bienenstock J, Forsythe P. Structural & functional consequences of chronic psychosocial stress on the microbiome & host. Psychoneuroendocrinology. 2016;63:217–227. doi:10.1016/j.psyneuen.2015.10.001
72. Delaroque C, Chervy M, Gewirtz AT, Chassaing B. Social overcrowding impacts gut microbiota, promoting stress, inflammation, and dysglycemia. Gut Microbes. 2021;13(1):2000275. doi:10.1080/19490976.2021.2000275
73. Dobbs CM, Feng N, Beck FM, Sheridan JF. Neuroendocrine regulation of cytokine production during experimental influenza viral infection: effects of restraint stress-induced elevation in endogenous corticosterone. J Immunol. 1996;157(5):1870–1877.
74. Dobbs CM, Vasquez M, Glaser R, Sheridan JF. Mechanisms of stress-induced modulation of viral Pathogenesis and immunity. J Neuroimmunol. 1993;48(2):151–160. doi:10.1016/0165-5728(93)90187-4
75. Engler A, Roy S, Sen CK, Padgett DA, Sheridan JF. Restraint stress alters lung gene expression in an experimental influenza A viral infection. J Neuroimmunol. 2005;162(1–2):103–111. doi:10.1016/j.jneuroim.2005.01.017
76. Hunzeker J, Padgett DA, Sheridan PA, Dhabhar FS, Sheridan JF. Modulation of natural killer cell activity by restraint stress during an influenza A/PR8 infection in mice. Brain Behav Immun. 2004;18(6):526–535. doi:10.1016/j.bbi.2003.12.010
77. Mercado AM, Padgett DA, Sheridan JF, Marucha PT. Altered kinetics of IL-1 alpha, IL-1 beta, and KGF-1 gene expression in early wounds of restrained mice. Brain Behav Immun. 2002;16(2):150–162. doi:10.1006/brbi.2001.0623
78. Tseng RJ, Padgett DA, Dhabhar FS, Engler H, Sheridan JF. Stress-induced modulation of NK activity during influenza viral infection: role of glucocorticoids and opioids. Brain Behav Immun. 2005;19(2):153–164. doi:10.1016/j.bbi.2004.07.001
79. Padgett DA, Marucha PT, Sheridan JF. Restraint stress slows cutaneous wound healing in mice. Brain Behav Immun. 1998;12(1):64–73. doi:10.1006/brbi.1997.0512
80. Silverman MN, Sternberg EM. Glucocorticoid regulation of inflammation and its functional correlates: from HPA axis to glucocorticoid receptor dysfunction. Ann N Y Acad Sci. 2012;1261:55–63. doi:10.1111/j.1749-6632.2012.06633.x
81. Hanke ML, Powell ND, Stiner LM, Bailey MT, Sheridan JF. Beta adrenergic blockade decreases the immunomodulatory effects of social disruption stress. Brain Behav Immun. 2012;26(7):1150–1159. doi:10.1016/j.bbi.2012.07.011
82. Avitsur R, Padgett DA, Dhabhar FS, et al. Expression of glucocorticoid resistance following social stress requires a second signal. J Leukoc Biol. 2003;74(4):507–513. doi:10.1189/jlb.0303090
83. Stark JL, Avitsur R, Padgett DA, Campbell KA, Beck FM, Sheridan JF. Social stress induces glucocorticoid resistance in macrophages. Am J Physiol Regul Integr Comp Physiol. 2001;280(6):R1799–1805. doi:10.1152/ajpregu.2001.280.6.R1799
84. Vagnerova K, Vodicka M, Hermanova P, et al. Interactions between gut microbiota and acute restraint stress in peripheral structures of the hypothalamic-pituitary-adrenal axis and the intestine of male mice. Front Immunol. 2019;10:2655. doi:10.3389/fimmu.2019.02655
