Back to Journals » Journal of Pain Research » Volume 14
Store-Operated Calcium Channels Contribute to Remifentanil-Induced Postoperative Hyperalgesia via Phosphorylation of CaMKIIα in Rats
Authors Zhou Z , Mao M, Cai X , Zhu W , Sun J
Received 9 August 2021
Accepted for publication 24 September 2021
Published 18 October 2021 Volume 2021:14 Pages 3289—3299
DOI https://doi.org/10.2147/JPR.S333297
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor E Alfonso Romero-Sandoval
Zhenhui Zhou,1,* Meng Mao,2,* Xuechun Cai,1 Wei Zhu,1 Jie Sun3
1Department of Anesthesiology and Perioperative Medicine, The First Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu, People’s Republic of China; 2Department of Anesthesiology, The Affiliated Stomatological Hospital of Nanjing Medical University, Nanjing, Jiangsu, People’s Republic of China; 3Department of Anesthesiology, Zhongda Hospital, Medical School, Southeast University, Nanjing, Jiangsu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jie Sun
Department of Anesthesiology, Zhongda Hospital, Medical School, Southeast University, Nanjing, 210009, People’s Republic of China
Tel +8615895971012
Email [email protected]
Wei Zhu
Department of Anesthesiology and Perioperative Medicine, The First Affiliated Hospital of Nanjing Medical University, Nanjing, 210009, People’s Republic of China
Tel +8618905182820
Email [email protected]
Purpose: The mechanisms of remifentanil-induced postoperative hyperalgesia (RIPH) remain unclear. Store-operated calcium channels (SOCCs) are mainly comprised of stromal interaction molecules 1 (STIM1) and pore-forming subunits (Orai1). They were found to take a pivotal part in Ca2+-dependent procedures and involved in the development of central sensitization and pain. Ca2+/calmodulin-dependent protein kinase IIα (CaMKIIα), regulated by Ca2+/calmodulin complex, has been shown to have a crucial role in RIPH. This study aims to determine whether SOCCs contribute to RIPH via activating CaMKIIα.
Materials and Methods: Intra-operative infusion of remifentanil (1.0 μg kg− 1 min− 1, 60 min) was used to establish a RIPH rat model. The SOCCs blocker (YM-58483) was applied intrathecally to confirm the results. Animal behavioral tests including paw withdrawal thermal latency (PWTL) and paw withdrawal mechanical threshold (PWMT) were performed at − 24, 2, 6, 24, 48 h after incision and remifentanil treatments. The protein expression of STIM1, Orai1, CaMKIIα, and p-CaMKIIα was assayed with Western blot, and the number of STIM1 and Orai1 positive cells was shown by immunofluorescence.
Results: Remifentanil administration significantly induced postoperative mechanical and thermal hyperalgesia, as well as increased STIM1 and Orai1 protein expression in the spinal dorsal horn. Furthermore, the intrathecal administration of YM-58483 effectively alleviated remifentanil-induced postoperative mechanical and thermal hyperalgesia according to the behavioral tests. In addition, YM-58483 suppressed the phosphorylation of CaMKIIα but had no effect on the expression of STIM1 and Orai1.
Conclusion: Our study demonstrated that SOCCs are involved in RIPH. The over-expressed STIM1 and Orai1 in the spinal cord contribute to RIPH via mediating the phosphorylation of CaMKIIα. Blockade of SOCCs may provide an effective therapeutic approach for RIPH.
Keywords: remifentanil, hyperalgesia, SOCCs, CaMKIIα
Introduction
Remifentanil is a potent short-acting μ-opioid receptor agonist that has unique pharmacokinetic properties with rapid metabolism, making it a common intraoperative analgesic.1 Although it is widely used in the clinic, increasing experimental and clinical researches suggested that remifentanil is associated with paradoxical nociceptive effect, known as remifentanil-induced postoperative hyperalgesia (RIPH).2–5 There have been studies investigating how remifentanil causes hyperalgesia; however, the specific mechanism remains unclear.
