Back to Journals » Therapeutics and Clinical Risk Management » Volume 18
Strategy to Reduce Hypercapnia in Robot-Assisted Radical Prostatectomy Using Transcutaneous Carbon Dioxide Monitoring: A Prospective Observational Study
Authors Lee HJ , Chae JS , An SM, Oh HW, Kim YJ, Woo JH
Received 8 November 2021
Accepted for publication 7 March 2022
Published 17 March 2022 Volume 2022:18 Pages 249—258
DOI https://doi.org/10.2147/TCRM.S347690
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Garry Walsh
Hyun Jung Lee,1 Ji Seon Chae,2 Sang-Mee An,2 Hye-Won Oh,1 Youn Jin Kim,1 Jae Hee Woo1
1Department of Anesthesiology and Pain Medicine, College of Medicine, Ewha Womans University, Seoul, South Korea; 2Department of Anesthesiology and Pain Medicine, Ewha Womans University Seoul Hospital, Seoul, South Korea
Correspondence: Jae Hee Woo, Department of Anesthesiology and Pain Medicine, College of Medicine, Ewha Womans University, 260 Gonghangdaero, Gangseo-gu, Seoul, 07804, South Korea, Tel +82-2-6986-4300, Fax +82-2-6986-4960, Email [email protected]
Purpose: Monitoring end-tidal carbon dioxide partial pressure (PETCO2) is a noninvasive, continuous method, but its accuracy is reduced by prolonged capnoperitoneum and the steep Trendelenburg position in robot-assisted radical prostatectomy (RARP). Transcutaneous carbon dioxide partial pressure (PTCCO2) monitoring, which is not affected by ventilator–perfusion mismatch, has been suggested as a suitable alternative. We compared the agreement of noninvasive measurements with the arterial carbon dioxide partial pressure (PaCO2) over a long period of capnoperitoneum, and investigated its sensitivity and predictive power for detecting hypercapnia.
Patients and Methods: The patients who underwent RARP were enrolled in this study prospectively. Intraoperative measurements of PETCO2, PTCCO2, and PaCO2 were analyzed. The primary outcome was the agreement of noninvasive monitoring with PaCO2 during prolonged capnoperitoneum. Bias and precision between noninvasive measurements and PaCO2 were assessed using Bland–Altman analysis. The bias and mean absolute difference were compared using a two-tailed Wilcoxon signed-rank test for pairs. The secondary outcome was the sensitivity and predictive power for detecting hypercapnia. To assess this, the Yates corrected chi-square test and the area under the receiver operating characteristic curve were used.
Results: The study analyzed 219 datasets from 46 patients. Compared with PETCO2, PTCCO2 had lower bias, greater precision, and better agreement with PaCO2 throughout the RARP. The mean absolute difference in PETCO2 and PaCO2 was larger than that of PTCCO2 and PaCO2, and continued to exceed the clinically acceptable range of 5 mmHg after 1 hour of capnoperitoneum. The sensitivity during capnoperitoneum and overall predictive power of PTCCO2 for detecting hypercapnia were significantly higher than those of PETCO2, suggesting a greater contribution to ventilator adjustment, to treat hypercapnia.
Conclusion: PTCCO2 monitoring measured PaCO2 more accurately than PETCO2 monitoring during RARP requiring prolonged capnoperitoneum and a steep Trendelenburg position. PTCCO2 monitoring also provides more sensitive measurements for ventilator adjustment and detects hypercapnia more effectively than PETCO2 monitoring.
