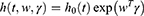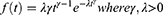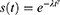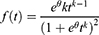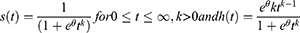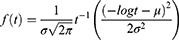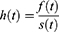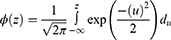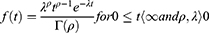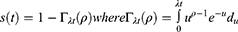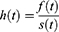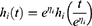Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 13
Statistical Analysis on Determinant Factors Associated with Time to Death of HIV/TB Co-Infected Patients Under HAART at Debre Tabor Referral Hospital: An Application of Accelerated Failure Time-Shared Frailty Models
Authors Birhan H , Derebe K , Muche S , Melese B
Received 11 May 2021
Accepted for publication 8 July 2021
Published 17 July 2021 Volume 2021:13 Pages 775—787
DOI https://doi.org/10.2147/HIV.S319745
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bassel Sawaya
Hailegebrael Birhan, Kenaw Derebe, Setegn Muche, Bezanesh Melese
Department of Statistics, Debre Tabor University Faculty of Natural and Computational Science, Debre Tabor, Amhara, Ethiopia
Correspondence: Hailegebrael Birhan Tel +251 9-15-28-91-30
Email [email protected]
Background: Human immune virus/tuberculosis co-infection in one’s immune system potentiates each other and hastening the weakening of the host’s immunological capabilities while growing active TB, which will increase susceptibility to primary contamination, re-contamination, and/or reactivation for sufferers with latent TB. The goal of this study was to identify determinant factors associated with the survival time to death of HIV/TB co-infected adult patients under HAART at Debre Tabor referral hospital.
Methods: A retrospective follow-up analysis was undertaken for 243 HIV/TB co-infected patients who were receiving ART treatment and had follow-ups between January 2014 and December 2019. To compare the survival experiences of different patient groups, the Log rank test was performed. The Weibull accelerated failure time gamma shared frailty model was used to find determinants of HIV/TB co-infected patients’ survival time.
Results: Among HIV/TB co-infected patients, 87 (35.39%) died of whom 77 (88.5%) patients were females. The Weibull AFT gamma shared frailty model showed that sex, baseline age, adherence status, educational status of respondents, functional status, WHO clinical stage, baseline hemoglobin and type of TB were among the potential determinants of survival time of HIV/TB co-infected patients. Furthermore, the findings of this study demonstrated that there is a clustering impact on patient time to death that results from the residency of HIV/TB co-infected patients’ survival time.
Conclusion and Recommendation: The majority of patients reside in rural area, have poor adherence to treatment, and have low CD4 cell counts. Educational status, WHO clinical stages, adherence status, and hemoglobin levels of patients are all important determinants of HIV/TB co-infected patients’ survival. As a result, to improve the survival of HIV/TB co-infected patients at the start of and during some stages of anti-TB treatment, the concerned body, FMOH, in collaboration with Regional Health Bureau, should emphasize the importance of following treatment for HIV/TB co-infected patients with poor adherence status, advanced WHO clinical stages, and a low CD4+ count.