Store-operated calcium channels (SOCCs) are Ca2+-selective cation channels, which are implicated in the Ca2+ influx pathway in many different cell types. The SOCCs system is comprised of stromal interaction molecules (STIM) and pore-forming subunits (Orai). The STIM is located on the surface of the endoplasmic reticulum (ER), and the Orai is located in the plasma membrane.6 Especially, STIM1 and Orai1, two key components of SOCCs, play a crucial role in mediating store-operated Ca2+ entry (SOCE) and maintaining resting calcium homeostasis.7 In neurons, Ca2+ is crucial to the development of long-term potentiation (LTP) in the synaptic plasticity, which leads to the formation and the maintenance of pain hypersensitivity.8 Accumulated evidence has been manifested that all the components of SOCCs are expressed in the central nervous system (CNS) and involved in neuronal functions and pathological status, such as Parkinson’s disease (PD),9 Alzheimer’s disease (AD),10 and chronic pain.11,12 In addition, SOCE is activated by the depletion of ER calcium stores and can be blocked by YM-58483, a potent SOCCs channel blocker.11 Recent studies reported that YM-58483 was able to reverse thermal and mechanical hypersensitivity in spared nerve injury (SNI) and inflammatory pain models.13,14 However, the relationship between SOCCs and RIPH remains to be elusive.
Ca2+/calmodulin-dependent protein kinase IIα (CaMKIIα), a multifunctional serine/threonine kinase, was gradually revealed a vital role in forming and maintaining RIPH.15,16 CaMKIIα is regulated by Ca2+/calmodulin complex. Elevated intracellular Ca2+ and calmodulin levels are essential in the activation of CaMKIIα.17 CaMKIIα is involved in LTP and synaptic plasticity by decoding synaptic Ca2+ oscillations.18,19 Temporarily elevated levels of p-CaMKIIα in the CNS are associated with central sensitization and pain hypersensitivity.20 Furthermore, SOCE induced by chronic ER Ca2+ depletion may lead to increased intracytoplasmic Ca2+ concentration, resulting in CaMKIIα activation.21 Therefore, we hypothesized that the inhibition of SOCE could reduce the phosphorylation of CaMKIIα in the spinal cord, thereby alleviating RIPH.
In the present study, we investigated the expression level of SOCCs in the spinal dorsal horn by establishing a rat model of RIPH. Since CaMKIIα is activated by Ca2+ and involved in RIPH, we next explored whether SOCCs contribute to RIPH via phosphorylation of CaMKIIα. Our results provide a molecular mechanism and therapeutic approach on RIPH.
Materials and Methods
Animals
Mature male Sprague Dawley rats (2–3 months old, 360–380 g) were purchased from the Zhejiang Academy of Medical Sciences. All procedures were performed following the approved guidelines of Southeast University, and all animal model experiments were tested with the approval of the Animal Experimental Ethics Committee of Southeast University (Ethical permission code: 20200310006). Rats were housed under controlled environmental conditions (room temperature of 24 ± 1 °C and relative humidity of 55% ± 10%) with a 12-h light/dark cycle. Five rats per cage were received standard rats chow and water ad libitum. The rats were randomly divided into four groups in each experiment, with 10 rats in each group before any assessment was performed.
Surgical Procedure
The plantar incision was performed as described in the previous article.22 A 1.0 cm longitudinal plantar incision through the skin and fascia of the right hind paw from the edge of the heel to the toe was made with a No. 11 blade. The plantaris muscle was separated by ophthalmic forceps and incised longitudinally, and the tendon should be protected from being severed. The skin was sutured with 4–0 sutures, and the wound site was covered with Aureomycin ointment to prevent infection. Rats in the control group received sham procedures without skin incisions.
Experiment Related Procedures
For intravenous drug administration, a 24‐gauge catheter rinsed with heparinized saline (100U/mL) was cannulated in the rat tail vein. For intrathecal drug administration, a lumbar puncture technique was used to accomplish a successful intrathecal injection.23 Sevoflurane anesthetized rats were placed in the prone position, exposing the lumbar vertebral space between L4 and L5. A 20 µL microinjector was inserted vertically into the lumbar intervertebral space. Brisk tail movement indicated successful intrathecal injection.
Drug Administration
Remifentanil (Yichang Renfu Pharmaceutical Co., Yichang, China) was dissolved in 0.9% saline and injected intravenously (i.v.) for 60 minutes, the infusion rate was 1.0 µg−1·kg−1·min. Rats in the control group received the same volume of saline (0.1 mL kg−1 min−1, 60 min). YM-58483 (MedChemexpress LLC, Princeton, NJ, USA) was dissolved in PEG300 (MedChemexpress LLC, Princeton, NJ, USA) and injected intrathecally (i.th.) at the concentration of 10 μL (1000 μmol/L) 2 h before each behavior test. The same volume of PEG300 was used as the control treatment. All drugs were administered under sevoflurane anesthesia (induction, 3.0%; maintenance, 1.0%; Hengrui Pharmaceutical Co., Ltd, Shanghai, China). The detailed process and grouping of the experiment are shown in Figure 1A–D.
