Back to Journals » Therapeutics and Clinical Risk Management » Volume 11
Statin therapy in patients with acute coronary syndrome: low-density lipoprotein cholesterol goal attainment and effect of statin potency
Authors Chinwong D, Patumanond J , Chinwong S, Siriwattana K, Gunaparn S, Joseph Hall J, Phrommintikul A
Received 9 October 2014
Accepted for publication 7 November 2014
Published 23 January 2015 Volume 2015:11 Pages 127—136
DOI https://doi.org/10.2147/TCRM.S75608
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Dujrudee Chinwong,1,2 Jayanton Patumanond,3 Surarong Chinwong,1 Khanchai Siriwattana,4 Siriluck Gunaparn,5 John Joseph Hall,6 Arintaya Phrommintikul5
1Department of Pharmaceutical Care, Faculty of Pharmacy, Chiang Mai University, Chiang Mai, Thailand; 2Clinical Epidemiology Program, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand; 3Center of Excellence in Applied Epidemiology, Faculty of Medicine, Thammasat University, Pathum Thani, Thailand; 4Division of Medicine, Nakornping Hospital, Chiang Mai, Thailand; 5Department of Internal Medicine, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand; 6Centre for Clinical Epidemiology and Biostatistics, School of Medicine and Public Health, Faculty of Health, University of Newcastle, Callaghan, NSW, Australia
Background: Elevated low-density lipoprotein cholesterol (LDL-C) is associated with an increased risk of coronary artery disease. Current guidelines recommend an LDL-C target of <70 mg/dL (<1.8 mmol/L) for acute coronary syndrome (ACS) patients, and the first-line treatment to lower lipids is statin therapy. Despite current guidelines and the efficacious lipid-lowering agents available, about half of patients at very high risk, including ACS patients, fail to achieve their LDL-C goal. This study assessed LDL-C goal attainment according to use of high and low potency statins in routine practice in Thailand.
Methods: A retrospective cohort study was performed by retrieving data from medical records and the electronic hospital database for a tertiary care hospital in Thailand between 2009 and 2011. Included were ACS patients treated with statins at baseline and with follow-up of LDL-C levels. Patients were divided into high or low potency statin users, and the proportion reaching the LDL-C goal of <70 mg/dL was determined. A Cox proportional hazard model was applied to determine the relationship between statin potency and LDL-C goal attainment. Propensity score adjustment was used to control for confounding by indication.
Results: Of 396 ACS patients (60% males, mean age 64.3±11.6 years), 229 (58%) were treated with high potency statins and 167 (42%) with low potency statins. A quarter reached their target LDL-C goal (25% for patients on high potency statins and 23% on low potency statins). High potency statins were not associated with increased LDL-C goal attainment (adjusted hazards ratio 1.22, 95% confidence interval 0.79–1.88; P=0.363).
Conclusion: There was no significant effect of high potency statins on LDL-C goal attainment. Moreover, this study showed low LDL-C goal attainment for patients on either low or high potency statins. The reasons for the low LDL-C goal attainment rate warrants further investigation.
Keywords: LDL-C goal attainment, statins, potency statins, high risk, propensity score
Introduction
Coronary artery disease is the leading cause of death globally,1 including in Thailand.2 The association between elevated low-density lipoprotein cholesterol (LDL-C) and increased risk of coronary artery disease is well established.3,4 Acute coronary syndrome (ACS) is an important clinical manifestation of coronary artery disease5 and usually occurs as a result of one of three problems, ie, unstable angina, non-ST segment elevation myocardial infarction, or ST segment elevation myocardial infarction, which is diagnosed by electrocardiography. Patients with ACS are at very high risk of further life-threatening cardiac events, so intensive LDL-C-lowering therapy is needed soon after diagnosis.3,4,6–8 Current guidelines therefore recommend more aggressive LDL-C targets for ACS patients compared with healthy patients (<200 mg/dL or <5.2 mmol/L) as per the updated National Cholesterol Education Program/Adult Treatment Panel III (NCEP/ATP III) guideline,3 and the guidelines of the European Society of Cardiology and the European Atherosclerosis Society (ESC/EAS)9 recommend an LDL-C goal of <70 mg/dL (<1.8 mmol/L).