Keywords: Weibull, accelerated failure time gamma shared frailty model, survival time, human immune virus/tuberculosis co-infection
Background
HIV/AIDS is one of the most common and fatal chronic illnesses in the world, accounting for a significant proportion of morbidity and mortality.1 Most HIV patients are exposed to infectious disease (TB), which is the most common infection once HIV infected persons and co-infected people are at high risk of death.2 TB can arise at any stage of HIV disease3,4 and people living with HIV are at a greater risk of acquiring TB than those who do not have HIV. Furthermore, when patients’ CD4 cell counts decline, their risk of mortality rises dramatically.4,5 A TB-infected individual has a 5–10% lifetime chance of developing clinical TB compared to an HIV-negative person and a 50% lifetime risk in an HIV-positive person. TB kills 90% of co-infected people if they are not treated within six months, even if they are taking their medication on a regular basis.2,6
Globally, more than 70 million individuals have been infected with HIV, and about 35 million have died as a result of the virus.1 According to a WHO report, Ethiopia is one of the world’s 20 high burden countries for HIV/TB co-infection.7 In Ethiopia, 40–70% of HIV patients are also infected with TB.8,9 However, to combat the transmission and deadliness of the diseases, WHO recommends different joint activities for HIV/TB co-infections like initiation of ART to reduce the risks of death and HIV-related morbidities, or improvement of quality of life for people living with HIV.10
HIV/TB co-infection constitutes several problems, including diagnostic and therapeutic challenges in healthcare settings and a person’s life.11–13 It is supported by a study conducted in the USA that revealed treatment of TB in co-infected patients differs from those patients who are infected with TB only.14 However, the number of people living with HIV/TB co-infection on ART is rapidly rising from year to year in the country, specifically in the northern part of Ethiopia. Due to this, this study has significant clinical merit because TB especially among HIV+ individuals may experience a more rapid development of the disease to acquired immune deficiency syndrome (AIDS) and lead to wasting, failure to thrive, and increased mortality due to TB itself or other opportunistic infections. Similarly, HIV increases the resistance of the TB mycobacterium to existing treatment regimens and leads to treatment failures and relapses. Therefore, identifying and understanding the factors affecting survival time could be of significant importance to any developing nation striving towards achieving adequate control over TB and HIV/AIDS. Additionally, this could also decrease the human suffering and economic burden associated with both diseases and to forward recommendation for the concerned body to realization of healthcare infrastructures to reduce diagnostic and clinical care challenges in the hospital.15
Moreover, numerous studies are being conducted associated with HIV/TB co-infection to investigate the risk factors associated with the survival time of HIV/TB co-infected patients in developing countries, including Ethiopia, and worldwide.12,16–23 While the majority of the studies used a small number of variables, this may have resulted in scholars making inefficient inferences about parameter estimates. In addition, most of these studies used survival time statistical analysis, which is assumption-based modeling without incorporating the existence of frailty. However, considering accelerated failure time shared frailty statistical modeling is more appropriate for such types of population data (survival correlated data) to account for association and unobserved heterogeneity rather than treating it as homogeneous by assuming that all individuals sampled in that study are subject in principle to the same risk as the risk of death.
Materials and Methods
Study Area and Study Design
A hospital-based retrospective follow-up study design was conducted on 243 HIV/TB co-infected patients under HAART at Debre Tabor referral Hospital, North central Ethiopia. The hospital serves all HIV/TB co-infected patients with mostly full-sized regional laboratory equipment.
Study Population and Data Collection
The target population for this study was all HIV/TB co-infected patients in the northern part of Ethiopia and the study population was HIV/TB co-infected patients receiving ART treatment at the Debre Tabor referral hospital. Secondary data is data that has been collected from individuals by health workers for treatment purposes. The data, therefore, is recorded on each patient’s card and documented in the ART section of the hospital, whose follow-ups are from January 2014 up to December 2019. A total of 1248 HIV/TB co-infected patients were under treatment whose follow-ups were from January 2014 to December 2019. Among these patients, 243 were HIV/TB co-infected patients and were considered as a sample. Hence, the sample size was determined using purposive sampling and taking 243 HIV/TB co-infected patients as eligible for the current study.
Study Variables and Inclusion Criteria
Outcome Variable
The current study’s outcome variable was the survival time to death in months of HIV/TB co-infected patients. Death is considered as the event of the study and the response time is the time when the patient dies. HIV/TB co-infected patients under ART who were alive up to the end of the study, lost, dropped out, transferred out to the nearest respective clinic for both cases and died due to other causes, are considered to be censored, while patients who took their treatment for either HIV or TB cases only in their respective nearest clinic and one case in Debre Tabor Referral hospital separately were not included in this study.
Independent Variables
Baseline CD4 cell count, body weight (baseline), age in years, marital status (single, married, separate, divorced, widowed), residence (urban, rural), educational level (non-educated, primary, secondary, tertiary), adherence status (good for patients with more than 85% treatment adherence, poor for patients with less than 85% treatment adherence), type of TB developed (pulmonary, extrapulmonary), WHO-clinical stages (stage-1, stage-2, stage-3, stage-4), functional status (ambulatory, bedridden, working), disclosure of the diseases (yes, no), sex (male, female), occupational status (unemployed, employed, others), social support (yes, no), opportunistic infectious disease (yes, no), baseline hemoglobin, BMI category (normal, underweight, overweight) and distance to ART clinic (less-than or equal to five kilometers (5Km), greater-than five kilometers (5Km)). The current study included all HIV/TB co-infected patients aged at least 15 years old who had at least two follow-ups at Debre Tabor referral hospital between January 2014 and December 2019.
