Back to Journals » OncoTargets and Therapy » Volume 9
Stereotactic body radiation therapy for patients with recurrent pancreatic adenocarcinoma at the abdominal lymph nodes or postoperative stump including pancreatic stump and other stump
Authors Zeng X, Wang H, Meng M, Wu Z, Song Y, Zhuang H, Dong Q, Li F, Zhao L, Yuan Z, Wang P
Received 18 December 2015
Accepted for publication 18 May 2016
Published 30 June 2016 Volume 2016:9 Pages 3985—3992
DOI https://doi.org/10.2147/OTT.S102784
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Min Li
Xian-Liang Zeng,* Huan-Huan Wang,* Mao-Bin Meng, Zhi-Qiang Wu, Yong-Chun Song, Hong-Qing Zhuang, Dong Qian, Feng-Tong Li, Lu-Jun Zhao, Zhi-Yong Yuan, Ping Wang
Department of Radiation Oncology, Tianjin’s Clinical Research Center for Cancer and Key Laboratory of Cancer Prevention and Therapy, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Tianjin, People’s Republic of China
*These authors contributed equally to this work
Background and aim: The aim of this study is to evaluate the efficacy and safety of stereotactic body radiation therapy (SBRT) using CyberKnife in the treatment of patients with recurrent pancreatic adenocarcinoma at the abdominal lymph node or stump after surgery.
Patients and methods: Between October 1, 2006 and May 1, 2015, patients with recurrent pancreatic adenocarcinoma at the abdominal lymph node or stump after surgery were enrolled and treated with SBRT at our hospital. The primary end point was local control rate after SBRT. Secondary end points were overall survival, time to symptom alleviation, and toxicity, assessed using the Common Terminology Criteria for Adverse Events version 4.0.
Results: Twenty-four patients with 24 lesions (17 abdominal lymph nodes and seven stumps) were treated with SBRT, of which five patients presented with abdominal lymph nodes and synchronous metastases in the liver and lung. The 6-, 12-, and 24-month actuarial local control rates were 95.2%, 83.8%, and 62.1%, respectively. For the entire cohort, the median overall survival from diagnosis and SBRT was 28.9 and 12.2 months, respectively. Symptom alleviation was observed in eleven of 14 patients (78.6%) within a median of 8 days (range, 1–14 days) after SBRT. Nine patients (37.5%) experienced Common Terminology Criteria for Adverse Events version 4.0 grade 1–2 acute toxicities; one patient experienced grade 3 acute toxicity due to thrombocytopenia.
Conclusion: SBRT is a safe and effective treatment for patients with recurrent pancreatic adenocarcinoma at the abdominal lymph node or stump after surgery. Further studies are needed before SBRT can be recommended routinely.
Keywords: stereotactic body radiation therapy, pancreatic adenocarcinoma, recurrent disease, overall survival
Introduction
The prognosis of pancreatic cancer remains poor with a 5-year overall survival (OS) rate <6%.1,2 Surgical resection is the only curative treatment, but unfortunately, only 20% of patients appear to be candidates for surgery at diagnosis.3 Even patients who undergo resection have a poor prognosis with a 5-year survival rate of 7%–25% due to frequent development of local and/or distant metastases.4 The pattern of recurrence for patients with pancreatic cancer after surgery is well known;5,6 however, the most effective therapeutic strategies are not well defined. Some surgeons attempted to re-resect in a small number of patients with recurrent pancreatic carcinoma but achieved a 5-year OS of only ≤5.6%.7,8
The National Comprehensive Cancer Network recommends chemoradiotherapy for patients with local recurrence if they have not received radiotherapy (RT), and alternative systemic chemotherapy or palliative and best supportive care if they have received prior RT.9 As in the case of local advanced pancreatic cancer, the outcomes of these treatment strategies remain poor due to suboptimal local control (LC) and a poor median OS.10 Therefore, treatment opinion for patients with recurrent pancreatic carcinoma, especially recurrent pancreatic adenocarcinoma, is deemed required.
Stereotactic body radiation therapy (SBRT) is a type of external beam RT that delivers radiation more accurately and precisely to the tumor than conventionally fractionated radiation therapy. SBRT can be delivered using either a traditional linear accelerator or a robotic arm (ie, CyberKnife). The purpose of this study is to evaluate the safety and efficacy of SBRT using CyberKnife for patients with recurrent pancreatic adenocarcinoma at the abdominal lymph node or stump after surgery.
