Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Starting Point for Protocols on the Use of Hyperdiluted Calcium Hydroxylapatite (Radiesse®) for Optimizing Age-Related Biostimulation and Rejuvenation of Face, Neck, Décolletage and Hands: A Case Series Report
Authors Massidda E
Received 18 July 2023
Accepted for publication 7 November 2023
Published 29 November 2023 Volume 2023:16 Pages 3427—3439
DOI https://doi.org/10.2147/CCID.S420068
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Anne-Claire Fougerousse
Enrico Massidda1,2
1Private Practice in Aesthetic Medicine, Milano, Cagliari, Italy; 2Member of Società Internazionale di Medicina Estetica, Rome, Italy
Correspondence: Enrico Massidda, Email [email protected]
Abstract: Radiesse® is a dermal filler made of calcium hydroxylapatite, a natural component of the human body, which, in diluted and hyperdiluted forms, promotes, unlike other fillers, neocollagenesis, neoelastinogenesis, fibroblast proliferation, and angiogenesis, leading to a long-term improved skin quality, elasticity, tightening, and firmness. This case series examined the use of Radiesse® (Merz, Frankfurt, Germany) for skin rejuvenation and regeneration through a long-lasting action of collagen biostimulation. The report explored for the first time the use of different dilution ratios of Radiesse® in 50 patients of varying ages and skin needs. By combining microboluses, tunneling, and/or fanning techniques, Radiesse® was superficially injected in different body regions, including the full-face, neck, décolletage, and hands. The treatment was effective in improving skin thickness, laxity, and wrinkles in 95% of 30– 40 year-olds, 80% of 40– 60 year-olds, and 70% of > 60 year-olds, with an average improvement of 81.6% for the general population. The treatment was well-tolerated with no significant adverse effects reported. The report also describes specific cases and includes pictures comparing the baseline condition to the changes obtained after different months and Radiesse® sessions. The patients reported a clear improvement in skin firmness and brightness, as well as a visible improvement in wrinkles’ appearance. This report found that diluting and hyperdiluting Radiesse® with flexible dilution ratios favoured a treatment’s individualization, providing improved skin quality, elasticity, tightening, and firmness, without volume augmentation. In conclusion, it highlights the versatility and flexibility of Radiesse® and emphasizes its efficacy and safety in skin rejuvenation and regeneration.
Keywords: aesthetics, biostimulation, calcium hydroxylapatite, hyperdilution, Radiesse, skin rejuvenation
Introduction
Human cutaneous aging is an unwelcome, but physiological, process which progressively leads to a reduction and deterioration of skin structure integrity, a reduced efficiency and an impairment of its function.1,2 These chronological and degenerative events are also adversely influenced and accelerated by environmental factors, among which the main driver is the natural and artificial exposure to ultraviolet radiations.1,2 These phenomena are externally recognizable with the onset and gradual worsening of wrinkles, folds and rhytids, along with a reduction or loss of skin firmness; these alterations are predictably prominent on the most uncovered and exposed body regions, such as the face, neck, décolletage and hands.3
To slow down and address the exacerbation of not extensive aging signs, cosmetic surgery is becoming an increasingly less performed practice since a wide range of nonsurgical and advanced therapeutic options have broken into the market.4 In the aesthetic setting, the large treatment armamentarium for skin rejuvenation includes, among others, injectable dermal fillers. The majority of these medical devices improve wrinkles and skin laxity by providing a volume restoration and augmentation and are, therefore, used for filling purposes only. The ideal agent should not limit its function to volume enhancement but should be able to counteract the underlying mechanisms involved in the skin aging process.