Data Entry and Statistical Analysis
Data was coded and double entered into SPSS version 20 by two trained data clerks and then cross checked for consistency. Data was exported to STATA version 13 (Stata Corp, College Station, TX, USA) and R version 4.1.1 for data checking, cleaning, and analysis. During the preliminary analysis, we looked for errors and corrected them by rechecking the data collection form. AFT shared frailty regression analysis was done to identify independent variables associated with time to death. Variables with a P-value of <0.05 in the analysis were considered as significant predictors.
Methods of Data Analysis
In the current study, for data analysis, descriptive statistics and inferential statistical analysis were employed to identify determinant predictors of the response variable. The two common functions, the survivor and the hazard function, are used to present numerical or graphical summaries of survival time and summarize survival data in a specific group, as in using the Log rank test, the median survival time for the existence of the censored and positively skewed nature of survival time data.24
Cox Proportional Hazard Model
One of the most popular types of regression models used in survival analysis is the Cox proportional hazard model introduced by Cox.25 The Cox model estimates the hazard ratio, which is always non-negative, as well as its confidence interval. It is also based on the assumption of proportional hazards, and no particular form of probability distribution is assumed for the survival times. The hazard function is 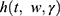 related to the covariates as a product of a baseline hazard
related to the covariates as a product of a baseline hazard 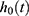 and a function of covariates
and a function of covariates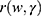 .The Cox proportional hazard function is given as:
.The Cox proportional hazard function is given as:
where 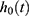 = the baseline hazard function that characterizes how the hazard function changes as a function of survival time;
= the baseline hazard function that characterizes how the hazard function changes as a function of survival time; 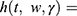 The hazard function at time t with covariates
The hazard function at time t with covariates 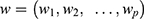 and a column vector of regression parameters
and a column vector of regression parameters ;
; 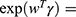 Characterizes how the hazard function changes as a function of subject covariates;
Characterizes how the hazard function changes as a function of subject covariates;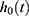 : is the baseline hazard function, and
: is the baseline hazard function, and 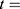 the failure time, where all values of the covariates are zero, i.e.
the failure time, where all values of the covariates are zero, i.e.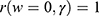 .
.
Parameter Estimation
Parameters in the current study were estimated using a maximum likelihood (ML) that can be obtained by maximizing the joint probability (likelihood function) for the values of the data.
Parametric Survival Models
Well-behaved, log-normal, log-logistic, and generalized gamma distributions are examples of popular parametric survival models.
Weibull Distribution
It is parameterized as both a proportional hazard (PH) and an accelerated failure time (AFT) model and it is suitable for modeling data with monotone hazard rates that either increase or decrease exponentially with time. The global distribution is as follows:
Log-Logistic Distribution
It is preferable in situations when the hazard function changes direction and could arise. The distribution is defined as:
The Lognormal Distribution
Defined for random variables that take positive values and so maybe used as a model for survival data. The distribution is given below as:
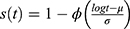 , where
, where 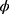 (.) is standard normal distribution function and given by:
(.) is standard normal distribution function and given by:
The Gamma Distribution
The probability density function of a gamma distribution is defined as follows.
Accelerated Failure Time Model
Is an alternative to Cox PH and parametric models for the analysis of survival time data. Unlike the proportional hazards model, the regression parameter estimates from AFT models are robust for omitted covariates, less affected by the choice of probability distribution, The effect of the covariates is a multiplication of the expected survival time and we measured the direct effect of the predictor variables on the survival time instead of hazard.26 The common distributions of the AFT model include exponential AFT, Weibull AFT, log-logistic AFT, log-normal and gamma AFT distributions. The following is a general formulation for the AFT hazard for an individual with covariates summarized in the vector.
