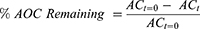Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 14
Standardized Terminalia chebula Fruit Extract: A Natural Ingredient That Provides Long-Lasting Antioxidant Protection and Reverses Visible Signs of Pollution-Induced Skin Damage
Authors Randhawa M , Meyer T, Sachdev M, Chaudhuri RK
Received 25 June 2021
Accepted for publication 26 August 2021
Published 17 September 2021 Volume 2021:14 Pages 1257—1269
DOI https://doi.org/10.2147/CCID.S326492
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Manpreet Randhawa,1 Thomas Meyer,2 Mukta Sachdev,3 Ratan K Chaudhuri1
1Sytheon Ltd, Boonton, NJ, USA; 2Meyer Sun & Skin Care Consulting, LLC, Memphis, TN, USA; 3MSCR Pvt Ltd, Bengaluru, India
Correspondence: Manpreet Randhawa
Sytheon Ltd, 315 Wootton Street, Boonton, NJ, USA
Tel +1 973 947 1109
Email [email protected]
Background: Identification of long-lasting natural antioxidants to protect against and repair skin damage induced by exposure to environmental pollution is in high demand.
Objective: To investigate a standardized Terminalia chebula (TC) fruit extract for its long-lasting antioxidant and anti-inflammatory properties and its ability to reverse the visible signs of pollution-induced skin damage in an 8-week clinical study.
Material and Methods: Chemical and cell-based in vitro studies were performed to characterize long-lasting antioxidant and anti-inflammatory properties; a clinical study with subjects with normal to dry skin living in a high-pollution city for the previous 5 years was conducted to assess if a formulation containing 1% standardized TC fruit extract affected significant improvements in skin’s visible condition and appearance compared with its placebo.
Results: The standardized TC fruit extract provided longer-lasting and more efficient neutralization of reactive oxygen species (ROS) than tocopherol; treatment of keratinocytes with the fruit extract prior to being stressed with urban dust safeguarded against increases in intracellular ROS, inhibited release of inflammatory cytokines IL-6 and IL-8 and protected membrane lipids against peroxidation. A clinical study yielded statistically significant improvements in dermatologist scores and subject self-assessments for skin texture, hydration, tone, firmness and radiance as compared to its placebo.
Conclusion: These studies validate the use of this standardized TC fruit extract not only as a restorative to diminish visible signs of existing damage but also as a preventative to help defend skin against damages caused by chronic exposure to environmental pollution.
Keywords: long-lasting antioxidant, pollution, skin hydration, skin radiance, Terminalia chebula
Introduction
A quest to identify plant extracts backed by ancient wisdom as sources of natural antioxidants with long-lasting performance is of high interest since research uncovered that environmental aggressors mediate a pivotal role in skin aging processes by inducing excess levels of reactive oxygen species (ROS) and by inciting inflammation.1,2 Of the many environmental aggressors cited, two of the most influential factors are UV radiation and particulate matter (PM) pollution. Excess formation of ROS causes oxidative stress wherein the buildup of ROS is free to oxidize major biomolecules (proteins, DNA, lipids) and organelles (mitochondria), influence gene expression patterns, alter cellular signaling and impair skin’s barrier function. In addition, based on their size, PM pollution can penetrate into the stratum corneum (SC) and into the deeper dermis to unleash an inflammatory cascade that increases the production of pro-inflammatory cytokines, including interleukin-6 and interleukin-8.3,4 This makes IL-6 and IL-8 important biomarkers for researchers trying to determine whether topical interventions can minimize the deleterious effects of inflammatory stress caused by PM pollution. While regular use of broad-spectrum sunscreens can help combat the deleterious effects of UV radiation exposure, research indicates that comprehensive protection against exposure to the UV radiation and PM pollution requires more holistic strategies to defend against (1) oxidative stress (2) inflammatory stress and (3) skin barrier disruption with concomitant reduction in hydration.5–7
Following this quest, and as reported previously,8 we developed a well-defined water-based extraction process to harvest phytochemicals from the fruit of Terminalia chebula (TC), a tall, deciduous tree grown throughout central and Southeast Asia that has been long recognized as a source of therapeutic agents to treat diverse health conditions (including diabetes, atherosclerosis, constipation, hemorrhoids, arthritis and allergies) and whose fruits have been reported to display anti-bacterial, anti-fungal, antioxidant and anti-inflammatory properties.9–12 Commercially available TC extracts typically contain ~33% total hydrolysable tannins but can be as low as 20%. Besides hydrolysable tannins the other phytonutrients present in the extract are flavonoids, steroids, amino acids, fructose, resins, fixed oils, anthraquinone, carbohydrates, glucose, sorbitol, etc.12 Our proprietary water-based process reproducibly yields TC extract standardized against total hydrolysable tannins (70% overall, typically ranging from 65% to 75%) including high levels of chebulinic acid (≥20%) and chebulagic acid (≥15%). Free gallic acid content was maintained below 5%.13