With aging, at skin tissue level, the activity of fibroblasts and the quality of collagen undergo a gradual downturn and degradation, respectively; over time, fibroblast function is compromised and neocollagenesis is suppressed, resulting in a decrease and loss of skin structural integrity and elasticity.1,2,5
Radiesse® (Merz, Frankfurt, Germany) contains synthetic 25–45 μm-sized calcium hydroxylapatite (CaHA) microspheres, equivalent to the 30% of its formulation, suspended in a 70% aqueous gel matrix including glycerin (6.4%) and sodium carboxymethylcellulose (1.3%). CaHA is a substance naturally present in the human body since it is the physiologic inorganic constituent of bones and teeth, meaning that the product is fully biocompatible, biodegradable, non-mutagenic, non-antigenic and highly safe locally and systemically.2,6–12
Radiesse® is the only dermal device characterized by a well-defined dual mechanism of action: when used in an undiluted formulation, it can offer an immediate and instant volumization and a subsequent long-lasting collagen biostimulation.6 Nonetheless, when volumization is not the primary treatment objective, the diluted and hyperdiluted forms of Radiesse® can bypass the first volume augmentation effect, allowing to immediately exploit the biostimulation property of the product. Therefore, diluted and hyperdiluted Radiesse® can be used in specific categories of patients, where the treatment goal is to manage laxity and improve skin quality and tightening by providing a recompacting effect on the tissues through the biostimulation of neocollagenesis and angiogenesis.13
CaHA has a unique property which gives Radiesse® the capability of a minor swelling compared to hyaluronic acid-based fillers: through its innovative composition, Radiesse® does not draw water, because it is less hygroscopic, and, consequently, it does not induce swelling after injection.14 Instead, hyaluronic acid-based fillers are hygroscopic and, by drawing water, they generate a turgor effect.15
Furthermore, when the pure formulation of Radiesse® is used, its high viscosity and cohesivity may prevent a proper spread and distribution of the product. The past approach based on forcing the extrusion of the product from needle or cannula may result in localized accumulation at the injection site.16 Conversely, when Radiesse® is administered in diluted or hyperdiluted forms, although these formulations are still off-label, the limitations due to the viscosity and cohesivity are overcome, also further enhancing the versatility and flexibility of the product.16,17
Indeed, based on these two properties of versatility and flexibility, remarked in a systematic review and according to the experience of clinicians, including Italian ones, Radiesse® should be the workhorse of most medical aesthetic practices and the first choice for a global improvement of skin quality, given the 100% satisfaction rate reported by treated individuals and physicians.5,6,18
The objective of this paper is to provide new data on the efficacy of Radiesse® for skin tightening by differentiating the product hyperdilution degrees for different age groups and body regions. The final purpose is to present new modulated protocols for the use of Radiesse® in order to better customize the product’s hyperdilutions for the skin improvement of face, neck, décolletage and hands by age, for early intervention and prevention in younger subjects and aesthetic restoration treatment in older patients.
Materials and Methods
Patient Population
All Caucasian patients treated with Radiesse® in 2022–2023 were evaluated retrospectively. All patients were informed of the off-label use of this dermal device and signed an informed consent to participate in the study and for their images to be used. They also gave consent for publication of their images and cases in this case series report.
Since this is a case series on patients seen at the author’s specialist practice, approval from the ethics committee was not required.
Three different age groups were identified: 30–40-year-old patients (prevention approach), 40–60-year-old patients and patients aged >60 years (treatment approaches).
Hyperdilution Degrees for Each Age Group
- Preventive approach in 30–40-year-old patients: 1.5 mL of Radiesse® + 6 mL of saline (final dilution, 1:4)
- Treatment approach in 40–60-year-old patients: 1.5 mL of Radiesse® + 3–6 mL of saline (final dilution, 1:2; 1:4)
- Treatment approach in >60-year-old patients: 1.5 mL of Radiesse® + 1.5–3 mL of saline (final dilution ~1:1; ~1:2)
Injection Procedures for Each Targeted Area
The injections were performed at a subepidermal level, combining microboluses, tunneling and/or fanning techniques.
For face, neck and décolletage, Radiesse® was injected with a 27–30 Gauge needle and a cannula with 50 mm-length and 25 Gauge-diameter, while for the hands a cannula with 50 mm-length and 25 Gauge-diameter was used only (no needle) under sterile conditions.
The injection procedures were immediately followed by an initial massage performed by the physician in order to facilitate the distribution of the product in the treated areas. Each patient was instructed to self-massage the injection site twice a day for 3–7 days after the product injection, as recommended by de Almeida and colleagues.12 The massage of the diluted and hyperdiluted product allows to prevent its localized accumulation in the injection site and favours a more homogeneous product distribution. More it is diluted, the more it spreads horizontally and vertically, acting on a larger surface. The massage contributes to counteract the potential volumization effect and to determine the recompaction of tissues through the formation of fibroblasts and the stimulation of neocollagenesis and neoangiogenesis. The extent of the dilution depends on patient’s age, size of the area to be treated, skin thickness, and treatment objectives.12
The quantity of injected product into each area is the smallest possible. The contribution of the massage allows to cover the largest possible area and to facilitate the product distribution within the tissues of interest.
No local anaesthetic cream was applied. In some cases, 0.5 mL of local anaesthetic was used to reduce the discomfort of the treatment.
Number and Timing of Sessions for Each Age Group and Targeted Area
For each patient, three sessions were performed at 30 day-intervals. The schedule was repeated after 6–12 months depending on the patient’s needs, as evaluated by the physician at the follow-up visits, when the practitioner evaluated the extent of the response which is variable among different patients. In case of unsatisfactory response, the practitioner injected the hyperdiluted product again, but always in the smallest quantity possible.