Keywords: intraoperative carbon dioxide monitoring, capnoperitoneum, robotic surgery, end-tidal carbon dioxide monitoring, general anesthesia
Introduction
Estimation of the arterial carbon dioxide partial pressure (PaCO2) by direct analysis of arterial blood gases is the gold standard for monitoring carbon dioxide during general anesthesia. However, because the direct measurement of PaCO2 is invasive and intermittent, end-tidal carbon dioxide partial pressure (PETCO2) monitoring is preferred for the continuous monitoring of carbon dioxide.1 An important limitation of PETCO2 monitoring is its reduced accuracy due to factors such as ventilation-perfusion mismatch, shunt, and the surgical position of the patient, including the Trendelenburg or lateral decubitus position.2
Transcutaneous carbon dioxide partial pressure (PTCCO2) monitoring offers an alternative, noninvasive method for the continuous measurement of PaCO2 from arterialized capillary blood in tissues. Unlike PETCO2 monitoring, it is not influenced by ventilation–perfusion mismatch. The accuracy of PTCCO2 has been proven in pediatric patients, thoracic anesthesia, and laparoscopic surgery.3–5
Robot-assisted radical prostatectomy (RARP) for the treatment of prostate cancer requires that the patient should be placed in the steep Trendelenburg position, followed by the inflation of carbon dioxide gas into the peritoneal cavity to improve the surgical view and reduce bleeding. As a result, the organs in the abdominal cavity are pushed towards the diaphragm, thereby reducing both the functional residual volume and lung compliance. In addition, in robot-assisted surgery, the angle of the surgical table is steeper than the angle used in other laparoscopic surgeries, which may worsen ventilation-perfusion mismatch and cause pronounced intraoperative hypercapnia.6,7 RARP is mainly performed in the elderly, in whom a higher risk for intraoperative hypercapnia has been attributed to the age-related decline in lung function.8 In addition, subcutaneous emphysema is more common in this population due to weakened tissue, which in turn also contributes to the development of hypercapnia, acidosis and therefore sympathetic excitation, tachycardia, hypertension, hyperkalemia, and other complications.9,10 As these complications may be lethal in older patients with preexisting cardiac and pulmonary disease, it is important to maintain normocapnia during RARP by accurately monitoring PaCO2.
We hypothesized that, compared to PETCO2 monitoring, PTCCO2 monitoring provides a more accurate approximation of PaCO2 and contributes more to ventilator adjustment during RARP performed in patients in a steep Trendelenburg position and under prolonged capnoperitoneum. Therefore, this prospective study evaluated the accuracy of two noninvasive monitoring systems, PTCCO2 and PETCO2, and the predictive power of each in detecting hypercapnia.
Materials and Methods
Participants
This study prospectively included 46 patients classified as American Society of Anesthesiologists physical status I–III scheduled for RARP from January 2020 to April 2021. Patients with a history of severe cardiovascular or respiratory disease, neuromuscular disease, a body mass index > 35 kg/m2, or who required a vasoconstrictor during surgery were excluded.
Anesthesia
General anesthesia was induced with propofol (1–2 mg/kg), fentanyl (1–2 µg/kg), and rocuronium (0.6 mg/kg). Then the trachea was intubated, and the lungs were mechanically ventilated under pressure-controlled ventilation volume-guaranteed with a tidal volume of 8 mL per predicted body weight, and an I:E ratio of 1:2 with a positive end-expiratory pressure of 5 cmH2O and a respiratory rate of 10 respirations per minute. The tidal volume was reduced by 25 mL to maintain a peak inspiratory pressure < 35 cmH2O. At PETCO2 > 40 mmHg or a PTCCO2 > 45 mmHg, the respiratory rate was increased appropriately and arterial blood gases (ABG) were simultaneously analyzed. Anesthesia was maintained using 1–1.5 minimum alveolar concentration of desflurane using 50% oxygen in air. A bolus of fentanyl or remifentanil infusion was administered as needed to ensure a bispectral index value between 40 and 60 and a systolic blood pressure within 20% of baseline. Patients who required a vasoconstrictor to increase blood pressure during surgery were removed from the study.
Intraoperative Monitoring
Intraoperative monitoring included an electrocardiogram, pulse oxygen saturation, noninvasive blood pressure, arterial blood pressure, peak airway pressure, and oropharyngeal body temperature. The patient’s body temperature was kept at 36–37°C. PETCO2 was monitored using a side-stream infrared CO2 analyzer (Avance CS2, GE Healthcare, Madison, WI, USA). PTCCO2 was measured using a TCM4 device (Radiometer, Copenhagen, Denmark). Before its placement, the electrode was cleaned, a new membrane was applied, and the device was calibrated according to the manufacturer’s recommendation. The electrode was placed on the upper left chest and set at a working temperature of 42°C. The skin where the electrode was placed was swabbed with alcohol to facilitate adhesion of the disc to the skin. Capnoperitoneum was established and the intra-abdominal pressure (IAP) was maintained at 15–20 mmHg at the surgeon’s discretion.