In past research, we documented that this standardized TC fruit extract exerts both preventative and restorative anti-aging skin benefits.8 Significant preventative anti-aging effects included proficient neutralization of all of the most reactive forms of ROS as an antioxidant, inhibition of matrix metalloproteinases (MMP), attenuated formation of advanced glycation endproducts (AGEs) that typically accumulate on collagen and elastin with age and inhibition of melanin formation that could help manage irregular pigmentation events (melasma, solar lentigines, post-inflammatory hyperpigmentation) and promote more even skin tones. Restorative anti-aging benefits were supported by significant stimulation of collagen production in normal human skin fibroblasts and further revealed in microarray studies13 which demonstrated that the TC fruit extract enhanced gene expression patterns associated with skin barrier establishment and water homeostasis as well as with increased expression of extracellular matrix (ECM) components, including collagens and proteoglycans, while inhibiting MMP enzymes that foster ECM degradation. Moreover, many of these benefits garnered with in vitro models translated into measurable benefits in photoaged subjects where application of a formulation containing 1% of the standardized TC fruit extract compared with its placebo stimulated significant and continual improvements over a 12-week clinical study in skin hydration, elasticity and firmness, skin tone and roughness while diminishing appearance of fine lines and wrinkles.8 Taken together, these results support that the standardized TC fruit extract as a skin care ingredient has capacity to exert both immediate actions to delay aging processes as well as long-term effects to maintain and promote a healthy skin architecture and appearance.
In conjunction with our previous research, the purpose of this study was to further characterize the performance of this standardized TC fruit extract with respect to its ability to provide long-lasting antioxidant protection, protect skin cells from oxidative and inflammatory stress induced by PM pollution and improve common visible signs of facial skin damage in subjects chronically exposed to a high pollution environment in an 8-week clinical study.
Materials and Methods
Standardized TC Fruit Extract
The standardized TC fruit extract used in this study is a commercially available material from Sytheon Ltd. called Synastol® TC, which is standardized against hydrolysable tannins (70%) containing two key bioactive phytochemicals, including chebulinic acid (≥20%) and chebulagic acid (≥10%) and having a free gallic acid content ≤5%.
ROS Neutralization
The in vitro methods followed to measure the performance of Standardized TC fruit extract to neutralize ROS were based on previously published methods.14–16
Determination of Long-Lasting Antioxidant Effectiveness
Standardized TC fruit extract and α-Tocopherol (α-TOC) were solubilized in 50% aqueous ethanol or ethanol, respectively, placed in quartz cuvettes and then exposed to solar-simulated UV radiation (UVR) at 6.51 mW/cm2 over 4 hours using a Rayonet RPR-100 photochemical reactor (Southern New England Ultraviolet Company), which simulates day light conditions. Aliquots of the solutions were removed at varying times (0, 15 min, 30 min, 1 hr, 2 hr and 4 hr) corresponding to UVR doses of 5.9, 11.7, 23.4, 46.9 and 93.7 J/cm2 and antioxidant capacities (AOC) of the solutions to neutralize peroxyl radicals were determined using Hydro ORAC method for the standardized TC fruit extract and lipophilic ORAC method for α-tocopherol as described previously. Long-lasting effectiveness was then equated with the extent to which AOC remained after each exposure to UVR and was calculated using the following equation:
Cell Culture
Human adult epidermal keratinocytes were purchased from Thermofisher (Catalog number: C0055C) and grown using EpiLife Media (60 μM calcium) supplemented with 0.2% v/v bovine pituitary extract, 1 μg/mL recombinant human insulin-like growth factor-I, 0.18 μg/mL hydrocortisone, 5 μg/mL bovine transferrin, 0.2 ng/mL human epidermal growth factor. The cells were cultured at 37±2°C and 5±1% CO2. The Keratinocyte cells were seeded at a density of 0.01x10^6 cells per well into either 96-well plates and grown overnight to allow the cells to adhere to the well plates. The cells were maintained in culture until they were 80% confluent. Urban dust was obtained from Sigma Chemicals (Standard Reference Material 1649b) and prepared at 10 mg/mL in unsupplemented EpiLife media. The urban dust solution was then sonicated for 10 minutes on ice. This stock solution was then diluted to 1 mg/mL with media. The respective test materials were prepared in hydrocortisone free media. At the end of the incubation period, the cell culture media was collected to assay for respective endpoints and the cells were subjected to an MTT assay.