Evaluation Instruments
Information on history of any previous aesthetic treatment or surgery and number of retreatments and complications or undesirable effects after injections were obtained from patient charts. Post-injection evaluations were made at 1 month, 2 months and 3 months and, if needed, at 6, 8, 10 and 12 months. All treated patients were invited to a free consultation in case of any problem. All patients had pre- and post-treatment photos for each targeted area.
At baseline and all follow-up visits, each patient’s skin thickness was evaluated by assigning a specific numeric grade on a qualitative scale, as reported in Table 1.
 |
Table 1 Scale for Skin Thickness Evaluation |
The practitioner’s evaluation was recorded at each visit with a descriptive assessment of the treatment results based on a numerical scale, as reported in Table 2.
 |
Table 2 Grading of the Improvement |
Baseline skin laxity and its improvement after Radiesse® treatment were graded by using the validated Merz Scale, based on a 5-point assessment from 0 (no wrinkles) to 4 (very severe wrinkles).19
Another valid indicator of the product effectiveness is the improvement in skin complexion and brightness, which can be evaluated by comparing the baseline skin condition, as visible in the photographic reports, with the skin appearance at the follow-up visits. This improvement reflects the expected angiogenic action exerted by the product and its favourable impact on the microcirculation.
Patients described their own perception of improvement and satisfaction verbally.
Results
Patient Case Studies
In total, 50 patients (45 female, 5 male) received Radiesse® injections, divided into age groups as follows:
- 30–40 year-old: 20 patients
- 40–60 year-old: 20 patients
- >60 year-old: 10 patients
Independent of the age groups, Radiesse® was used on 50 patients for face, and 30 patients for neck, décolletage and hands.
For each age group, the number of patients receiving Radiesse® for face, neck, décolletage and hands is reported in Table 3.
 |
Table 3 Number of Patients in Different Age Groups Receiving Radiesse® Divided by Targeted Areas |
In the general patient population, 10% of patients reported a mild improvement (grade 1) and 30% of patients experienced a moderate improvement (grade 2); improvement was graded as high (grade 3) and very high (grade 4) in 20% and 40% of the patients, respectively.
In the 20 patients included in the 30–40-year-old age group, the mean percentage of observed improvement in skin thickness and wrinkle change based on the Merz scale was 95%; 80% of the twenty 40–60-year-old patients achieved a visible improvement, which was also reported by 70% of the 10 patients in the >60-year-old group, with an average improvement for the general population of 81.6% (Figure 1).
 |
Figure 1 Percentage of patients improved after Radiesse® injection, as reported in the 3 age groups and in the general population. |
Some of the most representative cases of full-face intervention split for each age group are illustrated below.
Preventive Approach: 30–40-Year-Old Patients
Case 1
This female patient was administered 1.5 mL of Radiesse® + 6 mL of saline, for a final dilution of 1:4. At the baseline visit, she reported a facial skin thickness of grade 2 (thin) and a mild facial skin laxity and wrinkles (grade 1 on the Merz scale) (Figure 2a). The improvement achieved by the patient after 2 sessions of Radiesse® injection was graded as 4 (very good result) (Figure 2b).
 |
Figure 2 A 37-year-old female patient (P.M.) for a preventive approach. (a) Baseline visit. (b) At 8 months after 2 Radiesse® injection sessions. |
Case 2
This female patient was administered 1.5 mL of Radiesse® + 6 mL of saline, for a final dilution of 1:4. At the baseline visit, she presented with a facial skin thickness of grade 1 (very thin) and a mild facial skin laxity and wrinkles (grade 1 on the Merz scale) (Figure 3a). Radiesse® injection, after 1 session, allowed to achieve an improvement of 4 (very good result) (Figure 3b).
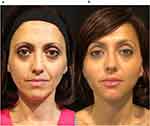 |
Figure 3 A 40-year-old female patient (F.F.) receiving the preventive approach. (a) Baseline visit. (b) At 6 months after one Radiesse® injection session. |
Treatment Approach: 40-60 Year-Old Patients
Case 3
This female patient was administered 1.5 mL of Radiesse® + 6 mL of saline, for a final dilution of 1:4. At the baseline visit, she reported a facial skin thickness of grade 2 (thin) and a mild facial skin laxity and wrinkles (grade 1 on the Merz scale) (Figure 4a). The improvement achieved by the patient after 2 sessions of Radiesse® injection was graded as 4 (very good result) (Figure 4b).