Outcome Assessment
The primary outcome was the agreement of noninvasive monitoring (PTCCO2 and PETCO2) with PaCO2. To assess of this, ABG were sampled and noninvasive monitoring measurements were recorded in the pre-capnoperitoneum state in a supine position, 30 minutes after CO2 insufflation in the Trendelenburg position and every hour thereafter or whenever the PTCCO2 was > 45 mmHg or the PETCO2 was > 40 mmHg, and 20 minutes after CO2 deflation on resumption of the supine position. The first ABG sampling was conducted when the patient’s blood pressure and heart rate had stabilized and the respiratory rate was constant for at least 5 minutes after tracheal intubation. The TCM4 device was calibrated in vivo based on the results of the first ABG analysis. We calculated the differences and absolute differences between the noninvasive monitoring values (PTCCO2 and PETCO2) and PaCO2. The absolute differences were determined because negative numbers would artificially lower the mathematical mean of the difference. An absolute difference of 5 mmHg was defined as within the clinically acceptable range indicative of the interchangeability of the two methods.10,11 The secondary outcomes were the sensitivity and predictive power for detecting hypercapnia, defined as PaCO2 > 45 mmHg, which would be the basis for deciding to adjust the ventilator settings. To assess these, PTCCO2 and PETCO2 were dichotomized at PTCCO2 > 45 mmHg and PETCO2 > 40 mmHg. No data were recorded within 30 minutes after the occurrence of an already-recorded hypercapnia event to avoid bias due to over-representation of any single hypercapnia event. PTCCO2, PaCO2, PETCO2, arterial blood pressure, heart rate, oropharyngeal temperature, and IAP were recorded simultaneously.
Statistical Analysis
The sample size was calculated using G*Power (ver. 3.1.4). This indicated that 48 subjects were required to achieve a 90% power to detect a 7.5 mmHg difference (with α = 0.05) between the two methods with an estimated standard deviation of 15 mmHg using a paired t-test and a 10% dropout rate. This difference was based on a previous study.12
The statistical analyses were performed using SPSS ver. 26.0 (SPSS, USA). Quantitative data are presented as the mean ± standard deviation (SD) or median (interquartile range [IQR]) depending on the normality of the distribution. Correlation analysis with Pearson’s correlation coefficient (r) was used to establish the relationship between the two noninvasive measurements (PTCCO2 and PETCO2) and PaCO2. To evaluate their agreement with PaCO2, the bias (mean difference between the noninvasive monitoring values and PaCO2) and precision (SD of the bias) were evaluated, using Bland–Altman analysis. The reliability of noninvasive monitoring was compared using the mean absolute difference, using a two-tailed Wilcoxon signed-rank test for pairs after assessing normality. Chi-square analysis or Fisher’s exact test was used to compare the number of data with an absolute difference exceeding 5 mmHg. The sensitivity of the two noninvasive CO2 monitoring systems for detecting hypercapnia was compared using Yates corrected chi-square method. The predictive power for detecting hypercapnia was compared by constructing a receiver operating characteristic curve and calculating the area under the curve (AUC). A P-value < 0.05 was considered statistically significant.
Results
Subject Characteristics
Of the 67 patients assessed for eligibility, 19 were excluded and 48 patients were enrolled in the study. Two patients were removed because they required vasoconstrictors during surgery to treat low blood pressure. Ultimately, the final analysis included 219 datasets from 46 patients (Figure 1).
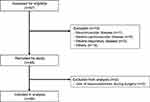 |
Figure 1 Flow chart of participant recruitment. |
All patients were male, with a mean age of 67.93 ± 9.30 years. Table 1 summarizes the patients’ demographic factors and surgical characteristics. All patients underwent RARP performed by either of two surgeons. The angle of the operation table was 28°–30°, which was the maximum angle of the two tables used in our hospital. Thirty-two patients were placed in 28° of Trendelenburg and 14 patients at 30°. The IAP was maintained between 15 and 20 mmHg according to the surgeon’s preference. The mean duration of capnoperitoneum was 175.93 ± 41.26 minutes and the mean body temperature of the patients during surgery was 36.10 ± 0.32°C. No complications related to prolonged PTCCO2 monitoring occurred.