The 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors, also known as statins, are considered the first-line pharmacological therapy for reducing LDL-C levels to prevent progression of coronary artery disease.3,9,10 Six statins are currently available in Thailand, including simvastatin, pravastatin, fluvastatin, atorvastatin, rosuvastatin, and pitavastatin. Although all statins have a similar therapeutic effect (class effect) by lowering lipids, their potency differs.11 Combination therapy with statins and other lipid-lowering agents (eg, ezetimibe, bile acid resins, or niacin) is recommended to achieve optimal reduction in LDL-C and minimize the risk of adverse effects from statin use. In addition, statins are one of the top groups with regard to drug expenditure in Thailand. Not all statins are listed in the National List of Essential Medicines (NLEM), which is used by public health insurance schemes in Thailand as the reference for the pharmaceutical benefit package.12,13 About 96% of the Thai population are covered by one of three public insurance schemes: a civil servant medical benefit scheme for government officers and their dependants; a social security scheme for private sector employees; and universal coverage for people who are not eligible for either the civil servant medical benefit scheme or social security scheme.12 Thus, the NLEM influences physicians’ choices of statins for LDL-C control in ACS patients.
Despite the current guidelines and efficacious lipid-lowering agents available, about half of very high-risk patients, including ACS patients, fail to achieve their LDL-C goal of <70 mg/dL.14–25 The highest success rate in achieving this goal came from a study in Hong Kong (83.1%),26 while the lowest reported success rate was in Greece (10%).22 However, studies of compliance with LDL-C levels of <70 mg/dL in very high-risk patients, especially ACS patients, are limited in Asia, especially in Thailand. Two observational studies in Thailand have shown a low proportion of attainment of an LDL-C goal <70 mg/dL in patients at very high risk for developing cardiovascular disease (Silaruks et al reported a rate of 11.6%21 and the CEPHEUS (CEntralized Pan-Asian survey on tHE Under-treatment of hypercholeSterolemia) Thailand survey reported 16.7%17).
Little is known about the effects of statins of differing potency with regard to achieving a target LDL-C <70 mg/dL in the real-world setting in Asia. This study investigated the success of ACS patients in achieving this goal, as well as any difference in effect of high potency statins versus low potency statins.
Materials and methods
Data source and data collection
This retrospective cohort study was performed at the Maharaj Nakorn Chiang Mai Hospital in the north of Thailand. This tertiary hospital serves patients in Chiang Mai province, which has a population of 1,600,000, and receives patients with complicated conditions referred from 17 other provinces in northern Thailand. The hospital has 1,400 patient beds, and provides care for an average of 1,300,000 outpatients and 48,000 inpatients annually. This study was approved by the research ethics committee, Faculty of Medicine, Chiang Mai University, Thailand, before commencement, and included patients diagnosed with ACS between January 2009 and December 2011.
Data were collected by a study nurse aware of the research protocol and a researcher. Patient information, including demographic data, comorbidities, risk factors for coronary artery disease, current medication, and laboratory results including lipid profiles (total cholesterol, LDL-C, high-density lipoprotein, and triglycerides) were retrieved from medical charts and the electronic hospital database.
We retrospectively selected all patients aged 18 years and over who were diagnosed with an ICD-10 (International Statistical Classification of Diseases and Related Health Problems, Tenth Revision) code of I20 (angina pectoris) or I21 (acute myocardial infarction) who were treated with statins during admission or from the discharge date between January 2009 and December 2011. All included patients needed to have two assessments of their LDL-C levels and had to have remained on statin therapy between the two assessments, ie, one assessment at baseline during their hospital admission (index date) and one at follow-up within 2 weeks to 1 year following the index date. Patients with a baseline LDL-C <70 mg/dL were excluded from the analysis (Figure 1).
  | Figure 1 Flow chart of patient selection. |
Exposure and outcome measurement
Patients were divided into two groups, as either high or low potency statin users. Patients in the high potency statin group were treated with simvastatin 40 mg, rosuvastatin 10 mg or 20 mg, atorvastatin 20 mg or 40 mg, or pitavastatin 2 mg daily which, based on previous studies, could be expected to achieve an LDL-C reduction of ≥40%.11,27,28 Patients on simvastatin 10 mg or 20 mg or pravastatin 40 mg daily were in the low potency statin group and had an expected LDL-C reduction <40%.11,27,28
The outcome target was achieving an LDL-C goal of less than 70 mg/dL (<1.8 mmol/L) according to the updated NCEP/ATP III3,10 and ESC/EAS guidelines9 during the follow-up period of 2 weeks to 1 year.