 |
Figure 4 A 42-year-old female patient (M.L.) for the treatment approach. (a) Baseline visit. (b) At 7 months after 2 Radiesse® injection sessions. |
Case 4
This female patient was treated with 1.5 mL of Radiesse® + 3 mL of saline (final dilution, 1:2). At baseline, her facial skin thickness was normal (grade 3) and the facial skin laxity and wrinkles were moderate (grade 2 on the Merz scale) (Figure 5a). The improvement reported after 3 sessions of Radiesse® injection was very good (grade 4) (Figure 5b).
 |
Figure 5 A 48-year-old female patient (A.S.) receiving a treatment approach. (a) Baseline visit. (b) At 10 months after 3 Radiesse® injection sessions. |
Treatment Approach: >60 Year-Old Patients
Case 5
This female patient was treated with 1.5 mL of Radiesse® + 3 mL of saline (final dilution, ~1:2). At baseline, her facial skin thickness was thin (grade 2) and the facial skin laxity and wrinkles were moderate (grade 2 on the Merz scale) (Figure 6a). The improvement reported after 3 sessions of Radiesse® injection was good (grade 3) (Figure 6b).
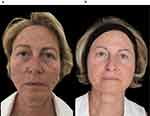 |
Figure 6 A 68-year-old female patient (M.R.) receiving a treatment approach. (a) Baseline visit. (b) At 12 months after 3 Radiesse® injection sessions. |
Neck, décolletage and hands were also targeted by using hyperdiluted Radiesse® according to the different approaches reported below, where results for each body region in three different patients are also presented (Figures 7–9).
Patients’ Perception and Satisfaction
Patients reported feeling a clearer and more glowing skin. They also declared to perceive a greater skin firmness and to observe a progressive reduction in skin laxity as well as a more harmonious facial contour. They did not experience any perception of swelling and observed gradual results, which stabilized after just a few days.
Safety
No relevant adverse effects were experienced by patients. Discomfort and downtime were minimal and determined by the injection act. All patients reported a reduction in discomfort in a few hours following the treatment, until it disappeared completely during the day of treatment. The observed tendency to bruising was minimal through the hyperdilution of Radiesse®, the slow administration and the cooling of the area of interest before and during the injection in all patients. In some cases, the topical use of an ointment for accelerating the bruising reduction was suggested.
Discussion
In the author’s experience, as herein reported, the objective examination of the patients treated with Radiesse® revealed an improvement in skin trophism, firmness and brightness, clearly attributable to the stimulating action of the newly formed collagen and the angiogenic action of Radiesse®, along with a recovery of facial profile convexities and an overall facial remodelling and reharmonization, as visible in the photographic reports.
Patients themselves reported appreciating a clearer and more glowing skin in the treated areas without swelling; they also stated that they had gradually observed the improvements and reached a final and stable result just a few days after treatment. The minimal discomfort and downtime and the reduced tendency to bruises can be explained by the recompacting of the tissues and, again, by the angiogenic action of Radiesse®.
In recent years, an increasing trend towards personalizing aesthetic treatments to make them minimally invasive and to match the individual patient’s needs has been observed.5 As herein reported and also demonstrated in different clinical trials, the unique dilution flexibility which characterizes Radiesse® allows the requirements of patients from different age groups to be addressed, providing optimal long-term improvements in skin tightening, texture, quality and rejuvenation.5 This has also been recently confirmed by the first Italian expert opinion on the properties, quality and reliability of Radiesse® based on a systematic literature review.5
Indeed, Radiesse® dilution and hyperdilution offer multiple advantages, fully observed and confirmed in this case series:
- As previously reported, the volumization effect, immediately observed by injecting the undiluted product, is bypassed and the less hygroscopicity determined by Radiesse® compared to hyaluronic acid-based fillers does not induce swelling or a turgor effect after injection.13–15 In specific categories of patients, this volume augmentation is not essential since the main treatment goal is to manage laxity and improve skin quality and tightening through the biostimulation of neocollagenesis.13
- When Radiesse® is used in diluted or hyperdiluted form, CaHA microspheres can be easily disseminated and spread in larger and deeper tissue areas. As a result, the concentration of particles within a short distance from the injection point and the consequent treatment compartmentalization are avoided.20 The greater lateral and deep dispersion and distribution of particles extend the benefits of the product to wider skin areas allowing for a full-face treatment.16
- When the primary treatment objective is not volume increase but the biostimulation of neocollagenesis and elastinogenesis, as in this case series, the diluted and hyperdiluted Radiesse® do not require to be injected deeply. Injections of diluted and hyperdiluted product in a more superficial plane (below the dermis in the dermal-subdermal plane) are feasible and highly effective, durable and safe, allowing the biostimulation and consequent remodelling and rejuvenation of thin skin surface, particularly in dynamic body regions, including neck and dorsal hands, without nodule formation, unevenness and product visibility.12,13,21–23