 |
Table 1 Clinical and Surgical Characteristics of the Patients (n = 46) |
Primary Outcome
Table 2 gives the mean values of PaCO2, PETCO2, and PTCCO2 before, during, and after capnoperitoneum. Correlation analysis at each time-point showed correlations between PTCCO2 and PaCO2 (r = 0.99, 0.84, 0.67, respectively; P < 0.05), and between PETCO2 and PaCO2 (r = 0.70, 0.70, 0.57, respectively; P < 0.05). Bland–Altman analysis indicated better agreement with lower bias and higher precision of PTCCO2 for PaCO2 compared with PETCO2 (Figure 2). As shown in Table 3, the mean absolute difference between PaCO2 and PTCCO2 was smaller than the difference between PETCO2 and PaCO2 at all time points, indicating better reliability. The PETCO2 continued to differ from PaCO2 by more than 5 mmHg after 1 h of CO2 insufflation. Of the 219 datasets, an absolute difference ≥ 5 mmHg was observed in 100 PETCO2 datasets (45.7%) and in 28 PTCCO2 datasets (12.79%).
 |
Table 2 Correlation Analysis of PETCO2 and PaCO2, PTCCO2, and PaCO2 at Three Time Points |
 |
Table 3 Bias and Mean Absolute Difference Between PaCO2 and Noninvasive Measurements |
Secondary Outcome
During surgery, the respiratory rate was adjusted 51 times in 31 patients, according to the CO2 management protocol. The increment in the respiratory rate was made based on the PTCCO2 in 23 times (45.10%), on the PETCO2 in 9 times (17.65%), and on both parameters in 19 times (37.25%). There were 37 hypercapnia events in 19 patients; 28 during capnoperitoneum and nine after CO2 deflation. Of the 28 events during capnoperitoneum, a PTCCO2 > 45 mmHg occurred in 23 events and a PETCO2 > 40 mmHg in 14 events. Using the predefined cut-off values of PTCCO2 > 45 mmHg and PETCO2 > 40 mmHg, the sensitivity of two monitoring methods was 82.14% and 50.0%, respectively (P = 0.024). Of the nine events after CO2 deflation, PTCCO2 > 45 mmHg occurred in seven events, and PETCO2 > 40 mmHg in two. The sensitivity of PTCCO2 and PETCO2 monitoring was 77.78% and 22.22%, respectively (P = 0.059).
The AUC [95% confidence interval (CI)] of PTCCO2 at detecting PaCO2 > 45 mmHg during and after capnoperitoneum was 0.88 (0.82–0.95) and 0.90 (0.78–1.00), respectively. The corresponding AUCs (95% CI) of the PETCO2 were 0.81 (0.71–0.90) and 0.80 (0.62–0.98), respectively. The overall AUC value of PTCCO2 was significantly higher than that of PETCO2 (0.916 vs 0.826; P = 0.044).
A Case Report
One case merits further discussion (Table 4). In an 82-year-old patient with moderate aortic regurgitation and mild chronic obstructive pulmonary disease (COPD), PTCCO2 increased suddenly after 2 minutes of CO2 gas insufflation, from 37 to 46 mmHg, whereas the PETCO2 increased slowly to 40 mmHg. Subcutaneous emphysema was detected by palpating the upper chest. After the surgeon was notified, the respiratory rate was adjusted from 10 to 12 respirations per minute. During the subsequent 30 minutes of capnoperitoneum, CO2 retention continued, resulting in PaCO2, PETCO2, and PTCCO2 values of 65.7, 55, and 78 mmHg, respectively, despite increasing the respiratory rate to 20 respirations per minute and lowering the IAP from 20 to 12 mmHg. The ABG analysis showed a pH of 7.186, indicative of acidosis. Approximately 1 h later, there was little change in his PaCO2 (66.1 mmHg); his PETCO2 decreased to 49 mmHg but his PTCCO2 decreased only slightly, to 72 mmHg. The highest PETCO2 value after the detection of subcutaneous emphysema was 55 mmHg, whereas the PTCCO2 peaked at 89 mmHg. Twenty minutes after CO2 deflation, the patient’s PaCO2 was 40.8 mmHg and his PETCO2 36 mmHg, but his PTCCO2 was still elevated at 64 mmHg. The patient completed RARP without conversion to open surgery.
 |
Table 4 PaCO2, PETCO2 and PTCCO2 in a Patient with Subcutaneous Emphysema |
Discussion
This study demonstrated the superior accuracy of transcutaneous carbon dioxide measurement over PETCO2 monitoring to estimate PaCO2 during RARP. PTCCO2 is more sensitive in detecting hypercapnia during prolonged capnoperitoneum, which can otherwise lead to respiratory acidosis. The combined use of PTCCO2 and PETCO2 monitoring may enable anesthesiologists to provide more meticulous ventilator management.