Patients and methods
Study design and eligible patients
We retrospectively queried our prospectively collected database of patients with recurrent pancreatic cancer. Patients were treated with SBRT using CyberKnife between October 1, 2006 and May 1, 2015. The inclusion criteria were the following: 1) any age; 2) Karnofsky performance score ≥70; 3) recurrent pancreatic adenocarcinoma after surgery, confirmed by biopsy and histology and either computed tomography (CT) or positron emission tomography/CT (PET/CT) images; and (4) written informed consent for the treatment. Exclusion criteria were 1) history of prior RT, 2) contraindication for receiving RT, and 3) uncontrolled comorbidities (metabolic or psychiatric). The study protocol was in accordance with the ethical guidelines of the Declaration of Helsinki and was approved by the independent ethics committees at Tianjin Medical University Cancer Institute and Hospital. In addition, all inclusion patients gave written informed consent.
Treatment schedule
The treatment planning and SBRT with CyberKnife was performed as previously described.11,12 The Xsight spine tracking system was used for patients with recurrent disease at the abdominal lymph node, and positional alignment was based on bony spinal skeletal structures. In contrast, it was recommended that gold fiducials were implanted at or near the recurrent stump for the gold fiducials tracking system which used B-ultrasound, endoscopic ultrasound, or CT guidance. Briefly, the patients were immobilized using a vacuum mattress before CT simulation.
A set of planning three-dimensional or four-dimensional CT images were obtained after intravenous radiographic contrast material infusion to highlight recurrent disease. The images included sufficient margins above and below the tumor, which were determined according to pretreatment planning CT and PET/CT images. The gross target volume (GTV) was defined as the volume of recurrent carcinoma, and the planning target volume (PTV) was defined as the GTV plus a margin of 0.3 cm in the x-, y-, and z-axis direction. The PTV was also amended to exclude the relevant anatomic boundaries when the target area was close to the duodenum, small bowel, or stomach in order to avoid damaging critical normal tissue. The normal tissue constraints were adopted from previous studies of advanced pancreatic carcinoma.13–15
Follow-up
The patients were observed at 1 month after completion of treatment, then every 3 months for the first year, and every 6 months thereafter until May 1, 2015. Imaging, adverse events, and compliance of all patients were monitored for the follow-up period using our clinical databases.
End points
The primary end point was the LC rate after SBRT, which was defined as complete response, partial response, or stable disease using the Response Evaluation Criteria in Solid Tumors version 1.1.16 Local failure was defined as evidence of increased tumor size in the treated region. PET/CT scan was employed to differentiate radiation-related changes from local or regional recurrence. LC of recurrent disease was assessed after a minimum of 6 months after SBRT in order to avoid uncertainty associated with early transient radiographic changes within the high-dose region. The secondary end points were the following: 1) OS from diagnosis and SBRT, defined as the time between diagnosis or SBRT and death or last follow-up for censored patients. 2) The time to symptom alleviation or pain reduction: the time to symptom alleviation was defined as the time between SBRT and the date at which symptoms were alleviated or the date of the last follow-up for censored patients, and pain was evaluated from the pain status rated by patients on a visual analog scale (VAS) before and after SBRT (a score of 0 indicated no pain and a score of 10 represented maximum pain). A researcher trained to conduct clinical interviews then contacted the patients to conduct clinical interviews in person via telephone at fixed intervals after their treatment sessions (at 1 day, 1 week, 2 weeks, and 1, 2, 3, and 6 months) or until the patients died or were lost to follow-up. 3) Toxicity was evaluated through Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Acute toxicities and late complications were recorded. All symptoms and complications documented in patient records during and following SBRT were recorded.
Statistical analysis
LC and OS rates were estimated using Kaplan–Meier analysis. Curves were compared using the stratified log-rank test. A P-value of ≤0.05 was considered to represent statistical significance. Data were analyzed using the statistical software Intercooled Stata version 8.2 for Windows (StataCorp LP, College Station, TX, USA).