- The efficacy of the superficial introduction of diluted or hyperdiluted product can be further increased when the treatment with Radiesse® is subdivided into 1–3 sessions per year depending on the patient’s degree of skin laxity and damage and on the patient’s age, with maintenance injections every subsequent 12 to 18 months.21,23,24 This approach has shown to provide optimal and more prolonged results, to minimize product waste and to fix a long-term, even life-long, therapeutic plan, by exploiting one of the further advantages offered by Radiesse®: after opening the syringe for the first time, the unadministered product can be saved for future uses with the same patient for up to 3 months.4,24
- The use of diluted and hyperdiluted Radiesse® allows to reduce the number of injection sites, since the administration of the product in these forms seems to be best performed by using cannulas instead of needles, providing a more precise product introduction.13
- Furthermore, because of the minimal invasiveness of hyperdiluted Radiesse® and the superficial administration, this technique does generally not require the use of any anaesthetic and is well tolerated: the minimal adverse events reported so far, including bruising, swelling, mild pain, and induration, are attributable to the injection not to the product, and no severe or persistent complications and, in particular, no vascular occlusions have ever been recorded.13,21,22 In particular, by using the hyperdiluted Radiesse®, the tendency to bruising is reduced compared to other fillers or by using pure formulation of Radiesse® which, due to the greater density, can cause a higher risk of trauma. Hyperdilution therefore contributes to reducing the compressive trauma on the areas subjected to injection treatment and determines minimal vascular suffering of the tissues. In this case series, the appearance of bruising was observed in all patients and is directly related to various individual factors, including the state of the skin microcirculation, the patient’s hormonal status, the intake of anti-inflammatory medicines, the environmental heat or recent food intake. Furthermore, a slow administration of the product, as occurred in the patients in this study, helped reduce the injection trauma.
These advantages are further reinforced when a massage is performed by the physician in the targeted area immediately after the injection for a smooth product distribution. The patient should be asked to massage the treated area twice a day for 3–7 days to ensure a more complete and even Radiesse® distribution.12 In this case series, this practice was strictly performed by the physician and, at home, by the patients and contributed to the high safety of the procedure: indeed, the risk of haematomas, embolizations and compressions was minimized, and oedemas and swelling did not occur. These benefits are not generally observed by the author when using hyaluronic acid-based dermal fillers.
Two consensuses were released, both in 2016, on combined procedures for dermal face and body rejuvenation including the use of hyperdiluted Radiesse®, which was suggested for dermal rejuvenation in large body areas.25,26 In the Fabi et al consensus, in particular, CaHA diluted 1:1 or 1:2 with sterile saline or lidocaine, in addition to other procedures, was indicated as an early intervention and prevention to stimulate neocollagenesis for reducing fine wrinkling and improving the skin texture of neck, décolletage and hand dorsum, and as a first- or second-line treatment for an aesthetic restoration in the same skin areas.25
In 2018, Goldie and colleagues produced global consensus guidelines for the novel off-label subdermal injection of diluted (1:1) and hyperdiluted (≥1:2) Radiesse® with lidocaine or saline for skin tightening in the face and body.21 They examined the clinical results obtained with diluted and hyperdiluted product injected in mid- and lower face, neck, décolletage, upper arms, abdomen, upper legs and buttocks, providing recommendations on the number of treatment sessions, duration of time intervals between sessions, injection techniques, follow-up visits and assessments and maintenance injections, for best treating laxity and superficial wrinkles and improving skin quality without causing adverse reactions related to the device implantation.21
Another consensus specifically dedicated to the use of hyperdiluted Radiesse® as a face and body biostimulatory agent to improve skin quality and firmness has been published in 2019 by de Almeida and other 9 international experts.12 The recommendations reported in this consensus were based on the authors’ expertise and on a literature review and illustrated the most appropriate injection techniques and dilution ratios for different face and body areas, such as face, neck, décolletage, buttocks, thighs, abdomen, arms, knees and elbows.12
A very recent clinical practice guidance on the use of hyperdiluted Radiesse® for skin tightening was provided by Lorenc and other 11 expert physician injectors in 2022.13 The authors specified that their article was not developed as a clinical study but derived from their extensive clinical experience.13 In addition to a very clear rationale to support the advantages of the hyperdilution of Radiesse®, this global consensus includes many helpful suggestions and indications to facilitate the injection procedures (mainly by cannulas) and to perform appropriate Radiesse® dilution and hyperdilution to promote neocollagenesis and elastogenesis and, as a result, to improve skin pliability and dermal thickness in different body regions.13
In accordance with these consensus recommendations, the injection modalities and dilution ratios adopted in the current report were strictly individualized based on the patient’s age, baseline skin thickness and laxity, and demonstrated high efficacy, reproducibility and stability of results and a strong safety profile, along with a relevant product manageability.