A previous study reported the inconsistent correlation between PETCO2 and PaCO2 after CO2 inflation in laparoscopic surgeries with prolonged capnoperitoneum.10 In morbidly obese patients undergoing laparoscopic bariatric surgery, capnoperitoneum may exacerbate a reduction in the functional residual capacity and increase ventilation–perfusion mismatch, thereby diminishing the accuracy of PETCO2.11 In our study, the correlation of PTCCO2 and PETCO2 with PaCO2 decreased as capnoperitoneum was prolonged. The mean absolute difference between PaCO2 and PETCO2 exceeded 5 mmHg after 1 h of capnoperitoneum.10,13,14 By contrast, the mean absolute difference between PaCO2 and PTCCO2 was < 5 mmHg throughout the period of capnoperitoneum. The scatter diagram of PETCO2 and PaCO2 on a Bland–Altman plot also showed many data points outside the acceptable range, indicating poor agreement between PaCO2 and PETCO2.
The bias in the PaCO2 and PTCCO2 in our study was much smaller than reported in previous studies.15 This was likely to be because none of our patients experienced severe hypercapnia and acidosis, which were avoided by the meticulous adjustments that were made based on both PTCCO2 and PETCO2 monitoring, with the exception of a single case of subcutaneous emphysema. The respiratory rate was adjusted in 45% of the cases based on the PTCCO2 values compared to 19% based on PETCO2 values, highlighting that the additional use of PTCCO2 monitoring can compensate for the deficiencies of PETCO2 monitoring. Considering previous reports of an increasing difference between PaCO2 and PTCCO2 along with an increase of PaCO2 levels,16,17 the relatively small change in the PaCO2 levels of our patients may account for the discrepancy. In addition, the stable cardiovascular function of the patients, the exclusion of those with severe lung diseases, and the maintenance of a constant body temperature during surgery may have reduced the bias in the PaCO2 and PTCCO2 values in this study.
Previous studies raised concerns about the limitations of PTCCO2 measurements, such as the relatively slow response time, difficulty maintaining good contact between the patient’s skin and the sensor, and the long warm-up time needed for the sensor to reach its final operating temperature. In addition, the variability in skin thickness at the sensor attachment site may affect the accuracy of the PTCCO2 measurements. Nishiyama et al18,19 reported that the PaCO2 was more precisely measured by attaching the electrode to the chest rather than to the upper arm, forearm, or earlobe. In our patients, the electrode was attached to the upper left area of the chest, where adherence of the disc to the skin could be checked by the anesthesiologist even when the patient was in the steep Trendelenburg position during RARP. Skin tissue perfusion is one of the most important factors determining the accuracy and precision of PTCCO2. A low environmental temperature causes vascular contraction in the skin, reducing blood flow. Bladder irrigation, which is frequently performed during urological surgery, may also cause a drop in the patient’s body temperature. In our patients, efforts were therefore made to maintain their body temperature, such as by using heated breathing circuits, an intravenous fluid warmer, and a forced-air warming system and by maintaining the temperature of the operating room above 23°C. The accuracy of PTCCO2 monitoring in patients administered vasoconstrictors is a matter of debate. Rodriguez et al20 reported that catecholamine support did not affect the accuracy PTCCO2 monitoring, but other studies came to the opposite conclusion.12,14,16,21 In our study, two patients who needed a vasoconstrictor to increase their intraoperative blood pressure were excluded from the final analysis, to eliminate a possible confounder.