Measurement of ROS Production
A stock DCF-DA (10 mM) was prepared in DMSO and then diluted in unsupplemented EpiLife media to a final concentration of 20 μM. The media in the well plates was then replaced with the DCF supplemented media and the well plate was incubated for 30 minutes at 37°C. At the end of the incubation period, the well plate was rinsed once with PBS. After loading the keratinocytes with DCF-DA, the cells were treated with three concentrations of Terminalia Chebula fruit extract 0.5 μg/mL, 1 μg/mL and 5 μg/mL prepared in 100 μL of unsupplemented EpiLife media. Trolox (150 μg/mL) was used as a positive control. Since one of the test materials was prepared in DMSO, 0.5% DMSO was used as a vehicle control. Cells treated with media alone were used as an untreated control. After the test materials were added to the cells, 10 μL of 1 mg/mL urban dust was added to each well. One set of cells was not exposed to urban dust (non-urban dust control). A baseline fluorescence measurement was then made using a Fluoroskan Ascent Fluorometer. Measurements were made on the underside of the 96-well plate to get direct measurements of the cells. The measurements were made using an excitation wavelength of 485 nm and an emission wavelength of 518 nm. After this baseline measurement was made, the cells were incubated for 3 hours at 37±2°C and 5±1% CO2, with additional fluorescence measurements made at the 1, 2 and 3-hour time points.
Measurement of Lipid Peroxidation
Keratinocytes were treated with three concentrations of Terminalia Chebula fruit extract 0.5 μg/mL, 1 μg/mL and 5 μg/mL prepared in 100 μL of unsupplemented EpiLife media and exposed to urban dust as described above. The cells were then cultured for 4 hours at 37±2°C and 5±1% CO2. At the 3.5-hour mark of the incubation 10 μL of 100 μM Image-It Lipid Peroxidation Sensor was added to each well and the cells were incubated for 30 minutes. One set of cells was not treated with the lipid peroxide sensor and was used as a blank to account for the background fluorescence of the cells. At the end of the incubation period, the wells were washed three times with PBS and then the plate was read using a fluorometer set to an excitation wavelength of 485 nm and an emission wavelength of 518 nm.
Measurement of IL-6 and IL-8
Respective treatments were applied to the keratinocytes, exposed to urban dust and then incubated for 24 hours as described above. Dexamethasone (39 ug/mL, 100 uM) is a corticosteroid known for anti-inflammatory properties that was used as a positive control, while cells treated with urban dust alone were used as an untreated control. Finally, one set of cells was not exposed to urban dust and was used to establish a baseline for each cytokine. A series of standards was prepared and 100 μL of each of these standards was dispensed into two wells (duplicates) in the ELISA well plate. Subsequently, 100 μL of each cell culture media sample was added to additional wells and the plate was incubated for two hours at room temperature. After the incubation, the plate was washed three times as described above. Once the last wash was removed, 100 μL of a biotin conjugated detection antibody was added. After incubating the plate for two hours at room temperature the plate was washed again as described above. 100 μL of HRP-streptavidin was then added to each well and the plate was incubated for 20 minutes at room temperature. Once the last wash was removed, 100 μL of substrate solution (hydrogen peroxide + tetramethylbenzidine as a chromagen) was added to each well. Once a sufficient level of color development had occurred, 50 μL of stop solution (2N sulfuric acid) was added to each well and the plate was read at 460 nm. Dexamethasone (39 ug/mL, 100 uM) was used as a positive control for inflammatory assays.
Measurement of Cell Viability (MTT Assay)
Cells were treated with 0.5 mg/mL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) in media for 30–60 min at 37°C. After the incubation, the wells were rinsed with 500 μL of phosphate buffered saline and then 400 μL of isopropanol was added to each well. After the extraction, a 200 μL sample of the isopropanol/MTT mixture was transferred to a 96-well plate and the absorbance of the sample was read at 540 nm with a plate reader using 200 μL of isopropanol as the blank.
Calculations
For the MTT assay, the mean absorbance value for the non-urban dust treated cells was calculated and used to represent 100% cell viability. The individual absorbance values from the cells undergoing the various treatments were then divided by the mean absorbance value representing 100% cell viability and expressed as a percent to determine the change in cell viability caused by each treatment.
For ROS assay, the baseline fluorescence measurements were subtracted from the subsequent time point measurements to correct for any background fluorescence of the test materials. For the lipid peroxidation assay, the mean fluorescence measurements from the non-dye loaded cells were subtracted from the lipid peroxidation sensor loaded cells to account for any non-specific fluorescence due to the cells themselves. After correcting the fluorescence measurements in the assays, mean fluorescence values were calculated for each treatment group and compared using an ANOVA.
For the ELISA-based assays, the absorbance values for the known standards were used to generate a standard curve. The values for the unknown samples were then determined from this standard curve.