Not least, indeed, in the current report, Radiesse® dilution and hyperdilution not only solved the issue of viscosity and resistant flow of the product during injection but also caused minor or absent post-injection oedema, bruising and erythema.
In a 2017 pilot study by Yutskovskaya and Kogan, 20 volunteers aged 35–45 years, with skin laxity in the neck and décolletage, received off-label multiple subdermal injections of Radiesse® diluted and hyperdiluted with preserved saline at baseline and 4 months as follows: 1:2 dilution (normal skin), 1:4 dilution (thin skin), and 1:6 dilution (atrophic skin). This study’s objectives were to assess the biostimulation effect on neocollagenesis and elastogenesis through immunohistochemical analyses on skin biopsies up to 7 months after Radiesse® treatment, to evaluate mechanical skin changes measured by ultrasound scanning and cutometry, and to assess the satisfaction of patients and practitioners based on the Global Aesthetic Improvement Scale. The authors reported a significant increase in type I and III collagen, with a decline of the latter after 7 months, in elastin production and angiogenesis compared with baseline, along with relevant improvements in skin elasticity and dermal thickness, high satisfaction levels among patients and professionals and a good tolerability profile. The authors were able to demonstrate the efficacy of this treatment approach with diluted and hyperdiluted Radiesse® in ameliorating skin quality, remodelling, tightening and durability in the targeted areas.3
Both Graivier and colleagues in 2018 and Figueredo et al in 2020 illustrated dilution strategies and injection techniques for hand rejuvenation using Radiesse® to optimize the aesthetic results and safety.22,27 Graivier et al reported a significant hand rejuvenation with Radiesse® diluted 1:1 with 1% lidocaine solution and the injection with a blunt cannula, which contributed to simplify the technique itself and to optimize the product safety profile and clinical outcomes.27 In the prospective, evaluator-blinded study by Figueredo et al, two injection techniques (through deep fat lamina or subdermal injection) for the treatment of dorsal hands with diluted Radiesse® were performed on 15 Brazilian 40–60-year-old women, demonstrating improvements in skin quality of aging hands and a high tolerability with diluted Radiesse®, regardless of the injection technique adopted.22
In 2020, Rovatti et al retrospectively explored the efficacy and safety of hyperdiluted Radiesse® 1:2 treatment for mid and lower face rejuvenation in 40 patients aged 38–72 years. The efficacy was evaluated with a validated severity 5-point scale based on external dermatologist assessments of subject pictures taken at baseline and at 4-month post-treatment. The dilution technique was described in detail by the authors: each 1.5 mL Radiesse® syringe was diluted with 0.5 mL of 1% lidocaine and 3 mL of 0.9% saline, and the final volume of the hyperdiluted product was injected for one half on the right side of the mid-lower face and the other half in the left side.28 According to the 2018 Goldie consensus guidelines, at least 20 passes between the syringes were performed to obtain the best product homogeneity.21,28 The combined mixture was immediately injected to avoid the separation of components. The treatment was performed with a 25 Gauge, 50-mm-long cannula: each injection released about 0.1 mL of mixture and all necessary measures and caution were adopted to avoid penetrating the vessels. The hyperdiluted Radiesse® 1:2 technique provided a significant improvement in 5 of 5 points of validated scales, with 87.5% of the patients very satisfied and the other 12.5% satisfied.28 Non-invasive analyses performed with a re-scan confocal microscope revealed variations in collagen morphology and increases in vascularization, supporting the clinical improvements and the successful decrease in aging severity scores observed in the patients. Mean pain score was low and only three minor adverse events (erythema, oedema and ecchymosis) were reported.28
In 2021, Fabi et al treated the décolletage of 20 women (mean age: 61.1 years) with a 1:2 dilution of Radiesse® (one 1.5 mL syringe of Radiesse® diluted with 3.0 mL bacteriostatic saline, immediately mixed prior to the injections) and evaluated the clinical outcomes at week 6, week 12, month 6 and month 12 using the validated Merz Décolletage Scales. Significant and durable improvements in décolleté dynamic and resting appearance of wrinkles were reported over time (P ≤ 0.01) and persisted for at least 1 year.23
A more recent study on the efficacy of hyperdiluted Radiesse® for the skin laxity of the neck, after the original pilot study by Yutskovskaya and Kogan, was carried out by Guida et al in 2021 on 20 patients with a mean age of approximately 55 years. A 1:2 dilution of Radiesse® (every 1.5 mL of Radiesse® syringe diluted with 2.5 mL of 0.9% saline solution and 0.5 mL of 1% lidocaine) was used and 20 passes were performed between the syringes to obtain a homogeneous mixture, which was immediately injected through a 25 Gauge, 50-mm-long cannula using a fanning technique. According to the neck skin laxity scale applied in this study, the hyperdiluted Radiesse® was significantly effective (P < 0.001) in improving the dermal thickness and the skin mechanical properties of the neck, also providing a good safety, with the occurrence of minor and self-limiting adverse events (redness, swelling, and bruising).29
Results as positive as these have not been reported by using other commercially available injectable dermal fillers, such as hyaluronic acid and poly-
Conclusions
To the best of the author’s knowledge, this is the first and largest case series of patients where Radiesse® was used in differentiated diluted and hyperdiluted forms to maximally exploit the biostimulation property of the product and to effectively and safely treat patients with different aesthetic needs in different body areas at any stage of the aging process.