Nociceptive Behavioral Tests
Paw withdrawal mechanical threshold (PWMT) was measured using von Frey filaments (Aesthesio, Danmic Global, LLC, Campbell, CA, USA) according to the “up-down” method.24 Briefly, each rat was placed in an individual plastic box with a metallic mesh bottom and acclimated for 30 min. The calibrated filaments were perpendicular to the plantar surface of the hind paw for 6 s, and the positive response was defined as a brisk paw withdrawal or flinching. Under the condition of a positive response, the next lower force filament was applied. If in a negative response, the next greater force filament was applied. The up-down algorithm was applied to calculate the 50% likelihood of the PWMT.25
Paw withdrawal thermal latency (PWTL) was measured using a thermal testing apparatus (BME-410C, Inst of Biomedical Engineering, CAMS, Tianjin, China). Each rat was placed in an individual plastic box on a glass surface and acclimated for 30 min. The targeted hind paw was focused by a mobile radiant heat source located under the glass until the positive response occurred, and the time was recorded as the PWTL. The test was repeated three times at 10-min intervals. The average value of the three trials was evaluated as the mean PWTL. To protect from potential tissue damage, a 30 s cut-off was set.25 The observer of behavioral tests was blinded to the treatment.
PWMT and PWTL were measured at 24 hours before remifentanil administration as baseline and then at 2, 6, 24, and 48 hours after remifentanil administration.
Western Blot
After the last behavioral test at 48 h, rats were deeply anesthetized with sevoflurane and spinal cord tissues were removed from the enlarged lumbar region (L4–L6) for Western blotting analysis. Spinal cord tissues were extracted in RIPA buffer (Beyotime Biotech Inc., Shanghai, China) containing protease and phosphatase inhibitors. After centrifuging (× 12,000 rpm) at 4°C for 20 min, the supernatant fluid of each sample was obtained and protein concentration was detected by BCA protein assay. Twenty-four micrograms of each protein sample was electrophoresis with 10% SDS-PAGE, then transferred to PVDF membrane (Millipore Corp, Billerica, MA, USA). The membrane was blocked with 5% bovine serum albumin for 1 h at room temperature and then incubated with the following primary antibodies: rabbit monoclonal anti-STIM1 (#5668,1:1000, CST, Danvers, MA, USA), mouse monoclonal anti-Orai1 (ab175040, 5 µg/µL, Abcam, Cambridge, UK), rabbit monoclonal anti-phospho-CaMKII (Thr286) (#12716,1:1000, CST, Danvers, MA, USA), mouse monoclonal anti- CaMKIIα (6G9) (#50049,1:1000, CST, Danvers, MA, USA) and rabbit anti-GADPH (#10494-1-AP, 1:20000, Proteintech, Wuhan, China) overnight at 4°C. The biotinylated secondary antibody (anti-rabbit IgG (GB23303,1:3000, Servicebio Technology Co., Ltd, Wuhan, China) or anti-mouse IgG (GB23301,1:3000, Servicebio Technology Co., Ltd, Wuhan, China)) was used to incubate the membranes for 1 h at room temperature. After rinsing in TBST and incubated in ECL liquid ((Beyotime Biotech Inc., Shanghai, China), target protein bands were detected using a ChemiScope6100 imaging system (Clinx Science Instruments, Co., Ltd, Shanghai, China) and quantified by the Image J software (National Institute of Health, Bethesda, MD, USA).
Immunofluorescence
After the last behavioral test at 48 h, rats were deeply anesthetized with sevoflurane and instilled with 0.1 M phosphate buffer saline (PBS) and 4% paraformaldehyde (PFA). The L4–L6 spinal cords were rapidly removed, post-fixed in 4% PFA for 24 h and dehydrated in 30% sucrose at 4 °C for 72 h. Spinal cord tissues were embedded with O.C.T. Compound (SAKURA Tissue-Tek® O.C.T. Compound, Sakura Finetek, USA) and cut into 30-μm-thick sections using a freezing microtome. Tissue sections were permeabilized in PBS containing 0.1% Triton X-100 for 2 h after being rinsed in PBS and then blocked in 10% bovine serum albumin for 1 h. Sections were then incubated with the mixed primary antibodies (rabbit anti-STIM1, #5668, 1:200, CST, Danvers, MA, USA, Orai1; mouse anti-Orai1 ab175040, 1 µg/µL, Abcam, Cambridge, UK) for 48 h at 4 °C. Subsequently, sections were washed in PBS and incubated with the blended secondary antibodies (Alexa Fluor 488 conjugated to goat anti-mouse IgG, 1:500, Proteintech, Wuhan, China; Alexa Fluor 532 conjugated to goat anti-rabbit IgG, 5 µg/mL, Invitrogen, Carlsbad, CA, USA) for 24 h. Images were acquired with a fluorescence microscope (MF31, Mshot, Guangzhou, China). Every slide was captured three selected fields under 10× magnification and counted the number of positive cells.