Results
Patients’ characteristics
Between January 10, 2011 and June 19, 2014, 24 patients with 24 lesions (17 abdominal lymph nodes and seven stumps) were treated with SBRT using CyberKnife (Table 1). Five patients presented with abdominal lymph nodes and synchronous metastasis in the liver or lung. In addition to SBRT for abdominal lymph nodes, patients with synchronous liver metastasis received sequential palliative localized treatment, four patients with synchronous liver and lung metastases received adjuvant chemotherapy, and one patient with synchronous liver metastasis received no treatment.
  | Table 1 Summary of patient characteristics |
Treatment characteristics
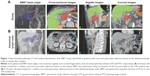
The treatment characteristics and treatment planning parameters for all patients are summarized in Table 2 and Figure 1. A total of 16 patients had negative margins (R0), four patients had microscopically positive margins (R1), and four patients had macroscopically positive margins (R2) after surgery. Eighteen patients had previously received gemcitabine-based chemotherapy, and three patients received other chemotherapy regimes.
The median PTV was 39.09 mL (range, 7.22–94.81 mL). Patients received a median of five fractions (range, five to eight fractions) with a median dose of 9 Gy per fraction (range, 6–10 Gy), and a total dose of 45 Gy (range, 42–50 Gy). The median biologically equivalent dose (α/β=10, linear-quadratic model used) was 85.50 Gy (range, 71.4–100 Gy). The dose was administered to the median 73% isodose line (range, 63%–79%), which encompassed 95% of the PTV (Table 3).
  | Table 3 Summary of SBRT parameters |
LC rate and OS
Out of 24 patients, five (20.8%) had a complete response, eight (33.3%) had a partial response, and nine (37.5%) had no response. The 6-, 12-, and 24-month actuarial LC rates were 95.2%, 83.8%, and 62.1%, respectively (Figure 2).
For the whole cohort, median follow-up time was 35.8 months (range, 14.2–72.7 months). The median OS from diagnosis and SBRT was 28.9 and 12.2 months, respectively (Figure 3A and B). It is worth noting that the rate of solitary recurrence and synchronous metastases did not differ significantly (P=0.31).
The time to symptom alleviation
Alleviation of symptoms was evaluated in 14 patients who reported symptoms (14/24, 58.33%, most commonly abdominal pain and back pain). Relief of symptoms was achieved in eleven patients (11/14, 78.57%), of which eight (8/14, 57.14%) reported complete relief of symptoms and discontinuation of medications. The remaining patients reported partial relief (3/14, 42.86%). Symptom improvement was reported after a median follow-up of 8 days (range, 1–14), and alleviation of symptoms continued throughout the follow-up period in all cases.
Pain was evaluated using a VAS score. VAS scores increased slightly from 7.2±2.5 at pre-SBRT to 8.9±1.2 24 hours after SBRT, and decreased from 7.2±2.5 at pre-SBRT to 2.7±1.3 1 week after SBRT. The VAS scores remained low throughout the follow-up period, 1.1±1.2 at 2 weeks, 1.4±1.3 at 1 month, 1.4±1.5 at 2 months, 1.3±0.9 at 3 months, and 1.2±1.4 at 6 months. Mean VAS differed significantly from the pre-SBRT baseline at each post-SBRT time point (all P<0.05).
Patterns of failure
Five patients (5/24, 20.8%) exhibited relapse within the PTV. Out-of-field progression was detected in 17 patients (17/24, 70.8%) within a median of 10.0 months after SBRT (range, 3.8–28.2 months), and in only two patients (2/24, 8.3%) was progression not observed after SBRT.
Toxicities
All patients completed SBRT without treatment breaks or dose reductions. As indicated in Table 4, nine patients (37.5%) experienced CTCAE version 4.0 grade 1–2 acute toxicities manifested as diarrhea, nausea, vomiting, abdominal pain, and agranulocytosis. One patient (4.2%) who received gemcitabine-based chemotherapy experienced grade 3 acute toxicity due to thrombocytopenia. These toxicities were generally transient and resolved with conservative management. No acute grade >3 toxicity or late toxicity was observed.
  | Table 4 Toxicities of pancreatic adenocarcinoma patients with recurrent disease treated with SBRT |
Discussion
The efficacy of treatments for recurrent pancreatic cancer following surgical resection is not well characterized. A majority of patients are not suitable candidates for surgery due to the presence of synchronous metastases, vascular involvement, or poor physical status, and for suitable patients, the rate of positive margin resection remains very high.7,8 To our knowledge, this is the first study to evaluate the safety and efficacy of SBRT for patients with recurrent pancreatic carcinoma at the abdominal lymph node or stump after surgery. Our results demonstrate that SBRT using CyberKnife is a safe and effective treatment for these patients. Further studies will be required to identify which patients benefit most from this treatment modality.