Data analysis
Applying a descriptive method, counts and percentages were reported for categorical variables, and the mean and standard deviation for continuous variables. Differences between groups were compared using Fisher’s Exact test for categorical variables or the independent t-test for continuous variables. Due to our use of an observational study design, which is prone to confounding factors, propensity scoring was used to adjust for confounding by indication.29–31 Using logistic regression, a propensity score was generated to estimate the probability of receiving high or low potency statins. The variables included in the propensity score were age, sex, diabetes mellitus, hypertension, serum creatinine, alanine aminotransferase, LDL-C at baseline, health insurance status of patients, and smoking status. The Cox proportional hazard model (adjusted for propensity score and stratified by spectrum of ACS) was used to assess the effect of statin potency on LDL-C goal attainment. In all cases, the statistical significance level was set as two-tailed and at a P-value <0.05. All statistical analyses were carried out using Stata version 12 software (StataCorp LP, College Station, TX, USA).
Results
A total of 1,089 patients diagnosed with ACS were identified. After excluding 693 patients (627 with missing data on LDL-C levels, and 66 patients with a baseline LDL-C <70 mg/dL), 396 patients were included in the final analysis (Figure 1). A comparison between the groups included and excluded from the final analysis showed no significant difference in demographics between the two groups, except that the included patients were younger than the excluded patients (64.4±11.9 years versus 67.8±12.7 years, respectively, P<0.001).
Sixty percent of the patients were men, about 60% were covered by the universal coverage scheme, and one-fifth were current smokers. Fifty-five percent were diagnosed as having ST segment myocardial infarction, 28% as having non-ST segment myocardial infarction, and 16% as having unstable angina. The top three reported atherosclerotic risk factors were hypertension (60%), dyslipidemia (38%), and diabetes mellitus (28%). Two-fifths were treated with percutaneous coronary intervention during their hospital stay. The most frequently used current medications were antiplatelet/anticoagulant drugs (97%), beta-blockers (84%), and angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (65%, Table 1). The baseline lipid profiles were 122.8±37.8 mg/dL for LDL-C, 40.3±11.1 mg/dL for high-density lipoprotein cholesterol, 143.4±79.1 mg/dL for triglycerides, and 191.5±46.3 mg/dL for total cholesterol (Table 2). Simvastatin was the most commonly prescribed statin and statin monotherapy was predominantly used in this study (Table 3).
  | Table 2 Baseline laboratory and lipid values of patients by statin potency (n=396) |
  | Table 3 Statin therapy in this study (n=396) |
Of the 396 ACS patients, 229 (57.8%) were treated with high potency statins and 167 (42.2%) with low potency statins. Both groups were similar with regard to demographic characteristics and risk factors for coronary artery disease. Patients covered by the universal coverage scheme were more often prescribed low potency statins, while those covered by the civil servant medical benefit scheme were more likely to receive high potency statins. Patients given high potency statins were more likely to have hypertension and dyslipidemia. Their pathology results and lipid profiles were similar, except that patients on high potency statins had higher total cholesterol and LDL-C levels at baseline (Tables 1 and 2). This suggests that the patients treated with high potency statins had more severe illness than those on low potency statins.
A quarter (24%) of the patients reached their target LDL-C, and there was no difference in LDL-C goal attainment between the high (24.9%) and low (23.4%) potency statin groups (Figure 2). The incidence rate of achieving the LDL-C goal was 2.0 per 1,000 person-days in patients with high potency statins and 1.7 per 1,000 person-days for those with low potency statins (Table 4). Patients using high potency statins were no more likely to reach their LDL-C target than patients on low potency statins (hazards ratio 1.15, 95% confidence interval 0.76–1.73, P=0.516), and the results remained the same after adjusting for propensity score (adjusted hazards ratio 1.22, 95% confidence interval 0.79–1.88, P=0.363, Table 5).