Clinical Study
Clinical Study Design
This study was conducted as a randomized, double‐blinded, placebo controlled 8‐week study and consisted of four visits. The study was performed in Bangalore, India, from September 2020 to December 2020. This study was approved by the Independent Ethics Committee (Clinicom Ethics Committee) conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent prior to participation. Fifty-two healthy female with skin type III, IV & V, between the ages of 35 to 50 yrs were recruited distributed at a ratio of 1:1 with placebo and investigational product, respectively. Female adult subjects in general good health as determined from a recent medical history, general physical examination, dermatological assessment were recruited. The inclusion criteria included chronic pollution exposure in the past 5 years, normal to dry skin with corneometer reading up to 35. Participants were excluded if they were pregnant or breastfeeding, had a known sensitivity to topical products or had a cutaneous disease that affected the face.
All treatments were pre-randomized using a computer‐based randomization generator with blinded allocation. Participants were enrolled and assigned interventions by the clinical research coordinator. The standardized TC fruit extract used in this study was isolated from the fruits of Terminalia Chebula as described previously. The extract is water soluble and was incorporated into an oil-in-water formulation for the clinical study. Participants were instructed to apply either the interventional formulation containing 1% standardized TC fruit extract or the placebo formulation to their full face twice a day as a thin layer.
Clinical Evaluation and Questionnaires
At each visit, the dermatologist assessed each subject’s facial skin on a 0 (best) to 9 (worse) scale of facial skin for radiance-related endpoints including visual texture, skin firmness, skin hydration and skin even tone. A subjective questionnaire made up of agree/disagree questions, and subjective attribute ratings were given to the subjects to complete at each visit after baseline. Subjects are graded on a 1 to 5 scale, with 1 being disagree completely and 5 being agree completely. All photographic images were captured with Canfield Visia CR system at baseline and respective time points through various modalities.
Statistical Analysis
For efficacy assessment, analysis of clinical evaluations was performed at week 2, 4 and week 8. Statistical analyses were performed using paired t‐tests (or Wilcoxon signed‐rank test for nonparametric measures) based on the normality of the data. Product comparison was done at each time point on change over baseline values. P‐values <0·05 were considered significant.
Outcomes Measured
The primary outcome measure was dermatologist assessment of five different skin benefits including skin texture, skin firmness, skin hydration and even tone and skin radiance at week 2, 4, 8, respectively. Secondary outcomes also included the subjective questionnaire at week 8. Participant‐reported tolerability (itching, burning and stinging) and in‐person clinical assessments (pigmentation, scaling and erythema) throughout the study. The authors do not intend to share individual deidentified participant data.
Results
Standardized TC Fruit Extract Exhibits Strong Antioxidant Properties That are Maintained During Exposure to UV for Long-Lasting Performance
The standardized TC fruit extract, a hydrophilic broad-action antioxidant, exhibited efficient capabilities to neutralize all of the reactive forms of ROS and was found to be generally more effective than α-tocopherol (Table I) with the exception of singlet oxygen neutralization. A comparative photostability study further demonstrated that the TC fruit extract sustained long-lasting antioxidant activity when exposed to solar-simulated UV radiation over a period of time equivalent to at least 4 hours of outdoor sunlight, in contrast to α-tocopherol which lost all of its antioxidant capacity within the first hour of exposure (Figure 1).
 |
Table 1 Comparison of Effectiveness of Standardized TC Fruit Extract with α-Tocopherol to Neutralize Different Chemical Forms of Reactive Oxygen Species (ROS) |
Standardized TC Fruit Extract Reduces Intracellular Levels of ROS and Protects Membrane Lipids Against Peroxidation in Keratinocytes Stressed with Urban Dust
Given that ROS has been reported to play a critical role in PM-induced inflammation and aging in skin,17 the generation of intracellular ROS was measured using 2′,7′-dichlorodihydrofluorescein diacetate (DCF-DA) fluorescence dye. As shown in (Figure 2), urban dust increased intracellular ROS levels whereas pre-treatment with standardized TC fruit extract showed ROS scavenging activity in a dose-dependent manner as compared to Trolox, a well-known antioxidant in human keratinocytes.
One of the consequences of ROS formation is lipid peroxidation. Lipid peroxidation was measured through lipid peroxide sensitive dye (Image-IT Lipid Peroxide Sensor). Urban dust increased lipid peroxidation and similar to radial scavenging properties, standardized TC fruit extract pre-treatments reduced lipid peroxidation in human keratinocytes in a dose-dependent manner as compared to Trolox (Figure 3). The results indicated that standardized TC fruit extract has the potential to reduce ROS levels that in turn decreases lipid peroxidation. Together, these results indicate that the standardized TC fruit extract maintains its ROS scavenging abilities effectively in biological systems.