Acknowledgments
The author expresses his appreciation to Brunilde Iovene for manuscript writing and editorial assistance.
Disclosure
The author reports no conflicts of interest in this work.
References
1. Fisher GJ, Varani J, Voorhees JJ. Looking older: fibroblast collapse and therapeutic implications. Arch Dermatol. 2008;144(5):666–672. doi:10.1001/archderm.144.5.666
2. Gonzaga da Cunha M, Gonzaga da Cunha AL, Garcia ME, da Silva Pinhal MA. Biostimulators and their mechanisms of action. Surg Cosmet Dermatol Rio de Janeiro. 2020;12(2):109–117.
3. Yutskovskaya YA, Kogan EA. Improved neocollagenesis and skin mechanical properties after injection of diluted calcium hydroxylapatite in the neck and décolletage: a pilot study. J Drugs Dermatol. 2017;16(1):68–74.
4. Jacovella PF. Use of calcium hydroxylapatite (Radiesse) for facial augmentation. Clin Interv Aging. 2008;3(1):161–174. doi:10.2147/CIA.S2065
5. Bartoletti E, Melfa F, Renzi M, Rovatti P. Systematic review of the literature on the properties, quality and reliability of calcium hydroxyapatite: results of an Italian experts’ meeting. Aesthetic Medicine. 2022;8(1):11–24.
6. Amselem M. Radiesse(®): a novel rejuvenation treatment for the upper arms. Clin Cosmet Investig Dermatol. 2015;9:9–14. doi:10.2147/CCID.S93137
7. Bernardini FP, Cetinkaya A, Devoto MH, Zambelli A. Calcium hydroxyl-apatite (Radiesse) for the correction of periorbital hollows, dark circles, and lower eyelid bags. Ophthalmic Plast Reconstr Surg. 2014;30(1):34–39. doi:10.1097/IOP.0000000000000001
8. Attenello NH, Maas CS. Injectable fillers: review of material and properties. Facial Plast Surg. 2015;31(1):29–34. doi:10.1055/s-0035-1544924
9. Corduff N, Chen JF, Chen YH, et al. Pan-Asian consensus on calcium hydroxyapatite for skin biostimulation, contouring, and combination treatments. J Clin Aesthet Dermatol. 2021;14(8):E76–E85.
10. Jansen DA, Graivier MH. Evaluation of a calcium hydroxylapatite-based implant (Radiesse) for facial soft-tissue augmentation. Plast Reconstr Surg. 2006;118(3):22S–30S. doi:10.1097/01.prs.0000234903.55310.6a
11. Sadick NS, Katz BE, Roy D. A multicenter, 47-month study of safety and efficacy of calcium hydroxylapatite for soft tissue augmentation of nasolabial folds and other areas of the face. Dermatol Surg. 2007;33(Suppl 2):S122–S126. doi:10.1111/j.1524-4725.2007.33351.x
12. de Almeida AT, Figueredo V, Gonzaga da Cunha AL, et al. Consensus recommendations for the use of hyperdiluted calcium hydroxyapatite (Radiesse) as a face and body biostimulatory agent. Plast Reconstr Surg Glob Open. 2019;7(3):e2160. doi:10.1097/GOX.0000000000002160
13. Lorenc ZP, Black JM, Cheung JS, et al. Skin tightening with hyperdilute CaHA: dilution practices and practical guidance for clinical practice. Aesthet Surg J. 2022;42(1):NP29–NP37. doi:10.1093/asj/sjab269
14. Alghoul MS, Vaca EE, Bricker JT, Mioton LM. Enhancing the lateral orbital “C-angle” with calcium hydroxylapatite: an anatomic and clinical study. Aesthet Surg J. 2021;41(8):952–966. doi:10.1093/asj/sjaa218
15. Kadouch J, Fakih-Gomez N. A hybrid filler: combining calcium hydroxylapatite and hyaluronic acid fillers for aesthetic indications. Am J Cosmet Surg. 2022;39(3):182–189. doi:10.1177/07488068211016135
16. Chao YY, Kim JW, Kim J, Ko H, Goldie K. Hyperdilution of CaHA fillers for the improvement of age and hereditary volume deficits in East Asian patients. Clin Cosmet Investig Dermatol. 2018;11:357–363. doi:10.2147/CCID.S159752
17. Halepas S, Christiansen C, Ferneini EM. Diluted and hyperdiluted calcium hydroxylapatite for skin tightening. J Oral Maxillofac Surg. 2022;80(9):S67–S68. doi:10.1016/j.joms.2022.07.091
18. Van Loghem J, Yutskovskaya YA, Werschler WP. Calcium hydroxylapatite: over a decade of clinical experience. J Clin Aesthet Dermatol. 2015;8(1):38–49.