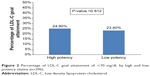  | Figure 2 Percentage of LDL-C goal attainment of <70 mg/dL by high and low potency statins (n=396). |
  | Table 4 Incidence of LDL-C goal attainment by statin potency (n=396) |
Discussion
LDL-C goal attainment
This clinic-based study in Thailand revealed that only a quarter of ACS patients (24%) attained their LDL-C goal of <70 mg/dL. Although the success rate of ACS patients achieving this goal was higher than in previous studies in Thailand,17,21 most did not achieve their LDL-C target with the statin therapy available at the hospital. These results are consistent with other studies in Asian countries and worldwide, ie, that less than half of patients at very high risk for cardiovascular disease attain their LDL-C target, even though there are several efficacious lipid-lowering medications available.14–25 Some studies have shown that less than 30% of high-risk patients reach their target LDL-C,17,21–24,32,33 while other studies reported that target LDL-C was achieved by 30%–45% of patients at high risk of cardiovascular disease.14–16,18–20,25
Inadequate statin therapy for lowering LDL-C might play a role in the failure of achieving target LDL-C. Most (98%) of the patients in our study used statin monotherapy, and simvastatin was the drug used most often, which is in line with other studies.17,18,26,34,35 According to the updated NCEP/ATP III and ESC/EAS guidelines, if the LDL-C goal is not achieved with statin monotherapy, combination therapy is recommended.3,9,10 Combination therapy that includes a statin plus another lipid-lowering agent (eg, ezetimibe, bile acid resins, or niacin) can achieve a considerable reduction in LDL-C levels, while also limiting the risk of dose-related adverse effects from statin therapy.3,9,10 Published studies have shown that the combination of a statin and ezetimibe is more effective than statin monotherapy in terms of lowering LDL-C and achieving the target of <70 mg/dL.36–42 Approximately 25% of patients in this study with LDL-C higher than 140 mg/dL at baseline would require combination therapy including a statin to achieve their target LDL-C. However, only seven patients (1.8%) were prescribed combination therapy. This is consistent with other studies reporting that statin combination therapy was used less frequently in routine practice.18,35,39,42 Although all treating physicians in this study were cardiologists, they were possibly reluctant to titrate statin doses upwards; there may be two reasons for this, ie, concern regarding potential adverse events, eg, an increase in muscle toxicity, and/or doubling the dose of a statin results in lowering LDL-C by only an additional 6%, ie, the “rule of 6”.43,44
The Thailand policy promoting the rational use of medicines may also play a role in the low rate of achievement of the LDL-C target. Thailand has adopted the NLEM to encourage rational drug use and to control drug cost in the country, so medicines listed in the NLEM can be prescribed for patients under the health insurance schemes, but patients have to pay for drugs not listed in the NLEM.12,13 Simvastatin was the only statin listed in the NLEM during the study period from 2009 to 2011.45 However, simvastatin 40 mg (the most commonly used high potency statin) can only reduce LDL-C by about 43%46 and cannot decrease the LDL-C level to <70 mg/dL in patients with a level >140 mg/dL at baseline. In this situation, atorvastatin, rosuvastatin or statin combination therapy should be used to lower LDL-C to the target level, but rosuvastatin and atorvastatin are not included in the NLEM. Our study suggests that physicians may have limited choices with regard to statin therapy for ACS patients, which impacts on LDL-C outcomes due to the regulations of the NLEM. A similar finding has been reported for Iceland, where a new reimbursement regulation was introduced in 2009 requiring patients to switch from atorvastatin, rosuvastatin, and pravastatin to simvastatin for the treatment of hyperlipidemia. After one year, the new reimbursement regulation resulted in an increase in cholesterol levels and decrease in the proportion of heart disease patients reaching the treatment goal.47
Poor adherence to statin therapy might explain the failure to attain the LDL-C goal in this real-world practice study, given that adherence to statins is positively related to achieving the LDL-C goal.48–50 Patients on statin therapy tend to decline in adherence after the initial prescription, and the 2-year adherence rate in ACS patients was reported to be only 40%.51 Patient adherence to statin therapy was not measured in this study, so further investigation of medication adherence in our population is warranted.