Where,
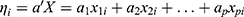 is the linear component of the model in which
is the linear component of the model in which 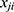 is the
is the 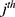 value of explanatory variable
value of explanatory variable 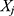 for the
for the 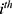 individual and
individual and 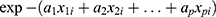 is acceleration factor, where
is acceleration factor, where 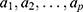 are unknown regression coefficients of the explanatory variables
are unknown regression coefficients of the explanatory variables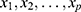 . The corresponding survivor function will be:
. The corresponding survivor function will be:
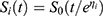 ; Where;
; Where; 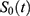 is the baseline survival function.
is the baseline survival function.
Results
Descriptive results: The baseline characteristics of participants for current investigation are indicated in Table 1.
 |
Table 1 Baseline Characteristics of Categorical Variables of HIV/TB Co-Infected Patients |
The characteristics of patients given in the table indicate that out of 243 patients, large numbers (78.6%) of patients were females of whom about 58.4% were rural residents and the majorities (46.5%) were married. Among the patients, most (44.4%) were employed, of whom 68.3% were able to work and 25.5% were ambulatory. 14.4% of co-infected patients were at clinical stage IV, 32.1% of the patients were non-educated, and 73.7% of the co-infected patients developed extrapulmonary Tuberculosis. In the study period, out of the patients under treatment, 35.8% of them died, whereas 64.2% were censored. Of all the patients who have died, the majority of them (31.7%) were females (Table 1).
The baseline characteristics of the continuous variables table also indicate that the average age of HIV/TB co-infected patients at enrollment in an ART clinic was 33.1 years with a standard deviation of 7.7 years. The average median initial CD4 cell count of HIV/TB co-infected patients was 357.1 with a standard deviation of 75.8 and the mean weight of HIV/TB co-infected patients at baseline was 53.3 with a standard deviation of 9.5. In addition, the mean baseline hemoglobin level of co-infected patients is 11.3 (g/dL) with a standard deviation of 2.5 (g/dL) (Table 2).
 |
Table 2 Baseline Characteristics of Continuous Variables of HIV/TB Co-Infected Patients |
Test of Proportional-Hazards Assumption
In order to validate the Cox proportional hazard model assumption, the Schoenfeld residuals and the formal statistical test were conducted to check the proportional assumption. In the plots of Schoenfeld residuals for each covariate against time in Figures 1–5 for covariates of HIV/TB co-infected patients showed that the existence of a pattern of randomness and the smooth curve is not like a horizontal straight line and the slope is far from zero. Therefore, the PH assumption is not satisfied. Even so, the graphical test is not enough to be certain of the proportionality assumption of the model. The reason for this is that it is open to different interpretations by different people. Hence, the proportional hazards assumption is tested using a formal statistical test that reveals the p-value of the rho-statistic (global test) is less than 5% for a given covariate, which indicates the rejection of the null hypothesis of the proportionality of the Cox-proportional hazard model (Table 3).
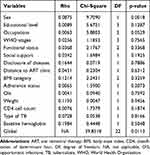 |
Table 3 Result for Test of Proportional-Hazards Assumption |
 |
Figure 1 Test of PH assumption for the covariate time versus educational level. |
 |
Figure 2 Test of PH assumption for the covariate time versus functional status. |
 |
Figure 3 Test of PH assumption for the covariate time versus occupational status. |
 |
Figure 4 Test of PH assumption for the covariate time versus WHO clinical stages. |
 |
Figure 5 Test of PH assumption for the covariate time versus religion. |
Accelerated Failure Time Model
Because the proportional hazard assumption was not met, we used a robust and alternative model, the accelerated failure time model, with a Weibull, log-normal, and log-logistic distribution as a baseline distribution, to analyze the survival data. Among those, the final reduced best model for describing the given HIV/TB co-infected patient data is the Weibull AFT model due to having the smallest AIC (219.057) and BIC (334.328) values (Table 4).
 |
Table 4 Result for Selection of Accelerated Time Failure Model |
Parametric Shared Frailty Model Results
Among the AFT baseline distributions, the selected appropriate distribution for the current study that was used to analyze the survival time of HIV/TB co-infected patients is the Weibull accelerated failure time shared frailty model, since it has smaller values of AIC (221.057) and BIC (339.821). In this study, the variance of frailty was significant for all baseline distributions with a gamma and an inverse Gaussian shared frailty distribution. This significance indicates the existence of random components (heterogeneity) in the population cluster, and it indicates that using a frailty model is appropriate (Table 5).