85. Bailey MT, Dowd SE, Parry NM, Galley JD, Schauer DB, Lyte M. Stressor exposure disrupts commensal microbial populations in the intestines and leads to increased colonization by Citrobacter rodentium. Infect Immun. 2010;78(4):1509–1519. doi:10.1128/IAI.00862-09
86. Galley JD, Nelson MC, Yu Z, et al. Exposure to a social stressor disrupts the community structure of the colonic mucosa-associated microbiota. BMC Microbiol. 2014;14:189. doi:10.1186/1471-2180-14-189
87. Tarr AJ, Galley JD, Fisher SE, Chichlowski M, Berg BM, Bailey MT. The prebiotics 3ʹSialyllactose and 6ʹSialyllactose diminish stressor-induced anxiety-like behavior and colonic microbiota alterations: evidence for effects on the gut-brain axis. Brain Behav Immun. 2015;50:166–177. doi:10.1016/j.bbi.2015.06.025
88. Bhattacharya T, Ghosh TS, Mande SS. Global profiling of carbohydrate active enzymes in human gut microbiome. PLoS One. 2015;10(11):e0142038. doi:10.1371/journal.pone.0142038
89. Lombard V, Golaconda Ramulu H, Drula E, Coutinho PM, Henrissat B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014;42(Databaseissue):D490–495. doi:10.1093/nar/gkt1178
90. Pereira FC, Wasmund K, Cobankovic I, et al. Rational design of a microbial consortium of mucosal sugar utilizers reduces Clostridiodes difficile colonization. Nat Commun. 2020;11(1):5104. doi:10.1038/s41467-020-18928-1
91. Marcobal A, Southwick AM, Earle KA, Sonnenburg JL. A refined palate: bacterial consumption of host glycans in the gut. Glycobiology. 2013;23(9):1038–1046. doi:10.1093/glycob/cwt040
92. Rausch P, Rehman A, Kunzel S, et al. Colonic mucosa-associated microbiota is influenced by an interaction of Crohn disease and FUT2 (Secretor) genotype. Proc Natl Acad Sci USA. 2011;108(47):19030–19035. doi:10.1073/pnas.1106408108
93. Sommer F, Adam N, Johansson ME, Xia L, Hansson GC, Backhed F. Altered mucus glycosylation in core 1 O-glycan-deficient mice affects microbiota composition and intestinal architecture. PLoS One. 2014;9(1):e85254. doi:10.1371/journal.pone.0085254
94. Agranyoni O, Meninger-Mordechay S, Uzan A, et al. Gut microbiota determines the social behavior of mice and induces metabolic and inflammatory changes in their adipose tissue. NPJ Biofilms Microbiomes. 2021;7(1):28. doi:10.1038/s41522-021-00193-9
95. Muniz LR, Knosp C, Yeretssian G. Intestinal antimicrobial peptides during homeostasis, infection, and disease. Front Immunol. 2012;3:310. doi:10.3389/fimmu.2012.00310
96. Natividad JM, Hayes CL, Motta JP, et al. Differential induction of antimicrobial REGIII by the intestinal microbiota and Bifidobacterium breve NCC2950. Appl Environ Microbiol. 2013;79(24):7745–7754. doi:10.1128/AEM.02470-13
97. Vreugdenhil AC, Dentener MA, Snoek AM, Greve JW, Buurman WA. Lipopolysaccharide binding protein and serum amyloid A secretion by human intestinal epithelial cells during the acute phase response. J Immunol. 1999;163(5):2792–2798.