Effect of statin potency on LDL-C goal attainment
This study showed that treatment with a high potency statin was not associated with an increased likelihood of attaining the LDL-C goal in routine clinical practice. The effect of statin potency on reduction of LDL-C remains controversial in observational studies, although a positive relationship between statin potency and LDL-C goal attainment is well established in randomized controlled trials.7,8
This study is in agreement with certain other studies showing that the potency of the statin used does not increase the likelihood of reaching the recommended goal.15,18,35,52 However, the results from yet other studies indicate that patients treated with high potency statins are significantly more likely to achieve LDL-C control.22,32,53,54 The difference in results between these carious studies might reflect differences in the definition of high or low potency of statins used in the studies, which could affect the percent of LDL-C reduction and lead to differences in successful goal attainment. For instance, a study by Rallidis et al32 showed a positive relationship between the potency of the statin and LDL-C control, and the definition of intensive lipid-lowering medication was a medication that could lower LDL-C by more than 50%. These drugs include rosuvastatin 20–40 mg, atorvastatin 40–80 mg, simvastatin 80 mg daily, and the combination of a statin at a moderate or high dose with ezetimibe, a bile acid sequestrant, or niacin. In our study, high potency statins were defined as simvastatin 40 mg, atorvastatin 20–40 mg, rosuvastatin 10–20 mg, and pitavastatin 2 mg daily, based on a percent LDL-C reduction of ≥40%.11,27,28 These treatment regimens fall mostly into the low to moderate potency category used in the study by Rallidis et al.32
Other factors may have also influenced the results, such as comorbidities (particularly hypertension and dyslipidemia), individual variation in response to statin therapy, and variation in lifestyle and food modification. Patients on high-intensity statins had higher baseline LDL-C levels and a higher prevalence of hypertension and dyslipidemia, resulting in poorer LDL-C control. Individual patients may respond to statin therapy differently even at the same statin dose, resulting in different degrees of LDL-C reduction. Further, patients may differ significantly in their extent of lifestyle and food modification, which can also result in differing degrees of LDL-C reduction.
The timing of the follow-up visit may have influenced the results with regard to LDL-C goal attainment. A single follow-up visit between 2 weeks and 1 year after hospitalization for ACS was used in this study, whereas the follow-up duration in a study conducted in Europe and Canada was at least 3 months.53 We carried out a further analysis examining the relationship between statin potency and LDL-C goal attainment by varying follow-up duration (eg, 1 month, 2 months, 3 months, and 6 months), but the results remained the same. As mentioned earlier, the nonlinear decline in statin adherence after the initial prescription is a concern that could affect LDL-C goal attainment.51
Further, high potency statins are recommended in the new 2013 American College of Cardiology/American Heart Association (ACC/AHA) guideline for the treatment of blood cholesterol to reduce the atherosclerotic cardiovascular risk in adults (ie, the 2013 ACC/AHA Cholesterol Guidelines)55 and lipid modification in the National Institute for Health and Care Excellence (NICE) clinical guideline 181,56 due to the results of randomized controlled trials considered by the guideline writers. Uncertainty appears to remain with regard to this policy. Our findings are at odds with the recommendations of the 2013 ACC/AHA Cholesterol Guidelines55 and the latest NICE guidelines from the UK,56 which no longer recommend use of both LDL-L goals and ongoing monitoring of LDL-C levels. Rather, our findings support the ESC/EAS9 and 2014 National Lipid Association57 recommendation that maintaining the LDL-C goal and monitoring of LDL-C levels are beneficial for physicians and patients in following the patient’s progress. In this study, for example, monitoring of LDL-C was essential for identifying the 75% of patients who fail to achieve their LDL-C goal, and without monitoring, many ACS patients will be at increased risk of future cardiovascular events.
Strengths and limitations
To the best of our knowledge, this is the first study in Thailand that assesses the attainment of LDL-C <70 mg/dL in those patients with ACS in routine clinical practice. Previous studies investigated reaching LDL-C <100, or <70 mg/dL in patients with cardiovascular risk. All patients were treated by a cardiologist. While previous studies in Thailand have had a cross-sectional design such that no causal relationship could be determined, our longitudinal study allows associations to be made.
This study has some limitations. First, its retrospective design may be distorted by confounding factors; however, we attempted to adjust for this by use of the propensity score to control for confounding. Second, inclusion of patients who had complete lipid profiles in their electronic medical records at both at baseline and follow-up resulted in fewer patients being included. However, a comparison between patients included and those excluded from the study found no significant difference. Third, the sample size in this study is too small for evaluation of the effect of statin potency on LDL-C goal attainment. However, it is still possible to legitimately establish an association between statin potency and LDL-C goal attainment in ACS patients. Fourth, these findings are limited in terms of their generalizability given that all patients were from a university affiliated hospital and all were managed by cardiologists. Therefore, our findings should not be generalized to ACS patients who were managed by primary care physicians or are from other parts of Thailand. Nonetheless, these findings are applicable in other Asian countries where physicians predominantly use statin monotherapy at low to medium potency in patients at high cardiovascular risk.24 Fifth, statin adherence and titration of the dose during treatment were beyond the scope of this study. If extremely low adherence is equally distributed between high and low potency statin users, our finding of no significant difference in LDL-C goal attainment between these two groups could be anticipated. There is a need for further assessment of medication adherence in statin users and the effect of statin dose adjustment to meet LCL-C goals in practice settings.