 |
Table 5 Result for Comparison of Gamma and Inverse Gaussian Shared Frailty Model with Different Baseline Distribution |
The Weibull Gamma Shared Frailty Model Result
Frailty is assumed to have a gamma distribution with a mean of one and a variance equal to theta (θ). The likelihood ratio test for the frailty parameter (θ) is significant with a P-value of less than 0.05 and a chi-square value of 1.38 with one degree of freedom. This shows that incorporating group variable (residence) as frailty component had a significant contribution to the model; in addition, the frailty term indicates that there is heterogeneity between residences. Our research also revealed the Kendall’s tau (τ=0.0061) value for the Weibull-gamma frailty model, which measures the presence of dependence within clusters. Moreover, this study shows that the shape of the hazard functions increases as time increases, since the estimated value of the shape parameter for the Weibull-gamma frailty model was greater than unity (p=6.4045) (Table 6).
 |
Table 6 Result for Weibull-Gamma Shared Frailty Model for HIV/TB Co-Infected Patients |
Sex
The estimated acceleration factor for patients who were male was ɸ = 1.1009 [95% CI: 1.0340–1.1720, p < 0.05]. This means that male co-infected patients’ survival has increased when compared to female patients.
Follow-Up Time
The estimated acceleration factor for follow-up time ɸ = 1.0187 [95% CI: 1.0122–1.0252, p < 0.05]. This shows that the survival time of co-infected patients can be prolonged as their follow-up time rises.
CD4+ Count
The estimated accelerated factor for patients CD4+ count was ɸ = 0.9998 [95% CI: 0.9979–0.9999, p < 0.05]. This reveals that the rate of survival for patients with HIV/TB co-infection decreases as the CD4+ count decreases.
Adherence Status
The estimated accelerated factor for patients who were good adherent ɸ = 0.9402 [95% CI: 0.9073–0.9743, p<0.05]. This indicates that the survival time of patients was shortened when they did not adhere regularly to the treatments in the ART clinic.
Hemoglobin Level
The estimated accelerated factor for patients’ hemoglobin level was ɸ = 0.9800 [95% CI: 0.9726–0.9875, p < 0.05]. This shows that, when the patients’ hemoglobin levels were reduced, the survival time of patients was shortened by a factor of 0.9800.
Distance to ART Clinic
The estimated accelerated factor for patients comes from far away more than 5km to ART clinic was ɸ = 0.8948 [95% CI: 0.8618–0.9389, p < 0.05]. This indicates HIV/TB co-infected patients who come from more-than 5km away from the hospital have a shortened survival time compared to patients who come from less-than 5km away from the hospital.
Functional Status
Patients with working and bedridden functional status had ɸ = 1.1398 [95% CI: 1.0933–1.1833, p < 0.05] and ɸ = 1.0673 [95% CI: 1.0221–1.1144, p < 0.05], respectively. These results showed that patients whose functional status was working and bedridden survival time was prolonged by a factor of 1.1398 and 1.0673 times greater-than those who were ambulatory at the initiation of treatment, respectively.
WHO Stages
For patients at advanced WHO stages III and IV stages, the estimated accelerated factor ɸ = 0.9079 [95% CI: 0.8667–0.951, p < 0.05] and ɸ = 0.6212 [95% CI: 0.5687–0.6785, p < 0.05] in comparison to that of stages I. This revealed that patients in stage III and IV have had survival time reduced by factors of 0.9079 and 0.6212, respectively.
Education Level
For patients’ educational status at primary, secondary, and tertiary level, the accelerated factor ɸ = 1.0539 [95% CI: 1.0153–1.0940, p < 0.05], ɸ = 1.1726 [95% CI: 1.1127–1.2355, p < 0.05] and ɸ = 1.1722 [95% CI: 1.1158–1.2295, p < 0.05], respectively, compared to non-educated implies that; increasing survival time of patients.