Statistical Analysis
Data were shown as means ± SEM. Two-way analysis of variance (ANOVA) followed by Dunnett’s post hoc test was applied to analyze the behavioral data. One-way ANOVA followed by Tukey’s post hoc test was applied to analyze the Western blot and immunohistochemistry data. All statistical analyses were performed using GraphPad Prism version 8.3 (GraphPad Software Inc., San Diego, CA, USA). P < 0.05 was considered to be statistically significant.
Result
Remifentanil-Induced Mechanical and Thermal Hyperalgesia in Rats
As shown in Figure 2A and B, there were no significant differences in basic values of PWMT and PWTL among all groups (P>0.05). Compared with baseline, PWMT and PWTL decreased in Inci, Remi and Inci + Remi groups (P<0.05) at different time points, and they did not show significant changes in NS group (P>0.05). Compared with NS group, PWMT decreased from 2 to 24 h and PWTL decreased from 2 to 48 h in Remi group (P<0.05). Furthermore, compared with Inci group, PWMT decreased from 6 to 48 h and PWTL decreased from 2 to 48 h in Inci + Remi group (P<0.05). These results indicated that the RIPH model was successfully established.
The Expression of STIM1 and Orai1 in the Spinal Dorsal Horn Increased After Remifentanil Administration
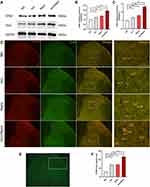
To determine the effect of remifentanil on STIM1 and Orai1 expression in the spinal cord, we detected the SOCCs expression at 48 h after the administration of remifentanil. The Western blot analysis of homogenized spinal tissues showed STIM1 and Orai1 levels were significantly increased in Inci (STIM1: P<0.05; Orai1: P<0.01), Remi (STIM1: P<0.01; Orai1: P<0.001), and Inci + Remi (STIM1: P<0.001; Orai1: P<0.001) groups as compared with NS group (Figure 3A–C). Compared with Inci group, the expression of STIM1 and Orai1 were increased in Inci + Remil group (STIM1: P<0.001; Orai1: P<0.001) (Figure 3A–C).
Since previous studies showed that STIM1 and Orai1 are mainly expressed in the spinal dorsal horn neurons, and the decreased concentration of ER Ca2+ stores increases STIM1 and Orai1 puncta formation in vitro.11,12 Thus, we detected the number of STIM1 and Orai1 in the spinal dorsal horn. As shown in Figure 3D–F, STIM1 and Orai1 collocated in the same spinal cord cells. Consistent with the Western blot results above, remifentanil administration significantly increased the number of STIM1 and Orai1 positive cells (P<0.001).
Blocking of SOCCs Reversed RIPH
To further verify the effect of SOCCs on RIPH, a specific blocker of the SOCCs, YM-58483 was intrathecally injected into rats 2 h before each behavior testing. As shown in Figure 4A and B, compared with Inci + Remi + Vehicle group, administration of YM-58483 (10 nmol) with incision and remifentanil significantly increased PWMT and PWTL (P<0.05). Interestingly, we did not detect a difference between Inci + Vehicle group and Inci+Remi+YM-58483 group (P>0.05).
YM-58483 Decreased Remifentanil-Induced Phosphorylation of CaMKIIα in the Spinal Cord
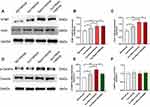
Next, Western blot data demonstrated that intrathecal treatment with SOCCs blocker, YM-58483, did not affect the expression of STIM1 and Orai1 in RIPH (P>0.05) (Figure 5A–C). Studies have manifested that the phosphorylation of CaMKIIα plays a crucial role in RIPH.20 Thus, we further determined whether the phosphorylation of CaMKIIα were involved in RIPH via increased Ca2+ induced by SOCE. Compared NS + vehicle group, the expression of p-CaMKIIα was significantly increased in Inci + vehicle group (P<0.01) and Inci + Remi + vehicle group (P<0.001) (Figure 5D and E). Moreover, YM-58483 suppressed the level of p-CaMKIIα compared with the Inci + Remi + vehicle group (P<0.01) (Figure 5D and E). In addition, the protein level of CaMKIIα did not demonstrate a significant difference among all groups (P>0.05) (Figure 5D and F).