Standardized TC Fruit Extract Reduces Urban Dust-Induced Inflammatory Stress Through Significant Reduction of Pro-Inflammatory Markers IL-6 and IL-8
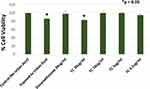
To investigate the mechanism of standardized TC fruit extract on inhibition of inflammatory mediator release, the effects of the fruit extract were compared with dexamethasone using human adult epidermal keratinocytes. Stress with urban dust stimulated a strong inflammatory response by significantly increasing levels of both IL-6 and IL-8, whereas pre-treatment with standardized TC fruit extract inhibited the release of IL-6 and IL-8 in a dose-dependent manner (Figure 4). While dexamethasone, used as a positive control, also inhibited the release of IL-6 and IL-8, the standardized TC fruit extract was about 4-fold better than the positive control. Notably, cell viability as determined by MTT assays was unaffected by any of these treatments (Figure 5).
Topical Treatment Containing 1% Standardized TC Fruit Extract Showed Significant Clinical Improvement in Radiance-Related Skin Health Benefits as Compared to Placebo
A total of 52 subjects were recruited and split in a ratio of 1:1 using placebo or the interventional product containing the standardized TC fruit extract, respectively. Fifty-one subjects completed the study, with one participant who failed to follow up. No subjects discontinued use of either placebo or interventional formulation due to irritation or discomfort. Subjects applied their respective formulation twice a day (morning and evening) for 8 weeks. No sunscreens were used during the study.
Subjects were graded by a dermatologist for clinical improvements in facial skin appearance, including skin texture, skin firmness, skin hydration, skin even tone and skin radiance (Figure 6A). Throughout the study, subjects showed significant improvement relative to placebo in all indicated efficacy parameters. No adverse effects related to the formulations were reported in the study. Treatment with the formulation containing 1% standardized TC fruit extract resulted in significant improvements as compared to placebo as early as week 2, with continued improvements throughout the study (Figure 6A). After 8 weeks application of the formulation containing 1% standardized TC fruit extract, 100% of subjects showed improvements in skin texture, skin firmness, skin hydration, even tone and skin radiance (Figure 6B).
Topical Treatment Containing 1% Standardized TC Fruit Extract Resulted in High Percentage of Agreement Between Dermatologist and Subject Self-Perceived Assessments
Self-agreement questions were included as a part of the clinical study to determine the extent to which improvements documented by a dermatologist agreed with improvements detected by the panelists themselves. The self-agreement questions were created along the same lines as clinically assessed benefits by a dermatologist. Subjects did not perceive much improvement at the earlier timepoints (data not shown), but positive results were obtained by week 8. Continued use of the formula containing 1% standardized TC fruit extract over 8 weeks resulted in over 80% of subjects in agreement with all questions. Notably, 85% of the panelists agreed that the formulation containing standardized TC fruit extract made their skin feel firmer while 92% agreed that their skin felt tighter and younger; additionally, 81% agreed their skin looked even-toned, 81% agreed their skin looked brighter and 92% agreed their skin looked radiant (Table 2).
Discussion
Use of natural phytochemicals to help skin defend against or treat common clinical signs of aging caused by exposure to environmental aggressors represents a promising anti-aging strategy. The present study culminated in a clinical trial that showcased the ability of a formulation containing 1% standardized TC fruit extract as a natural ingredient compared with its placebo to affect significant improvements in the visible signs of facial damage caused by chronic exposure to a high pollution environment in Bengaluru, India. In addition, using in vitro chemical and cell culture assays, we established that this standardized TC fruit extract possesses powerful long-lasting antioxidant and anti-inflammatory properties, which are believed to help account for the observed clinical improvements in skin’s condition and appearance.
Terminalia Chebula (TC), also called the “King of Medicine” in Tibet, is at the top of the list of “Ayurvedic Materia Medica” because of its extraordinary powers of healing. Numerous studies support multiple skin benefits of extracts prepared from the fruit of TC, from antioxidant, anti-inflammatory and anti-microbial effects to depigmentation, wound healing and rejuvenating effects.18–21 However, many of the studies were performed using crude extracts prepared with non-aqueous or aqueous-alcoholic solvents. This lies in sharp contrast to the study described here and previously reported that utilized a TC fruit extract prepared from a well-defined aqueous process that reliably yields high levels of the bioactive phytochemicals known to account for its biological properties. Indeed, standardization against hydrolysable tannins (70%), including chebulinic acid (≥20%) and chebulagic acid (≥15%), advantageously guarantees reproducibility of the skin benefits provided by this TC fruit extract.
Environmental pollutants like PM commonly mediate their effects within skin by inducing oxidative and inflammatory stress.22,23 Oxidative stress occurs following formation of excess levels of ROS, which can be managed with the use of efficacious antioxidants. However, in order to be effective within skin, antioxidants must have capacity to neutralize all of the different reactive forms of ROS that are associated with skin damage, which comprise free radicals (•OH, •OOH, •OOR), reactive anions (ONOO‒), neutral species (H2O2) and electronic excited states (1O2). That the standardized TC fruit extract as a natural antioxidant possesses high efficiencies to neutralize all of these different forms of ROS is exemplified by the data in Table I, which further illustrates that with the exception of 1O2, the TC fruit extract is generally more effective than α-tocopherol, an important constituent of skin’s non-enzymatic antioxidant defenses24 and one of the most common antioxidants used in topical formulations.