In the aforementioned patient with subcutaneous emphysema, PTCCO2 increased more rapidly than PETCO2 because the former was measured in an area of subcutaneous emphysema. Likewise, the PTCCO2 decreased slowly because of the accumulated CO2 at that site. Because the development of subcutaneous emphysema in the chest area is common during robotic surgery with the patient in the Trendelenburg position, the attachment of a sensor to the chest area would allow the rapid detection of subcutaneous emphysema. However, once the PTCCO2 increases, it would overestimate the PaCO2 due to the accumulation of CO2 in the subcutaneous layer.
We initially attempted to compare the decreasing trends in PETCO2 and PTCCO2 with the decrease in PaCO2 during CO2 elimination. However, the brief time (< 30 minutes) from the end of capnoperitoneum to extubation was insufficient for the cutaneous capillary and systemic PaCO2 to reach equilibrium, thus ruling out a comparison. Nonetheless, our results showed that the superiority of PTCCO2 over PETCO2 was maintained even during the CO2 elimination period after CO2 insufflation was ended. Further studies are needed to determine whether the good correlation between PTCCO2 and PaCO2 is retained after CO2 elimination.
The limitations to our study include the following. First, only healthy male patients without significant co-morbidities were enrolled, such that our findings may not be generalizable to patients with severe coexisting conditions. In addition, patients treated with vasoactive drugs were excluded from our study, although transcutaneous monitoring might be advantageous for sedated patients in the intensive care unit who often need vasoactive drugs.
Conclusion
In conclusion, PTCCO2 monitoring was more accurate than PETCO2 monitoring for measuring PaCO2 and was better able to detect hypercapnia in surgery requiring prolonged capnoperitoneum and the steep Trendelenburg position. Because capnography enables the detection of acute life-threatening events, such as apnea, airway obstruction, ventilator disconnection, or esophageal intubation, PTCCO2 cannot substitute for PETCO2. Nonetheless, it is valuable as an adjunct method in situations in which ventilation–perfusion mismatch interferes with the gradient between PETCO2 and PaCO2.
Abbreviations
PETCO2, end-tidal carbon dioxide partial pressure; PTCCO2, transcutaneous carbon dioxide partial pressure; RARP, robot-assisted radical prostatectomy; PaCO2, arterial carbon dioxide partial pressure; AUC, area under the curve; ABG, arterial blood gases; IAP, intra-abdominal pressure; SD, standard deviation; IQR, interquartile range; CI, confidence interval; COPD, chronic obstructive pulmonary disease.
Data Sharing Statement
The individual deidentified raw data that support the study findings are available from the corresponding author upon reasonable request.
Ethics Approval and Consent for Publication
This study was approved by the Ethics Committee of Ewha Womans University, Seoul, South Korea (SEUMC 2019–10–013–002). This study was registered with the Clinical Trial Registry of Korea (registration identifier: KCT0004680, cris.nih.go.kr) before patient recruitment. The written informed consent was obtained from all patients. It was also obtained for publication of case details with unidentifiable information. This study was conducted in accordance with the Declaration of Helsinki.
Acknowledgments
We thank our colleagues and staff of the Department of Urology for their cooperation with this study.
Funding
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
All authors report no conflicts of interest arising from this work.