Discussion
Our results suggested that SOCCs play a crucial role in RIPH. The increase of the STIM1 and Orai1 protein was seen in remifentanil used rats’ spinal dorsal horn. Furthermore, remifentanil infusion downregulated the nociceptive thresholds (PWMT and PWTL) at different time points, which could be partly improved by YM-58483, a potent SOCCs channel blocker. The expression of p-CaMKIIα was partly reversed by YM-58483. It is indicated that blockade of SOCCs could regulate the expression of p-CaMKIIα to prevent the RIPH.
Hyperalgesia refers to increased pain from a stimulus that usually provokes pain, including a reduced pain threshold and an increased response to subnormal stimuli.26 Opioid-induced hyperalgesia (OIH) is known as a paradoxical increase in sensitivity to painful stimuli associated with opioid therapy, resulting in slower patient’s recovery after surgery and more consumption of analgesics.27,28 As a kind of opioid, remifentanil is widely used in general anesthesia due to its unique properties. Indeed, remifentanil has high lipid solubility, allowing for a rapid onset of effect; It is rapidly metabolized by non-specific plasma and tissue esterases and hence a rapid recovery.1 Although remifentanil offers significant advantages in the clinic, compared with other opioids, it has the highest reported incidence of hyperalgesia, known as remifentanil-induced hyperalgesia (RIH).29 With regard to the postoperative period, RIH results in increased opioid consumption and pain sensibility, which causes discomfort in patients and longer stay in hospital than expected.
Evidence from both human and animal models has reported that RIH is caused by exposure to a high dosage of remifentanil.30 Clinical trials are difficult to draw conclusions about the incidence of RIH due to heterogeneity of remifentanil infusion regimens, maintenance of anesthesia, duration of infusion, cumulative dose of remifentanil, and pain measures. However, it appears that intraoperative remifentanil infusion rates greater than 0.2 µg−1·kg−1·min is associated with lower pain thresholds, which suggests hyperalgesia.1 On the other hand, animal researches showed that 1.0 µg−1·kg−1·min for 60 min could induce hyperalgesia in rats. Therefore, the dosage of remifentanil (1.0 µg−1·kg−1·min, 60 min) was chosen to establish a rat model of hyperalgesia, which approximates the equivalent dose conversed between humans and rats.31 Furthermore, remifentanil anesthesia during operation is more common in clinical practice; thus, we investigated the effects of incision plus remifentanil on pain sensitivity. Interestingly, intra-operative exposure to remifentanil apparently increased the incision-induced mechanical and thermal hyperalgesia.
SOCCs are activated by the release or depletion of Ca2+ from the ER, influencing neurotransmitter release and synaptic plasticity.32,33 SOCE is a major mechanism for triggering Ca2+ enter into cells, which is required for many Ca2+-dependent cellular functions, such as enzymatic activity. There are two key components of SOCCs–stromal interaction molecule 1 (STIM1) and Orai1.34 Growing evidence manifests that SOCCs play a crucial role in pain disorders, such as SNI, inflammation, and acute pain.13,35,36 Our results indicated that continuous remifentanil infusion increases the expression level of STIM1 and Orai1 in the spinal dorsal horn, which has a vital effect on maintained remifentanil-induced thermal and mechanical hypersensitivities. Furthermore, YM-58483, a potent SOCCs channel blocker, was reported to have analgesic actions in both acute and chronic pain.13 Our behavior testing results confirmed that intrathecal administration of YM-58483 reversed thermal and mechanical hyperalgesia, suggesting an action of SOCCs in regulating RIPH.
However, we further tested the protein expression of STIM1 and Orai1 by Western blot and found that YM-58483 could not decrease their levels during the development of RIPH. It is well documented that a decrease in ER Ca2+ leads to the intracellular redistribution of STIM1 at the ER membrane. The STIM1 puncta activates the Orai1 channel at the plasma membrane, allowing Ca2+ entry into the cellular.12 However, the core mechanisms of action remain to be determined. Previous studies have manifested that YM-58483 blocks SOCE and dose-dependently inhibits the CaMKIIα activation in arthritic pain models.14 Our results showed that the analgesic effect of YM-58483 in RIPH is not through impacting the expression of SOCCs. Thus, we further detected the protein expression of CaMKIIα and p-CaMKIIα.