SBRT is an attractive therapeutic option due to its short duration and proven efficacy and safety, which has been reported in many earlier studies on patients with localized advanced pancreatic cancer.13–15,17–22 In the present study, the median OS of patients with recurrent pancreatic carcinoma treated with SBRT was 12.2 months; this is superior to the OS of 7.6–11.8 months of patients with localized advanced pancreatic carcinoma treated using SBRT,17–19 and also superior to the OS of patients with recurrent pancreatic carcinoma who underwent re-resection.7,8 However, the OS did not differ significantly between patients with solitary recurring and synchronous metastases, perhaps due to the small number of samples included, patients’ status, and/or the characteristic of recurrent pancreatic adenocarcinoma.
SBRT achieves promising LC rates in patients with recurrent pancreatic cancer. The 6-, 12-, and 24-month actuarial LC rates were 95.2%, 83.8%, and 62.1%, respectively. However, a total of 22 patients (22/24, 91.67%) exhibited either relapse within the PTV or progression outside of the field. Of these patients, five exhibited relapse within the PTV, and 17 patients exhibited out-of-field progression within a median of 10.0 months of SBRT. Therefore, SBRT should be combined with systemic therapies to reduce recurrence of pancreatic cancer after surgery, and more studies will be required to confirm these findings.
The treatment of recurrent pancreatic cancer is a continuing challenge because the disease typically causes symptoms that negatively impact patients’ quality of life, such as abdominal and back pain. These symptoms are not typically transient and cannot be resolved with conservative management. However, in this study, the transient increase in pain observed 24 hours after SBRT could be attributed to edema, and the majority of patients in our study achieved complete symptoms relief, typically within 2 weeks of treatment. Our findings concur with published studies suggesting that SBRT can reduce pain and improve quality of life of patients with localized advanced pancreatic cancer.23
The low-toxicity profile observed in our study is of particular importance in cancer patients, who have received or will receive additional oncologic therapies. In previous trials in which 25 Gy was delivered in one fraction, grade ≥3 toxicities were reported in 5%–18.8% of patients.18,24–26 However, some reports suggested that regimens of SBRT including two to five fractions for localized advanced pancreatic cancer are associated with better LC rates than those using single-fraction SBRT, and a lower incidence of high-grade toxicities.14,15,27 In this study, most patients experienced CTCAE grade 1–2 acute toxic events, and most of these symptoms were transient and resolved with conservative management. No late toxicity was reported, although the relatively short median OS may have underestimated the rate of late toxicities following SBRT.
To our knowledge, our study is the first to demonstrate the efficacy and safety of SBRT using CyberKnife for patients with recurrent disease at the abdominal lymph node or stump after surgery originating from pancreatic cancer. This study is limited by its retrospective nature, and as our patient group encompassed a range of treatment sites, disease severities, fractionation regimens, and systemic therapies, interpretation of the results is somewhat difficult. However, in light of the paucity of literature on the outcomes of SBRT for these patients, our results further recommend the clinical use of SBRT. Further investigation will be required to identify which patients benefit most from this treatment modality, particularly in combination with other treatment modalities.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No 81472797 and No 81201754) and the National Key Clinical Specialist Construction Programs of China (No N14B04). No benefits in any form have been or will be received from a commercial party directly or indirectly related to the subject of this article. We also thank the anonymous reviewer for his/her very helpful comments, which improved the quality of this paper.
Disclosure
The authors report no conflicts of interest in this work.