98. Wlodarska M, Willing B, Keeney KM, et al. Antibiotic treatment alters the colonic mucus layer and predisposes the host to exacerbated Citrobacter rodentium-induced colitis. Infect Immun. 2011;79(4):1536–1545. doi:10.1128/IAI.01104-10
99. Tobias PS, Soldau K, Gegner JA, Mintz D, Ulevitch RJ. Lipopolysaccharide binding protein-mediated complexation of lipopolysaccharide with soluble CD14. J Biol Chem. 1995;270(18):10482–10488. doi:10.1074/jbc.270.18.10482
100. Zweigner J, Schumann RR, Weber JR. The role of lipopolysaccharide-binding protein in modulating the innate immune response. Microbes Infect. 2006;8(3):946–952. doi:10.1016/j.micinf.2005.10.006
101. Tsukamoto H, Takeuchi S, Kubota K, et al. Lipopolysaccharide (LPS)-binding protein stimulates CD14-dependent Toll-like receptor 4 internalization and LPS-induced TBK1-IKK-IRF3 axis activation. J Biol Chem. 2018;293(26):10186–10201. doi:10.1074/jbc.M117.796631
102. Gupta D, Kirkland TN, Viriyakosol S, Dziarski R. CD14 is a cell-activating receptor for bacterial peptidoglycan. J Biol Chem. 1996;271(38):23310–23316. doi:10.1074/jbc.271.38.23310
103. He Y, Liu S, Kling DE, et al. The human milk oligosaccharide 2’-fucosyllactose modulates CD14 expression in human enterocytes, thereby attenuating LPS-induced inflammation. Gut. 2016;65(1):33–46. doi:10.1136/gutjnl-2014-307544
104. Guo S, Al-Sadi R, Said HM, Ma TY. Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD14. Am J Pathol. 2013;182(2):375–387. doi:10.1016/j.ajpath.2012.10.014
105. Chen SJ, Chi YC, Ho CH, Yang WS, Lin CH. Plasma lipopolysaccharide-binding protein reflects risk and progression of Parkinson’s disease. J Parkinsons Dis. 2021;11:1129–1139. doi:10.3233/JPD-212574
106. Andre P, Samieri C, Buisson C, et al. Lipopolysaccharide-binding protein, soluble CD14, and the long-term risk of Alzheimer’s disease: a nested case-control pilot study of older community dwellers from the three-city cohort. J Alzheimers Dis. 2019;71(3):751–761. doi:10.3233/JAD-190295
107. Alvarez-Mon MA, Gomez AM, Orozco A, et al. Abnormal distribution and function of circulating monocytes and enhanced bacterial translocation in major depressive disorder. Front Psychiatry. 2019;10:812. doi:10.3389/fpsyt.2019.00812
108. Ambree O, Ruland C, Scheu S, Arolt V, Alferink J. Alterations of the innate immune system in susceptibility and resilience after social defeat stress. Front Behav Neurosci. 2018;12:141. doi:10.3389/fnbeh.2018.00141
109. Menard C, Pfau ML, Hodes GE, et al. Social stress induces neurovascular pathology promoting depression. Nat Neurosci. 2017;20(12):1752–1760. doi:10.1038/s41593-017-0010-3
110. Pfau ML, Menard C, Cathomas F, et al. Role of monocyte-derived microRNA106b approximately 25 in resilience to social stress. Biol Psychiatry. 2019;86(6):474–482. doi:10.1016/j.biopsych.2019.02.023
111. Shaftel SS, Carlson TJ, Olschowka JA, Kyrkanides S, Matousek SB, O’Banion MK. Chronic interleukin-1beta expression in mouse brain leads to leukocyte infiltration and neutrophil-independent blood brain barrier permeability without overt neurodegeneration. J Neurosci. 2007;27(35):9301–9309. doi:10.1523/JNEUROSCI.1418-07.2007
112. Stankiewicz AM, Goscik J, Swiergiel AH, et al. Social stress increases expression of hemoglobin genes in mouse prefrontal cortex. BMC Neurosci. 2014;15:130. doi:10.1186/s12868-014-0130-6
113. Roy U, Panwar A, Pandit A, Das SK, Joshi B. Clinical and neuroradiological spectrum of metronidazole induced encephalopathy: our experience and the review of literature. J Clin Diagn Res. 2016;10(6):OE01–09. doi:10.7860/JCDR/2016/19032.8054
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.