Conclusion
Three-quarters of ACS patients failed to achieve their recommended LDL-C goal of <70 mg/dL, and use of high potency statins was not associated with increased LDL-C control. We believe that this study reflects the real-world practice situation of suboptimal LDL-C goal achievement in ACS patients who are at high cardiovascular risk. Hence, we encourage cardiologists to use LDL-C goal attainment as a target for therapy, and to monitor LDL-C levels in ACS patients in order to prevent further cardiovascular events. Improvement in achieving the LDL-C goal is required in clinical practice to improve outcomes in ACS patients. Further studies are needed to identify the reasons for low LDL-C control rates. In addition, the impact of the NLEM on LDL-C control in very high-risk patients (eg, those with ACS) needing more intensive statin therapy requires further evaluation.
Acknowledgments
The authors thank the Graduate School, Chiang Mai University, Thailand, for its financial support of this study, the authorities of Maharaj Nakorn Chiang Mai Hospital for their permission to use the data, Claudia Koller for assistance with editing this manuscript, and Yodi Christiani, Research Centre for Gender, Health and Ageing, University of Newcastle, for her valuable suggestions regarding preparation of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367(9524):1747–1757. | ||
Porapakkham Y, Rao C, Pattaraarchachai J, et al. Estimated causes of death in Thailand, 2005: implications for health policy. Popul Health Metr. 2010;8:14. | ||
Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110(2):227–239. | ||
Schwartz GG, Olsson AG, Ezekowitz MD, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA. 2001;285(13):1711–1718. | ||
Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005;111(25):3481–3488. | ||
Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350(15):1495–1504. | ||
de Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292(11):1307–1316. | ||
Ray KK, Cannon CP, McCabe CH, et al. Early and late benefits of high-dose atorvastatin in patients with acute coronary syndromes: results from the PROVE IT-TIMI 22 Trial. J Am Coll Cardiol. 2005; 46(8):1405–1410. | ||
Reiner Z, Catapano AL, De Backer G, et al. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J. 2011;32(14):1769–1818. | ||
Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486–2497. | ||
Weng TC, Yang YH, Lin SJ, Tai SH. A systematic review and meta-analysis on the therapeutic equivalence of statins. J Clin Pharm Ther. 2010;35(2):139–151. | ||
Tarn YH, Hu S, Kamae I, et al. Health-care systems and pharmacoeconomic research in Asia-Pacific region. Value Health. 2008;11 Suppl 1: S137–S155. | ||
Anothaisintawee T, Leelahavarong P, Ratanapakorn T, Teerawattananon Y. The use of comparative effectiveness research to inform policy decisions on the inclusion of bevacizumab for the treatment of macular diseases in Thailand’s pharmaceutical benefit package. Clinicoecon Outcomes Res. 2012;4:361–374. | ||
Chin CW, Gao F, Le T, Tan R. Lipid goal attainment and prescription behavior in asian patients with acute coronary syndromes: experience from a tertiary hospital. Clin Med Insights Cardiol. 2013;7:51–57. | ||
Kauffman AB, Olson KL, Youngblood ML, Zadvorny EB, Delate T, Merenich JA. Attainment of low-density lipoprotein cholesterol goals in coronary artery disease. J Clin Lipidol. 2010;4(3):173–180. | ||
Kitkungvan D, Lynn Fillipon NM, Dani SS, Downey BC. Low-density lipoprotein cholesterol target achievement in patients at high risk for coronary heart disease. J Clin Lipidol. 2010;4(4):293–297. | ||
Sukonthasarn A, Homsanit M, Prommete B, Chotinaiwattarakul C, Piamsomboon C, Likittanasombat K. Lipid-lowering treatment in hypercholesterolemic patients: the CEPHEUS Thailand survey. J Med Assoc Thai. 2011;94(12):1424–1434. | ||