Of interest was the finding that the standardized TC fruit extract was more effective than α-tocopherol at neutralizing hydrogen peroxide. Excess formation of hydrogen peroxide within cells is normally removed by catalase, one of skin’s key antioxidant enzymes that converts it into water and molecular oxygen. However, of the key antioxidant enzymes, research shows that catalase becomes easily depleted in skin upon exposure to environmental stressors like UV radiation and undergoes a seasonal variation with low activities in summer and higher activities in winter.25,26 Moreover, following acute UVA exposure, catalase activity was slower to recover in skin of older subjects, which has further implications for aging. This suggests that regular use of ingredients in topical applications with heightened capacity to neutralize hydrogen peroxide may represent a useful tool to bolster skin’s internal antioxidant defenses over long periods. In addition, this study also confirms that the standardized TC fruit extract maintains its antioxidant capacity over 4 hours of continuous exposure to simulated day light, which easily extrapolates to a full day of intermittent UV radiation exposures that typify non-extreme conditions.27 In contrast, α- tocopherol lost its antioxidant properties quickly within the first hour of exposure (Figure 1). This ability to sustain long-lasting antioxidant performance is particularly important for maintaining benefits on areas of skin that are commonly exposed to the sun exposure, especially the face, during the course of a normal day.
The high efficiency of the standardized TC fruit extract to neutralize ROS observed in chemical assays also translated to biological systems. As shown in Figure 2, treatment of keratinocytes with the TC fruit extract prior to being stressed with urban dust significantly reduced intracellular levels of ROS in a dose-dependent manner. Even the lowest dose of the TC fruit extract yielded significant reduction in ROS levels, while higher doses conferred complete protection against increases in intracellular ROS induced by the stressor. In a separate experiment, the standardized TC fruit extract was also found to protect keratinocyte membrane lipids against peroxidation in a dose-dependent manner that mirrored its ability to reduce intracellular levels of ROS (Figure 3) when the cells were stressed with urban dust. This indicates that the TC fruit extract can function efficiently to intercept and neutralize ROS before they can react with and oxidize the lipids. While these results are entirely consistent with its ROS neutralization capabilities as an antioxidant, the possibility that the standardized fruit extract could also function at least partially by inhibiting cellular production of ROS cannot be completely ruled out.
Another consequence of exposure to PM pollution is that the excess ROS levels they spawn triggers inflammation by activating redox-sensitive transcription factors such as NF-κB.4,28 Once activated, NF-kB can translocate to the nucleus where it transcribes pro-inflammatory genes. This consistently leads to increased gene expression of NF-κB as well as increased production of IL-6 and IL-8, resulting in inflammatory stress.3,4 As shown in Figure 4, treatment of keratinocytes with the standardized TC fruit extract before being stressed with urban dust significantly reduced IL-6 and IL-8 production in a dose-dependent fashion, with the fruit extract appearing to be about 4-times more effective than dexamethasone used as a positive control. Importantly, cell viability was unaffected by all of these treatments (Figure 5). Together with previous results, this demonstrates overall that the standardized TC fruit extract effectively protects cells against oxidative as well as inflammatory stress induced by PM pollution.
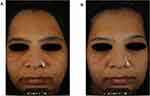
The 8-week clinical study demonstrated significant improvements as compared to placebo in pollution-related skin improvements within as early as 2 weeks of daily applications. Furthermore, significant and continued improvements continued to occur over the entire 8-week study based on dermatologist assessments of skin hydration, skin texture, skin tone, skin firmness and skin radiance (Figure 6). It has been scientifically documented that women exposed to high levels of environmental pollution tend to have less radiant skin with less homogeneous complexions and are perceived as less “healthy” and older as compared to their counterparts in non-polluted environments.29 We hypothesize that treatment with 1% standardized TC fruit extract resulted in clinical improvements in the appearance of facial skin radiance and youthfulness owing mainly to improvements in skin hydration and skin tone. Both of these attributes positively affect light’s interaction with skin at and below the surface.27,28 Hydrated skin enhances radiance by reducing surface roughness, which results in less scattering and deeper penetration of incident light. Skin that is deeply hydrated also facilitates degradation of corneodesmosomes and aids desquamation, processes that additionally promote skin smoothness and that further contribute to radiance and glow.30,31 These observations are also supported by our previous mechanistic research, which found that the standardized TC fruit extract significantly increased the expression of genes associated with water homeostasis and late keratinocyte differentiation marker genes (FLG, LOR) that directly correlate with increased skin hydration. In addition, the TC fruit extract was also found [14] to stimulate regulators of collagens (COL1A1, COL1A2), proteoglycans (PRELP, OGN), and the extracellular matrix (TGM5, SERPIN512) that correlate with improvements in skin texture and firmness, all of which help promote a more youthful appearance. Moreover, increases in skin radiance are associated with a compact and well-organized ECM because it enhances subsurface reflection of light from the dermis.26 Indeed, the typical improvements in skin tone, localized pigmentation, brightness and radiance cited by subjects for the formulation with TC fruit extract are illustrated by the photos taken before and after the 8-week study in Figure 7 using cross-polarized photography.