References
1. Breen PH. Arterial blood gas and pH analysis. Clinical approach and interpretation. Anesthesiol Clin North Am. 2001;19(4):885–906. doi:10.1016/S0889-8537(01)80014-6
2. Whitesell R, Asiddao C, Gollman D, Jablonski J. Relationship between arterial and peak expired carbon dioxide pressure during anesthesia and factors influencing the difference. Anesth Analg. 1981;60(7):508–512. doi:10.1213/00000539-198107000-00008
3. Oshibuchi M, Cho S, Hara T, Tomiyasu S, Makita T, Sumikawa K. A comparative evaluation of transcutaneous and end-tidal measurements of CO2 in thoracic anesthesia. Anesth Analg. 2003;97(3):776–779. doi:10.1213/01.ANE.0000074793.12070.1E
4. Tobias JD. Transcutaneous carbon dioxide monitoring in infants and children. Pediatr Anaesth. 2009;19(5):434–444. doi:10.1111/j.1460-9592.2009.02930.x
5. May A, Humston C, Rice J, Nemastil CJ, Salvator A, Tobias J. Non-invasive carbon dioxide monitoring in patients with cystic fibrosis during general anesthesia: end-tidal versus transcutaneous techniques. J Anesth. 2020;34(1):66–71. doi:10.1007/s00540-019-02706-5
6. Kalmar AF, Foubert L, Hendrickx JF, et al. Influence of steep Trendelenburg position and CO2 pneumoperitoneum on cardiovascular, cerebrovascular, and respiratory homeostasis during robotic prostatectomy. Br J Anaesth. 2010;104(4):433–439. doi:10.1093/bja/aeq018
7. McLarney JT, Rose GL. Anesthetic implications of robotic gynecologic surgery. J Gynecol Endosc Surg. 2011;2(2):75–78. doi:10.4103/0974-1216.114077
8. Murdock CM, Wolff AJ, Van Geem T. Risk factors for hypercarbia, subcutaneous emphysema, pneumothorax, and pneumomediastinum during laparoscopy. Obstet Gynecol. 2000;95(5):704–709. doi:10.1016/s0029-7844(00)00781-x
9. Weinberg L, Lee D-K, Gan C, et al. The association of acute hypercarbia and plasma potassium concentration during laparoscopic surgery: a retrospective observational study. BMC Surg. 2021;21(1):1–8. doi:10.1186/s12893-020-01034-w
10. Xue Q, Wu X, Jin J, Yu B, Zheng M. Transcutaneous carbon dioxide monitoring accurately predicts arterial carbon dioxide partial pressure in patients undergoing prolonged laparoscopic surgery. Anesth Analg. 2010;111(2):417–420. doi:10.1213/ANE.0b013e3181e30b54
11. Liu S, Sun J, Chen X, Yu Y, Liu X, Liu C. The application of transcutaneous CO2 pressure monitoring in the anesthesia of obese patients undergoing laparoscopic bariatric surgery. PLoS One. 2014;9(4):e91563. doi:10.1371/journal.pone.0091563
12. De Oliveira GS
13. Bland JM, Altman D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;327(8476):307–310. doi:10.1016/S0140-6736(86)90837-8
14. Zhang H, Wang D-X. Noninvasive measurement of carbon dioxide during one-lung ventilation with low tidal volume for two hours: end-tidal versus transcutaneous techniques. PLoS One. 2015;10(10):e0138912. doi:10.1371/journal.pone.0138912
15. Poerner TC, Goebel B, Kralev S, et al. Impact of mitral E/A ratio on the accuracy of different echocardiographic indices to estimate left ventricular end-diastolic pressure. Ultrasound Med Biol. 2007;33(5):699–707. doi:10.1016/j.ultrasmedbio.2006.11.014
16. Griffin J, Terry B, Burton R, et al. Comparison of end‐tidal and transcutaneous measures of carbon dioxide during general anaesthesia in severely obese adults. Br J Anaesth. 2003;91(4):498–501. doi:10.1093/bja/aeg217
17. Reid CW, Martineau RJ, Miller DR, Hull KA, Baines J, Sullivan PJ. A comparison of transcutaneous, end-tidal and arterial measurements of carbon dioxide during general anaesthesia. Can J Anaesth. 1992;39(1):31–36. doi:10.1007/BF03008669
18. Nishiyama T, Kohno Y, Koishi K. Comparison of ear and chest probes in transcutaneous carbon dioxide pressure measurements during general anesthesia in adults. J Clin Monit Comput. 2011;25(5):323–328. doi:10.1007/s10877-011-9311-3
19. Nishiyama T, Nakamura S, Yamashita K. Effects of the electrode temperature of a new monitor, TCM4, on the measurement of transcutaneous oxygen and carbon dioxide tension. J Anesth. 2006;20(4):331–334. doi:10.1007/s00540-006-0422-9
20. Rodriguez P, Lellouche F, Aboab J, Buisson CB, Brochard L. Transcutaneous arterial carbon dioxide pressure monitoring in critically ill adult patients. Intensive Care Med. 2006;32(2):309–312. doi:10.1007/s00134-005-0006-4
21. Casati A, Squicciarini G, Malagutti G, Baciarello M, Putzu M, Fanelli A. Transcutaneous monitoring of partial pressure of carbon dioxide in the elderly patient: a prospective, clinical comparison with end-tidal monitoring. J Clin Anesth. 2006;18(6):436–440. doi:10.1016/j.jclinane.2006.02.007
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