Marital Status
The covariate marital status was statistically determined for time to death of HIV/TB co-infected patients. The acceleration factor and its 95% confidence interval of marital status for a group of married, separate, divorced, and widowed was ɸ = 1.0887 [95% CI: 1.0460–1.1330, p < 0.05], ɸ = 0.8071 [95% CI: 0.7366–0.8843, p < 0.05], ɸ = 1.2113 [95% CI: 1.1505–1.2753, p < 0.05] and ɸ = 0.9018 [95% CI: 0.8491–0.9576, p < 0.05], respectively. At a 5% level of significance, this showed that patients who were married and divorced had a longer survival time, whereas patients who were separated and widowed by their married parents had a shorter survival time compared to singles.
Opportunistic Infectious Diseases
Acceleration factor and its 95% CI for patients who have previous opportunistic infectious disease were ɸ = 0.9252 [95% CI: 0.8872–0.9646, p < 0.05] who had previously developed opportunistic infectious diseases were reduced by a factor of 0.9252 when compared to patients who had not developed them.
Discussion
Our finding revealed that age is an important socio-demographic predictor for the survival of co-infected patients, which is supported by a study conducted by Gezie and Mageda et al.17,18 This study also showed that CD4+ count was a significant predictor of survival of co-infected patients, in which as the CD4 cell count decreases, the survival of patients will reduce in line with another study.16–19 Furthermore, patients with good adherence to their treatment had a higher survival rate than those with poor adherence. This could be due to the immune system being rebuilt and the current CD4+ level being raised as a result of strict adherence to ART medications. This result is correlated with a study.20 Our study revealed that the TB site (type of TB developed) was a significant determinant factor for HIV/TB co-infection supported by a study.16,19
The significant result of our study demonstrates that WHO clinical stages (IV, III, and II) are factors of in the survival time of co-infected patients who have had a shortened survival time than patients who were at stage I. According to our findings, patients with advanced WHO clinical stages have a higher risk of developing tuberculosis and other opportunistic infections and this leads to a shorter survival time, which is also supported by previous longitudinal studies.12,18,20,27–29 This study also found that the functional status of the co-infected patients was a significant predictor of the survival time of patients, supported by another study.16,17,22,23,30 This finding found that male patients have a longer survival time than females. This finding is consistent with those of Taha et al and Mashimbye.21,31 This finding, however, contradicts other studies18,20 found no association between gender and patient survival time. This discrepancy might be due to sample size differences, study population and study periods or statistical models built to estimate the coefficients. For instance, the study was conducted in Biharamulo Tanzania using Cox regression analysis.18 This study also revealed that the patient’s educational level was the major significant determinant factor that affected the survival of the HIV/TB co-infected patients. This finding is consistent with the findings.21,31 In our study, being employed and other occupations were among the determinant factors that increased the survival times of HIV/TB co-infected patients. This result was supported by a study.31 Our findings again showed that patients’ hemoglobin status was the significant determinant factor that affected the survival time for HIV/TB co-infected patients, supported by Taha et al.21
Conclusion
In this study, the majority of the HIV/TB co-infected patients were found in rural areas. The Weibull AFT gamma shared frailty model showed that sex, baseline age, adherence status, educational status of respondents, functional status, WHO clinical stage, baseline hemoglobin and type of TB were significantly associated with HIV/TB co-infected patients’ survival time. Furthermore, the outcomes of this study showed that the residency of HIV/TB co-infected patients has a clustering effect on patient survival time at 5% level of significance.
Recommendation
As a result, to improve the survival of HIV/TB co-infected patients at the start of and during some stages of anti-TB treatment, the concerned body, FMOH, in collaboration with Regional Health Bureau and other health-related agencies, should emphasize the importance of following treatment for HIV/TB co-infected patients with poor adherence status, advanced WHO clinical stages, and a low CD4+ count.
Strengths and Limitations of the Study
In its design, we properly formulate the subcategories of the independent variables and covariates to control the existence of confounding. To remove the clustering effect, we used the AFT shared frailty model. Even if maximum effort has been done to maintain the data quality standards, data completeness could be an issue because of the lack of laboratory equipment and, due to this, some important socioeconomic and clinical variables, like viral load from the HIV/TB co-infected patients’ data, could be missed.