CaMKIIα, as a major CaMKII isoform expressed in the CNS, is an essential cellular mechanism leading to and maintaining OIH.37 CaMKIIα is activated by increased intracellular Ca2+. Phosphorylated CaMKIIα is a part of LTP hypersensitivity signaling, which is involved in the sensitization of homosynapses leading to an enhanced strength of the synapse and its signal transduction. Indeed, cumulative studies confirmed that inhibiting the activation of CaMKIIα attenuates RIPH.38,39 Furthermore, SOCCs have been shown implicated in CaMKIIα activation and YM-58483 effectively inhibits the CaMKIIα activation in the spinal cord in different pain models.14,40 Our results indicated that CaMKIIα becomes activated after the intra-operative infusion of remifentanil and that this increased phosphorylation of CaMKIIα was significantly suppressed by YM-58483.
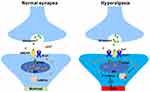
Together, these results above indicated that exposure to remifentanil up-regulates STIM1 and Orai1 protein expression, further enhancing the SOCE. Increased intracellular Ca2+ leads to the phosphorylation of CaMKIIα, which is part of LTP hypersensitivity signaling contributing to RIPH (Figure 6).
Conclusion
In summary, our study demonstrated that SOCCs in rat spinal dorsal horn contribute to the development of RIPH via activation of CaMKIIα. The pharmacologic intervention of SOCCs protected against the development of hyperalgesia. Thus, the identification of SOCCs opens the way for targeted treatment and the blockade of the SOCCs/p-CaMKIIα pathway is a new therapeutic target. However, how SOCCs are activated after the administration of remifentanil is unclear. Further studies on the upstream mechanisms of SOCCs are encouraged to exam the relationship with RIPH.
Abbreviations
RIPH, remifentanil-induced postoperative hyperalgesia; SOCCs, store-operated calcium channels; STIM1, stromal interaction molecules 1; CaMKIIα, Ca2+/calmodulin-dependent protein kinase IIα; PWTL, paw withdrawal thermal latency; PWMT, paw withdrawal mechanical threshold; ER, endoplasmic reticulum; SOCE, store-operated Ca2+ entry; LTP, long-term potentiation; CNS, central nervous system; PD, Parkinson’s disease; AD, Alzheimer’s disease; SNI, spared nerve injury; PBS, phosphate buffer saline; PFA, paraformaldehyde; SEM, standard error of measurement; ANOVA, analysis of variance; OIH, opioid-induced hyperalgesia; RIH, remifentanil-induced hyperalgesia.
Data Sharing Statement
The datasets used during the present study are available from the corresponding author upon reasonable request.
Author Contributions
Zhenhui Zhou: This author helped design the study, conduct the study, analyze the data, and write the manuscript; Meng Mao: This author helped conduct the study, analyze the data, and prepare the manuscript; Xuechun Cai: This author helped analyze the data and prepare the manuscript; Wei Zhu: This author helped design the study, prepare the manuscript; Jie Sun: This author helped design the study, analyze the data, prepare the manuscript, and obtain the research fund. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China (No.82071196), the Jiangsu Commission of Health (No. z201949) and the Basic Research Grant of Southeast University.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Yu EHY, Tran DHD, Lam SW, Irwin MG. Remifentanil tolerance and hyperalgesia: short-term gain, long-term pain? Anaesthesia. 2016;71(11):1347–1362. doi:10.1111/anae.13602
2. Comelon M, Raeder J, Stubhaug A, Nielsen CS, Draegni T, Lenz H. Gradual withdrawal of remifentanil infusion may prevent opioid-induced hyperalgesia. Br J Anaesth. 2016;116(4):524–530. doi:10.1093/bja/aev547
3. Zhang L, Shu R, Zhao Q, Li Y, Yu Y, Wang G. Preoperative butorphanol and flurbiprofen axetil therapy attenuates remifentanil-induced hyperalgesia after laparoscopic gynaecological surgery: a randomized double-blind controlled trial. Br J Anaesth. 2016;117(4):504–511. doi:10.1093/bja/aew248
4. Zhang L, Guo S, Zhao Q, et al. Spinal protein kinase mzeta regulates alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking and dendritic spine plasticity via kalirin-7 in the pathogenesis of remifentanil-induced postincisional hyperalgesia in rats. Anesthesiology. 2018;129(1):173–186. doi:10.1097/ALN.0000000000002190
5. Gao Y, Zhou S, Pan Y, Gu L, He Y, Sun J. Wnt3a inhibitor attenuates remifentanil-induced hyperalgesia via downregulating spinal NMDA receptor in rats. J Pain Res. 2020;13:1049–1058. doi:10.2147/JPR.S250663