Along with dermatologist assessments, we also assessed subjects’ self-perceived improvements in facial skin appearance over the course of the clinical study. While many subjects did not perceive improvements at earlier time points, by week 8, with continual improvement in all of the endpoints, all subjects perceived that their skin felt firmer and tighter and looked brighter, more radiant and lifted (Table 2). Indeed, the self-perceived improvements encouraged compliance during the study. That the majority of subjects’ perceptions of improvement paralleled those assessed by the dermatologist also attest to the true efficacy of the topical treatment with 1% standardized TC fruit extract.
Conclusion
Overall, our results suggest that use of the standardized TC fruit extract as a natural phytochemical mixture functions effectively as a preventative as well as a restorative skincare treatment against the aging effects caused by chronic exposure to environmental pollution. Owing to its immediate and long-lasting performance, use of the standardized TC extract in topical formulations also aligns well with the minimalist approach of making only one application each morning to fight off external aggressors throughout the day while continuing to provide reparative and strengthening skin care benefits throughout the night. In conclusion, the standardized TC fruit extract not only as a restorative to diminish visible signs of existing damage but also as a preventative to help defend skin against damages caused by chronic exposure to environmental pollution.
Acknowledgments
We thank Tony Chang and Marsha Sintara (International Chemistry Testing, Milford, MA) and Robert Holtz (BioInnovation Laboratories, Inc., Lakewood, CO) for excellent technical assistance in carrying out experiments.
Funding
This study was funded by Sytheon Ltd (Boonton NJ).
Disclosure
The standardized TC extract analyzed in this study (Synastol® TC) was developed and commercialized by Sytheon Ltd (Boonton, NJ).
Ratan K Chaudhuri is president and CEO of Sytheon with ownership interest. Manpreet Randhawa is an employee of Sytheon Ltd (Boonton, NJ). Thomas Meyer has received consulting reimbursement from Sytheon. Mukta Sachdev is the medical director of MSCR Pvt Ltd and has received consulting reimbursement from Sytheon.
References
1. Passersonon T, Krutmann J, Andersen ML, Katta R, Zouboulis CC. Clinical and biological impact of the exposome on the skin. JEADV. 2020;34(4):4–25.
2. Parrado C, Mercado-Saenz S, Perez-Davo A, Gilaberte Y, Gonazlez S, Juarranz A. Environmental stressors on skin aging. Mechanistic insights. Front Pharmacol. 2019;10:759. doi:10.3389/fphar.2019.00759
3. Jin S, Li Z, Choi EK, et al. Urban particulate matter in air pollution penetrates into the barrier-disrupted skin and produces ROS-dependent cutaneous inflammatory response in vivo. J Dermatol Sci. 2018;91(2):175–183. doi:10.1016/j.jdermsci.2018.04.015
4. Ryu YS, Kang KA, Piao MJ, et al. Particulate matter induces inflammatory cytokine production via activation of NFκB by TLR5-NOX4-ROS signaling in human skin keratinocyte and mouse skin. Redox Biol. 2019;21:101080. doi:10.1016/j.redox.2018.101080
5. Mancebo SE, Wang BS, Wang SQ. Recognizing the impact of ambient air pollution on skin health. J Eur Acad Dermatol Venereol. 2015;29(12):2326–2332. doi:10.1111/jdv.13250
6. Meyer T, Beasley D, Hanson K. Augmenting skin photoprotection beyond sunscreens. In: Wang S, Lim H, editors. Principles and Practice of Photoprotection. Adis and Cham; 2016:439–460.
7. Lee CW, Lin ZC, Hu SC, Chiang YC, Hsu LF, Lin YC. Urban particulate matter down-regulates filaggrin via COX2 expression/PGE2 production leading to skin barrier dysfunction. Sci Rep. 2016;6(6):27995. doi:10.1038/srep27995
8. Chaudhuri RK, Lascu Z, Marchio F. Terminalia chebula for preventive and restorative anti-aging benefits. Cosm Toil. 2015;130:32–48.