Abbreviations
AFT, accelerated failure time; AIC, Akaike’s information criteria; AIDS, acquired immune deficiency syndrome; ART, antiretroviral treatment; BIC, Bayesian information criteria; BMI, body mass index; CD4, classification determinant four; FMOH, Federal Ministry of Health; HAART, highly active antiretroviral treatment; HGB, hemoglobin; HIV, human immune deficiency virus; LRT, likelihood ratio test; OIS, opportunistic infectious diseases; PH, proportional hazard; TB, tuberculosis; WHO, World Health Organization.
Data Sharing Statement
We confirm that the research is based on secondary data obtained from Debre Tabor referral Hospital. The corresponding author can avail the data up on request.
Ethical Consideration
The data used in this study was collected previously by the health staff for treatment purpose/for diagnosis HIV/TB co-infection and to start ART. To use this previously collected data, Ethical approval certificate had been obtained from Debre Tabor University with reference number RCS/1221/2020. In data collection, there was no written or verbal consent from participants. The reason was, investigators did not get participants rather, and secondary data was obtained in patients’ chart. The Ethical approval committee approved for the use of this secondary data for current investigation. This study was conducted in accordance with the Declaration of Helsinki.
Acknowledgments
We are highly grateful to all the staff members of the ART clinic at Debre Tabor referral hospital for their unreserved support during the data collection. Furthermore, we would like to express our heartfelt appreciation to Debre Tabor University.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research received no specific grant from any funding agency in the public commercial, or not for profit sectors.
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. World Health Organization. Tuberculosis Report 2019. World Health Organization; 2019.
2. Corbett EL, Watt CJ, Walker N, et al. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163(9):1009–1021. doi:10.1001/archinte.163.9.1009
3. Havlir DV, Getahun H, Sanne I, Nunn P. Opportunities and challenges for HIV care in overlapping HIV and TB epidemics. JAMA. 2008;300(4):423–430.
4. Sonnenberg P, Glynn J, Fielding K, et al. How soon after infection with HIV does the risk of tuberculosis start to increase? A retrospective cohort study in South African gold miners. J Infect Dis. 2005;191(2):150–158. doi:10.1086/426827
5. Getahun H, Gunneberg C, Granich R, et al. HIV infection—associated tuberculosis: the epidemiology and the response. Clin Infect Dis. 2010;50(Supplement_3):S201–S207. doi:10.1086/651492
6. World Health Organization. Strategic Framework to Decrease the Burden of TB/HIV. Geneva: World Health Organization; 2002.
7. World Health Organization. Global Tuberculosis Report 2019, in Global Tuberculosis Report 2019. 2019.
8. Kassu A, Mengistu G, Ayele B, et al. Coinfection and clinical manifestations of tuberculosis in human immunodeficiency virus-infected and-uninfected adults at a teaching hospital, northwest Ethiopia. J Microbiol Immunol Infect. 2007;40(2):116.
9. Demissie M, Lindtjørn B, Tegbaru B. Human immunodeficiency virus (HIV) infection in tuberculosis patients in Addis Ababa. Ethiop J Health Dev. 2000;14(3). doi:10.4314/ejhd.v14i3.9900
10. Nachega JB, Uthman OA, Ho Y-S, et al. Current status and future prospects of epidemiology and public health training and research in the WHO African region. Int J Epidemiol. 2012;41(6):1829–1846. doi:10.1093/ije/dys189
11. Nglazi MD, Bekker L-G, Wood R, et al. The impact of HIV status and antiretroviral treatment on TB treatment outcomes of new tuberculosis patients attending co-located TB and ART services in South Africa: a retrospective cohort study. BMC Infect Dis. 2015;15(1):536. doi:10.1186/s12879-015-1275-3
12. Wondimeneh Y, Muluye D, Belyhun Y. Prevalence of pulmonary tuberculosis and immunological profile of HIV co-infected patients in Northwest Ethiopia. BMC Res Notes. 2012;5(1):331. doi:10.1186/1756-0500-5-331
13. Swaminathan S, Padmapriyadarsini C, Narendran G. HIV-associated tuberculosis: clinical update. Clin Infect Dis. 2010;50(10):1377–1386. doi:10.1086/652147
14. Sterling TR, Pham PA, Chaisson RE. HIV infection—related tuberculosis: clinical manifestations and treatment. Clin Infect Dis. 2010;50(Supplement_3):S223–S230. doi:10.1086/651495
15. Reda AA, Biadgilign S, Deribew A, et al. Predictors of change in CD4 lymphocyte count and weight among HIV infected patients on anti-retroviral treatment in Ethiopia: a Retrospective Longitudinal Study. PLoS One. 2013;8(4):e58595. doi:10.1371/journal.pone.0058595
16. Hailu R, Eshetu W. Survival of HIV-TB co-infected adult patients under ART in Ambo referral hospital, Ethiopia. Ethiop J Health Dev. 2013;27(2):88–93.