6. Munoz F, Hu H. The role of store-operated calcium channels in pain. Adv Pharmacol. 2016;75:139.
7. Desvignes L, Weidinger C, Shaw P, et al. STIM1 controls T cell–mediated immune regulation and inflammation in chronic infection. J Clin Invest. 2015;125(6):2347–2362. doi:10.1172/JCI80273
8. Wei F, Vadakkan KI, Toyoda H, et al. Calcium calmodulin-stimulated adenylyl cyclases contribute to activation of extracellular signal-regulated kinase in spinal dorsal horn neurons in adult rats and mice. J Neurosci. 2006;26(3):851–861. doi:10.1523/JNEUROSCI.3292-05.2006
9. Kuang X, Liu Y, Chang Y, et al. Inhibition of store-operated calcium entry by sub-lethal levels of proteasome inhibition is associated with STIM1/STIM2 degradation. Cell Calcium. 2016;59(4):172–180. doi:10.1016/j.ceca.2016.01.007
10. Ryazantseva M, Goncharova A, Skobeleva K, et al. Presenilin-1 delta E9 mutant induces STIM1-driven store-operated calcium channel hyperactivation in hippocampal neurons. Mol Neurobiol. 2018;55(6):4667–4680. doi:10.1007/s12035-017-0674-4
11. Qi Z, Wang Y, Zhou H, et al. The central analgesic mechanism of YM-58483 in attenuating neuropathic pain in rats. Cell Mol Neurobiol. 2016;36(7):1035–1043. doi:10.1007/s10571-015-0292-5
12. Gao X, Xia J, Munoz FM, et al. STIMs and Orai1 regulate cytokine production in spinal astrocytes. J Neuroinflamm. 2016;13:1–13. doi:10.1186/s12974-016-0594-7
13. Gao R, Gao X, Xia J, et al. Potent analgesic effects of a store-operated calcium channel inhibitor. Pain. 2013;154(10):2034–2044. doi:10.1016/j.pain.2013.06.017
14. Gao XH, Gao R, Tian YZ, et al. A store-operated calcium channel inhibitor attenuates collagen-induced arthritis. Brit J Pharmacol. 2015;172(12):2991–3002. doi:10.1111/bph.13104
15. Qi F, Liu T, Zhang X, et al. Ketamine reduces remifentanil-induced postoperative hyperalgesia mediated by CaMKII-NMDAR in the primary somatosensory cerebral cortex region in mice. Neuropharmacology. 2020;162:107783. doi:10.1016/j.neuropharm.2019.107783
16. Cui W, Wang S, Han R, Wang Q, Li J. CaMKII phosphorylation in primary somatosensory cortical neurons is involved in the inhibition of remifentanil-induced hyperalgesia by lidocaine in male Sprague-Dawley rats. J Neurosurg Anesthesiol. 2016;28(1):44–50. doi:10.1097/ANA.0000000000000177
17. Zhou Y, Liu D, Chen S, et al. Cellular and molecular mechanisms of calcium/calmodulin-dependent protein kinase II in chronic pain. J Pharmacol Exp Ther. 2017;363(2):176–183. doi:10.1124/jpet.117.243048
18. Herring BE, Nicoll RA. Long-term potentiation: from CaMKII to AMPA receptor trafficking. Annu Rev Physiol. 2016;78(1):351–365. doi:10.1146/annurev-physiol-021014-071753
19. Cai Q, Zeng M, Wu X, et al. CaMKIIalpha-driven, phosphatase-checked postsynaptic plasticity via phase separation. Cell Res. 2021;31(1):37–51. doi:10.1038/s41422-020-00439-9
20. Wang Q, Zhao X, Li S, Han S, Peng Z, Li J. Phosphorylated CaMKII levels increase in rat central nervous system after large-dose intravenous remifentanil. Med Sci Monit Basic Res. 2013;19:118–125. doi:10.12659/MSMBR.883866
21. Ali ES, Rychkov GY, Barritt GJ. Metabolic disorders and cancer: hepatocyte store-operated Ca(2+) channels in nonalcoholic fatty liver disease. Adv Exp Med Biol. 2017;993:595–621.
22. Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996;64(3):493–501. doi:10.1016/0304-3959(95)01441-1
23. Mestre C, Pelissier T, Fialip J, Wilcox G, Eschalier A. A method to perform direct transcutaneous intrathecal injection in rats. J Pharmacol Toxicol Methods. 1994;32(4):197–200. doi:10.1016/1056-8719(94)90087-6
24. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Meth. 1994;53(1):55–63. doi:10.1016/0165-0270(94)90144-9
25. Sun J, Chen SR, Chen H, Pan HL. μ-Opioid receptors in primary sensory neurons are essential for opioid analgesic effect on acute and inflammatory pain and opioid-induced hyperalgesia. J Physiol. 2019;597(6):1661–1675. doi:10.1113/JP277428
26. Jensen TS, Finnerup NB. Allodynia and hyperalgesia in neuropathic pain: clinical manifestations and mechanisms. Lancet Neurol. 2014;13(9):924–935. doi:10.1016/S1474-4422(14)70102-4
27. Mercadante S, Arcuri E, Santoni A. Opioid-induced tolerance and hyperalgesia. CNS Drugs. 2019;33(10):943–955. doi:10.1007/s40263-019-00660-0
28. Santonocito C, Noto A, Crimi C, Sanfilippo F. Remifentanil-induced postoperative hyperalgesia: current perspectives on mechanisms and therapeutic strategies. Local Reg Anesth. 2018;11:15–23. doi:10.2147/LRA.S143618
29. Martorano PP, Aloj F, Baietta S, et al. Sufentanil-propofol vs remifentanil-propofol during total intravenous anesthesia for neurosurgery. A multicentre study. Minerva Anestesiol. 2008;74(6):233–243.
30. Roeckel L, Le Coz G, Gavériaux-Ruff C, Simonin F. Opioid-induced hyperalgesia: cellular and molecular mechanisms. Neuroscience. 2016;338:160–182.
31. Shu RC, Zhang LL, Wang CY, et al. Spinal peroxynitrite contributes to remifentanil-induced postoperative hyperalgesia via enhancement of divalent metal transporter 1 without iron-responsive element-mediated iron accumulation in rats. Anesthesiology. 2015;122(4):908–920. doi:10.1097/ALN.0000000000000562
32. Berna-Erro A, Braun A, Kraft R, et al. STIM2 regulates capacitive Ca2+ entry in neurons and plays a key role in hypoxic neuronal cell death. Sci Signal. 2009;2(93):a67. doi:10.1126/scisignal.2000522
33. Baba A, Yasui T, Fujisawa S, et al. Activity-evoked capacitative Ca2+ entry: implications in synaptic plasticity. J Neurosci. 2003;23(21):7737–7741. doi:10.1523/JNEUROSCI.23-21-07737.2003
34. Stathopulos PB, Schindl R, Fahrner M, et al. STIM1/Orai1 coiled-coil interplay in the regulation of store-operated calcium entry. Nat Commun. 2013;4:2963. doi:10.1038/ncomms3963
35. Gemes G, Bangaru ML, Wu HE, et al. Store-operated Ca2+ entry in sensory neurons: functional role and the effect of painful nerve injury. J Neurosci. 2011;31(10):3536–3549. doi:10.1523/JNEUROSCI.5053-10.2011
36. Szteyn K, Gomez R, Berg KA, Jeske NA. Divergence in endothelin-1- and bradykinin-activated store-operated calcium entry in afferent sensory neurons. Asn Neuro. 2015;7:2. doi:10.1177/1759091415578714
37. Chen Y, Yang C, Wang ZJ. Ca2+/calmodulin-dependent protein kinase II is required for the initiation and maintenance of opioid-induced hyperalgesia. J Neurosci. 2010;30(1):38–46. doi:10.1523/JNEUROSCI.4346-09.2010
38. Yuan Y, Sun Z, Chen Y, et al. Prevention of remifentanil induced postoperative hyperalgesia by dexmedetomidine via regulating the trafficking and function of spinal NMDA receptors as well as PKC and CaMKII level in vivo and in vitro. PLoS One. 2017;12(2):e171348.
39. Zhou J, Qi F, Hu Z, et al. Dezocine attenuates the remifentanil-induced postoperative hyperalgesia by inhibition of phosphorylation of CaMKIIα. Eur J Pharmacol. 2020;869:172882. doi:10.1016/j.ejphar.2019.172882
40. Voelkers M, Salz M, Herzog N, et al. Orai1 and Stim1 regulate normal and hypertrophic growth in cardiomyocytes. J Mol Cell Cardiol. 2010;48(6):1329–1334. doi:10.1016/j.yjmcc.2010.01.020
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.