References
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. | ||
Thomas RM, Truty MJ, Nogueras-Gonzalez GM, et al. Selective reoperation for locally recurrent or metastatic pancreatic ductal adenocarcinoma following primary pancreatic resection. J Gastrointest Surg. 2012;16(9):1696–1704. | ||
Hartwig W, Werner J, Jäger D, Debus J, Büchler MW. Improvement of surgical results for pancreatic cancer. Lancet Oncol. 2013;14(11):e476–e485. | ||
Moningi S, Dholakia AS, Raman SP, et al. The role of stereotactic body radiation therapy for pancreatic cancer: a single-institution experience. Ann Surg Oncol. 2015;22(7):2352–2358. | ||
Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297(3):267–277. | ||
Sperti C, Pasquali C, Piccoli A, Pedrazzoli S. Recurrence after resection for ductal adenocarcinoma of the pancreas. World J Surg. 1997;21(2):195–200. | ||
Zacharias T, Oussoultzoglou E, Jaeck D, Pessaux P, Bachellier P. Surgery for recurrence of periampullary malignancies. J Gastrointest Surg. 2009;13(4):760–767. | ||
Kleeff J, Reiser C, Hinz U, et al. Surgery for recurrent pancreatic ductal adenocarcinoma. Ann Surg. 2007;245(4):566–572. | ||
NCCN clinical practice guidelines in pancreatic adenocarcinoma (Version 1). 2015. Available from: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf. Accessed June 21, 2016. | ||
Wilkowski R, Thoma M, Bruns C, Dühmke E, Heinemann V. Combined chemoradiotherapy for isolated local recurrence after primary resection of pancreatic cancer. JOP. 2006;7(1):34–40. | ||
Yuan ZY, Meng MB, Liu CL, et al. Stereotactic body radiation therapy using the CyberKnife system for patients with liver metastases. Onco Targets Ther. 2014;7:915–923. | ||
Meng MB, Wang HH, Zaorsky NG, et al. Clinical evaluation stereotactic radiation therapy for recurrent or second primary mediastinal lymph node metastases originating from non-small cell lung cancer. Oncotarget. 2015;6(17):15690–15703. | ||
Herman JM, Chang DT, Goodman KA, et al. Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer. 2015;121(17):1128–1137. | ||
Chuong MD, Springett GM, Freilich JM, et al. Stereotactic body radiation therapy for locally advanced and borderline resectable pancreatic cancer is efficacy and well tolerated. Int J Radiat Oncol Biol Phys. 2013;86(3):516–522. | ||
Mahadevan A, Miksad R, Goldstein M, et al. Induction gemcitabine and stereotactic body radiotherapy for locally advanced nonmetastatic pancreas cancer. Int J Radiat Oncol Biol Phys. 2011;81(4):e615–e622. | ||
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumors: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. | ||
Koong AC, Le QT, Ho A, et al. Phase I study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2004;58(4):1017–1021. | ||
Koong AC, Christofferson E, Le QT, et al. Phase II study to assess the efficacy of conventionally fractionated radiotherapy followed by a stereotactic radiosurgery boost in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2005;63(2):320–323. | ||
Schellenberg D, Goodman KA, Lee F, et al. Gemcitabine chemotherapy and single-fraction stereotactic body radiotherapy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2008;72(3):678–686. | ||
Mahadevan A, Jain S, Goldstein M, et al. Stereotactic body radiotherapy and gemcitabine for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2010;78(3):735–742. | ||
Goyal K, Einstein D, Ibarra RA, et al. Stereotactic body radiation therapy for nonresectable tumors of the pancreas. J Surg Res. 2012;174(2):319–325. | ||
Wei Q, Yu W, Rosati LM, Herman JM. Advances of stereotactic body radiotherapy in pancreatic cancer. Chin J Cancer Res. 2015;27(4):349–357. | ||
Polistina F, Costantin G, Casamassima F, et al. Unresectable locally advanced pancreatic cancer: a multimodal treatment using neoadjuvant chemoradiotherapy (gemcitabine plus stereotactic radiosurgery) and subsequent surgical exploration. Ann Surg Oncol. 2010;17(8): 2092–2101. | ||
Schellenberg D, Kim J, Christman-Skieller C, et al. Single-fraction stereotactic body radiation therapy and sequential gemcitabine for the treatment of locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2011;81(1):181–188. | ||
Didolkar MS, Coleman CW, Brenner MJ, et al. Image-guided stereotactic radiosurgery for locally advanced pancreatic adenocarcinoma results of first 85 patients. J Gastrointest Surg. 2010;14(10):1547–1559. | ||
Pollom EL, Alagappan M, von Eyben R, et al. Single- versus multifraction stereotactic body radiation therapy for pancreatic adenocarcinoma: outcomes and toxicity. Int J Radiat Oncol Biol Phys. 2014;90(4):918–925. | ||
Rwigema JC, Parikh SD, Heron DE, et al. Stereotactic body radiotherapy in the treatment of advanced adenocarcinoma of the pancreas. Am J Clin Oncol. 2011;34(1):63–69. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.