19. Stella E, Di Petrillo A. Standard evaluation of the patient: the Merz scale. In: Goisis M, editor. Injections in Aesthetic Medicine. Milano: Springer; 2014:33–50.
20. Nowag B, Casabona G, Kippenberger S, et al. Calcium hydroxylapatite microspheres activate fibroblasts through direct contact to stimulate neocollagenesis. J Cosmet Dermatol. 2023;22(2):426–432. doi:10.1111/jocd.15521
21. Goldie K, Peeters W, Alghoul M, et al. Global consensus guidelines for the injection of diluted and hyperdiluted calcium hydroxylapatite for skin tightening. Dermatol Surg. 2018;44(Suppl 1):S32–S41. doi:10.1097/DSS.0000000000001685
22. Figueredo VO, Miot HA, Soares Dias J, de Barros Nunes GJ, Barros de Souza M, Bagatin E. Efficacy and safety of 2 injection techniques for hand biostimulatory treatment with diluted calcium hydroxylapatite. Dermatol Surg. 2020;46(Suppl 1):S54–S61. doi:10.1097/DSS.0000000000002334
23. Fabi SG, Alhaddad M, Boen M, Goldman M. Prospective clinical trial evaluating the long-term safety and efficacy of calcium hydroxylapatite for chest rejuvenation. J Drugs Dermatol. 2021;20(5):534–537. doi:10.36849/JDD.5680
24. Eviatar J, Lo C, Kirszrot J. Radiesse: advanced techniques and applications for a unique and versatile implant. Plast Reconstr Surg. 2015;136(5 Suppl):164–170. doi:10.1097/PRS.0000000000001825
25. Fabi SG, Burgess C, Carruthers A, et al. Consensus recommendations for combined aesthetic interventions using botulinum toxin, fillers, and microfocused ultrasound in the neck, décolletage, hands, and other areas of the body. Dermatol Surg. 2016;42(10):1199–1208. doi:10.1097/DSS.0000000000000869
26. Carruthers J, Burgess C, Day D, et al. Consensus recommendations for combined aesthetic interventions in the face using botulinum toxin, fillers, and energy-based devices. Dermatol Surg. 2016;42(5):586–597. doi:10.1097/DSS.0000000000000754
27. Graivier MH, Lorenc Z, Bass P, Fitzgerald LM, Goldberg L. Calcium hydroxyapatite (CaHA) indication for hand rejuvenation. Aesthet Surg J. 2018;38(suppl_1):S24–S28. doi:10.1093/asj/sjy013
28. Rovatti PP, Pellacani G, Guida S. Hyperdiluted calcium hydroxylapatite 1:2 for mid and lower facial skin rejuvenation: efficacy and safety. Dermatol Surg. 2020;46(12):e112–e117. doi:10.1097/DSS.0000000000002375
29. Guida S, Longhitano S, Spadafora M, et al. Hyperdiluted calcium hydroxylapatite for the treatment of skin laxity of the neck. Dermatol Ther. 2021;34(5):e15090. doi:10.1111/dth.15090
30. Lorenc ZP, Bass LM, Fitzgerald R, et al. Physiochemical characteristics of calcium hydroxylapatite (CaHA). Aesthet Surg J. 2018;38(suppl_1):S8–S12. doi:10.1093/asj/sjy011
31. Bass LS, Smith S, Busso M, McClaren M. Calcium hydroxylapatite (Radiesse) for treatment of nasolabial folds: long-term safety and efficacy results. Aesthet Surg J. 2010;30(2):235–238. doi:10.1177/1090820X10366549
32. Wollina U, Goldman A. Long lasting facial rejuvenation by repeated placement of calcium hydroxylapatite in elderly women. Dermatol Ther. 2020;33(6):e14183. doi:10.1111/dth.14183
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