Karalis DG, Victor B, Ahedor L, Liu L. Use of lipid-lowering medications and the likelihood of achieving optimal LDL-cholesterol goals in coronary artery disease patients. Cholesterol. 2012;2012:861924. | ||
Karalis DG, Subramanya RD, Hessen SE, Liu L, Victor MF. Achieving optimal lipid goals in patients with coronary artery disease. Am J Cardiol. 2011;107(6):886–890. | ||
Park JE, Chiang CE, Munawar M, et al. Lipid-lowering treatment in hypercholesterolaemic patients: the CEPHEUS Pan-Asian survey. Eur J Prev Cardiol. 2012;19(4):781–794. | ||
Silaruks S, Sriratanasathavorn C, Rawdaree P, Kunjara-Na-Ayudhaya R, Thinkhamrop B, Sritara P. Lipid-lowering therapy using statins in patients with cardiovascular risk in clinical practice in Thailand. Heart Asia. 2011;3:99–103. | ||
Xanthopoulou I, Davlouros P, Siahos S, Perperis A, Zaharioglou E, Alexopoulos D. First-line treatment patterns and lipid target levels attainment in very high cardiovascular risk outpatients. Lipids Health Dis. 2013;12(170):12–170. | ||
Andrikopoulos G, Tzeis S, Nikas N, et al. Short-term outcome and attainment of secondary prevention goals in patients with acute coronary syndrome-Results from the countrywide TARGET study. Int J Cardiol. 2013;168(2):922–927. | ||
Kim HS, Wu Y, Lin SJ, et al. Current status of cholesterol goal attainment after statin therapy among patients with hypercholesterolemia in Asian countries and region: the Return on Expenditure Achieved for Lipid Therapy in Asia (REALITY-Asia) study. Curr Med Res Opin. 2008;24(7):1951–1963. | ||
Melloni C, Shah BR, Ou FS, et al. Lipid-lowering intensification and low-density lipoprotein cholesterol achievement from hospital admission to 1-year follow-up after an acute coronary syndrome event: results from the Medications ApplIed aNd SusTAINed Over Time (MAINTAIN) registry. Am Heart J. 2010;160(6):1121–1129. | ||
Chan RH, Chan PH, Chan KK, et al. The CEPHEUS Pan-Asian survey: high low-density lipoprotein cholesterol goal attainment rate among hypercholesterolaemic patients undergoing lipid-lowering treatment in a Hong Kong regional centre. Hong Kong Med J. 2012;18(5):395–406. | ||
Mukhtar RY, Reid J, Reckless JP. Pitavastatin. Int J Clin Pract. 2005;59(2):239–252. | ||
Masana L. Pitavastatin – from clinical trials to clinical practice. Atheroscler Suppl. 2010;11(3):15–22. | ||
Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. | ||
Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. | ||
D’Agostino RB. Propensity scores in cardiovascular research. Circulation. 2007;115(17):2340–2343. | ||
Rallidis LS, Kotakos C, Sourides V, et al. Attainment of optional low-density lipoprotein cholesterol goal of less than 70 mg/dL and impact on prognosis of very high risk stable coronary patients: a 3-year follow-up. Expert Opin Pharmacother. 2011;12(10):1481–1489. | ||
Hermans MP, Castro Cabezas M, Strandberg T, et al. Centralized Pan-European survey on the under-treatment of hypercholesterolaemia (CEPHEUS): overall findings from eight countries. Curr Med Res Opin. 2010;26(2):445–454. | ||
Chaiyakunapruk N, Asuphol O, Dhippayom T, Poowaruttanawiwit P, Jeanpeerapong N. Statins utilisation pattern: a retrospective evaluation in a tertiary care hospital in Thailand. Int J Pharm Pract. 2011;19(2): 129–135. | ||
Van Ganse E, Laforest L, Alemao E, Davies G, Gutkin S, Yin D. Lipid-modifying therapy and attainment of cholesterol goals in Europe: the Return on Expenditure Achieved for Lipid Therapy (REALITY) study. Curr Med Res Opin. 2005;21(9):1389–1399. | ||
Stein E, Stender S, Mata P, et al. Achieving lipoprotein goals in patients at high risk with severe hypercholesterolemia: efficacy and safety of ezetimibe co-administered with atorvastatin. Am Heart J. 2004;148(3):447–455. | ||
Davidson MH, McGarry T, Bettis R, et al. Ezetimibe coadministered with simvastatin in patients with primary hypercholesterolemia. J Am Coll Cardiol. 2002;40(12):2125–2134. | ||