17. Gezie LD. Predictors of CD4 count over time among HIV patients initiated ART in FelegeHiwot Referral Hospital, Northwest Ethiopia: multilevel analysis. BMC Res Notes. 2016;9(1):1–9. doi:10.1186/s13104-016-2182-4
18. Mageda K, Leyna GH, Mmbaga EJ. High initial HIV/AIDS-Related mortality and-its predictors among patients on antiretroviral therapy in the Kagera region of Tanzania: a five-year retrospective cohort study. AIDS Res Treat. 2012;2012:1–7. doi:10.1155/2012/843598
19. Nansera D, Bajunirwe F, Elyanu P, et al. Mortality and loss to follow-up among tuberculosis and HIV co-infected patients in rural southwestern Uganda. Int J Tuberc Lung Dis. 2012;16(10):1371–1376. doi:10.5588/ijtld.11.0589
20. Seyoum D, Degryse J-M, Kifle Y, et al. Risk factors for mortality among adult HIV/AIDS patients following antiretroviral therapy in Southwestern Ethiopia: an assessment through survival models. Int J Environ Res Public Health. 2017;14(3):296. doi:10.3390/ijerph14030296
21. Taha M, Deribew A, Tessema F, et al. Risk factors of active tuberculosis in people living with HIV/AIDS in southwest Ethiopia: a Case Control Study. Ethiop J Health Sci. 2011;21(2):131–140. doi:10.4314/ejhs.v21i2.69053
22. Temesgen A, Gurmesa A, Getchew Y. Joint modeling of longitudinal CD4 count and time-to-death of HIV/TB co-infected patients: a case of Jimma University Specialized Hospital. Ann Data Sci. 2018;5(4):659–678. doi:10.1007/s40745-018-0157-0
23. Sinshaw Y, Alemu S, Fekadu A, et al. Successful TB treatment outcome and its associated factors among TB/HIV co-infected patients attending Gondar University Referral Hospital, Northwest Ethiopia: an Institution Based Cross-Sectional Study. BMC Infect Dis. 2017;17(1):132. doi:10.1186/s12879-017-2238-7
24. Hosmer DW
25. Cox DR. Regression models and life‐tables. J R Stat Soc Series B Stat Methodol. 1972;34(2):187–202.
26. Lambert P, Collett D, Kimber A, et al. Parametric accelerated failure time models with random effects and an application to kidney transplant survival. Stat Med. 2004;23(20):3177–3192. doi:10.1002/sim.1876
27. Seid A, Getie M, Birlie B, Getachew Y. Joint modeling of longitudinal CD4 cell counts and time-to-default from HAART treatment: a comparison of separate and joint models. Electron J Appl Stat Anal. 2014;7(2):292–314.
28. Adams M, Luguterah A. Longitudinal analysis of change in CD4+ cell counts of HIV-1 patients on antiretroviral therapy (ART) in the Builsa district hospital. Eur Sci J. 2013;9(33).
29. Seyoum A, Zewotir T, Zewotir T. Quasi-Poisson versus negative binomial regression models in identifying factors affecting initial CD4 cell count change due to antiretroviral therapy administered to HIV-positive adults in North–West Ethiopia (Amhara region). AIDS Res Ther. 2016;13(1):36. doi:10.1186/s12981-016-0119-6
30. Tarekegn S. The Effect of HAART on Incidence of Tuberculosis Among HIV Infected Patients in Hawassa University Referral Hospital. Addis Ababa: Addis Abba University; 2011.
31. Mashimbye L. Tuberculosis (TB) Treatment Outcomes in Adult TB Patients Attending a Rural HIV Clinic in South Africa (Bushbuckridge). 2009.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.