Friedman HS, Rajagopalan S, Barnes JP, Roseman H. Combination therapy with ezetimibe/simvastatin versus statin monotherapy for low-density lipoprotein cholesterol reduction and goal attainment in a real-world clinical setting. Clin Ther. 2011;33(2):212–224. | ||
Foody JM, Toth PP, Tomassini JE, et al. Changes in LDL-C levels and goal attainment associated with addition of ezetimibe to simvastatin, atorvastatin, or rosuvastatin compared with titrating statin monotherapy. Vasc Health Risk Manag. 2013;9:719–727. | ||
Davidson MH. Reducing residual risk for patients on statin therapy: the potential role of combination therapy. Am J Cardiol. 2005;96(9A):3K–13K. | ||
Mikhailidis DP, Lawson RW, McCormick AL, et al. Comparative efficacy of the addition of ezetimibe to statin vs statin titration in patients with hypercholesterolaemia: systematic review and meta-analysis. Curr Med Res Opin. 2011;27(6):1191–1210. | ||
Toth PP, Foody JM, Tomassini JE, et al. Therapeutic practice patterns related to statin potency and ezetimibe/simvastatin combination therapies in lowering LDL-C in patients with high-risk cardiovascular disease. J Clin Lipidol. 2014;8(1):107–116. | ||
Vaughan CJ, Gotto AM Jr. Update on statins: 2003. Circulation. 2004;110(7):886–892. | ||
Illingworth DR. Management of hypercholesterolemia. Med Clin North Am. 2000;84(1):23–42. | ||
National Drug Committee. National List of Essential Medicines 2008. Bangkok, Thailand: Sri-muang Kanpim; 2008. | ||
Ose L, Budinski D, Hounslow N, Arneson V. Comparison of pitavastatin with simvastatin in primary hypercholesterolaemia or combined dyslipidaemia. Curr Med Res Opin. 2009;25(11):2755–2764. | ||
Gizurarson S, Bjornsdottir LR, Einarsdottir R, Halldorsson M, Andersen K. Clinical consequences following regulatory changes in respect to reimbursement of statins cost by the Icelandic Social Insurance Administration. Scand J Public Health. 2012;40(7):663–667. | ||
Parris ES, Lawrence DB, Mohn LA, Long LB. Adherence to statin therapy and LDL cholesterol goal attainment by patients with diabetes and dyslipidemia. Diabetes Care. 2005;28(3):595–599. | ||
Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation. 2009;119(23):3028–3035. | ||
Bermingham M, Hayden J, Dawkins I, et al. Prospective analysis of LDL-C goal achievement and self-reported medication adherence among statin users in primary care. Clin Ther. 2011;33(9):1180–1189. | ||
Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA. 2002;288(4):462–467. | ||
Laforest L, Moulin P, Souchet T, et al. Correlates of LDL-cholesterol goal attainment in patients under lipid lowering therapy. Atherosclerosis. 2008;199(2):368–377. | ||
Gitt AK, Drexel H, Feely J, et al. Persistent lipid abnormalities in statin-treated patients and predictors of LDL-cholesterol goal achievement in clinical practice in Europe and Canada. Eur J Prev Cardiol. 2012;19(2):221–230. | ||
Ho KT, Chin KW, Ng KS, Alemao E, Rajagopalan S, Yin D. The A-SACT (Achievement in Singapore of Cholesterol Targets) study in patients with coronary heart disease. Am J Cardiovasc Drugs. 2006;6(6):383–391. | ||
Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2889–2934. | ||
National Institute for Health and Care Excellence. Lipid Modification: Cardiovascular Risk Assessment and the Modification of Blood Lipids for the Primary and Secondary Prevention of Cardiovascular Disease. London, UK: National Clinical Guideline Centre; 2014. Available from: http://www.nice.org.uk/guidance/cg181/resources/guidance-lipid-modification-cardiovascular-risk-assessment-and-the-modification-of-blood-lipids-for-the-primary-and-secondary-prevention-of-cardiovascular-disease-pdf. Accessed November 27, 2014. | ||
Jacobson TA, Ito MK, Maki KC, et al. National Lipid Association recommendations for patient-centered management of dyslipidemia: Part 1 – executive summary. J Clin Lipidol. 2014;8(5):473–488. |



 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at