9. Gupta PC. Biological and pharmacological properties of Terminalia chbula Retz. (Haritaki) – an overview. Int J Pharm Pharm Sci. 2011;4(3):62–68.
10. Bag A, Bhattacharyya SK, Chattopadhyay RR. The development of Terminalia chebula Retz (combretaceae) in clinical research. Asian Pac J Trop Biomed. 2013;3(3):244–252. doi:10.1016/S2221-1691(13)60059-3
11. Singh P, Malhotra H. Terminalia chebula: a review pharmacognistic and phytochemical studies. Intern J Recent Sci Res. 2017;8(11):21496–21507.
12. Rubini B, Santhi G, Soundhari C, Rajarajan S. Antifungal activity of Terminalia chebula and Terminalia catappa on two dermatophytes. J Med Aromat Plants. 2013;4(2):15–19.
13. Swindell WR, Bojanowski K, Chaudhuri RK. A standardized Terminalia chebula fruit extract alters the expression of genes associated with skin architecture and barrier formation. Eur J Dermatol. 2020;30(5):469–492. doi:10.1684/ejd.2020.3882
14. Chaudhuri RK, Meyer T, Premi S, Brash D. Acetyl zingerone: an efficacious multifunctional ingredient for continued protection against ongoing DNA damage in melanocytes after sun exposure ends. Int J Cosmet Sci. 2020;42(1):36–45. doi:10.1111/ics.12582
15. Ou B, Hampsch-Woodill M, Prior RL. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J Agric Food Chem. 2001;49(10):4619–4626. doi:10.1021/jf010586o
16. Ruch RJ, Cheng S, Klaunig JE. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis. 1989;10(6):1003–1008. doi:10.1093/carcin/10.6.1003
17. Kim M, Kim JH, Jeong GJ, Park KY, Lee MK, Seo SJ. Particulate matter induces pro-inflammatory cytokines via phosphorylation of p38 MAPK possibly leading to dermal inflammaging. Exp Dermatol. 2019;28(7):809–815. doi:10.1111/exd.13943
18. Saha S, Verma RJ. Antioxidant activity of polyphenolic extract of Terminalia chebula Retzius fruits. J Taiba Univ Sci. 2015:10(6):805–812.
19. Yakaew S, Itsarasook K, Ngoenkam J, Jessadayannamaetha A, Vyoch J, Ungsurungsie M. Ethanol extract of Terminalia chebula fruit protects against UVB-induced skin damage. Pharm Biol. 2016;54(11):2701–2707. doi:10.1080/13880209.2016.1179768
20. Akhtar N, Khan AB, Muhannad S, et al. Formulation and characterization of a cream containing Terminalia chebula extract. Forsch Komplementmed. 2012;19(1):20–25. doi:10.1159/000335823
21. Manosroi A, Jantrawut P, Akihisa T, Manosroi W, Manosroi J. In vitro anti-aging activities of Terminalia chebula gall extract. Pharm Bio. 2010;48(4):469–481. doi:10.3109/13880200903586286
22. Magnani ND, Muresan XM, Belmonte G, et al. Skin damage mechanisms related to airborne particulate matter exposure. Toxicol Sci. 2016;149(1):227–236. doi:10.1093/toxsci/kfv230
23. Rembiesa J, Ruzgas T, Engblom J, Holefors A. The impact of pollutants on skin and proper efficacy testing for anti-pollution claims. Cosmetics. 2018;5(1):4. doi:10.3390/cosmetics5010004
24. Thiele JJ, Traber MG, Packer L. Depletion of human stratum corneum vitamin e: an early and sensitive in vivo marker of UV induced photo-oxidation. J Invest Dermatol. 1998;110(5):756–761. doi:10.1046/j.1523-1747.1998.00169.x
25. Maresca V, Flori E, Briganti S, et al. UVA-induced modification of catalase charge properties in the epidermis is correlated with the skin phototype. J Invest Dermatol. 2006;126(1):182–190. doi:10.1038/sj.jid.5700021
26. Kim HJ, Baek JH, Eo JE, Choi KM, Shin MK, Koh JS.Dermal matrix affects translucency of incident light on the skin. Skin Res Technol. 2015;21(1):41–46. doi:10.1111/srt.12154
27. Marionnet C, Tricaud C, Bernerd F. Exposure to non-extreme solar UV daylight: spectral characterization, effects on skin and photoprotection. Int J Mol Sci. 2015;16(1):68–90. doi:10.3390/ijms16010068
28. Marchini T, Magnani ND, Paz ML, et al. Time course of systemic oxidative stress and inflammatory response induced by an acute exposure to residual oily fly ash. Toxicol Appl Pharmacol. 2014;274(2):274–282. doi:10.1016/j.taap.2013.11.013
29. Flament F, Bourokba N, Nouveau S, Li J, Charbonneau A. A severe chronic outdoor urban pollution alters some facial aging signs in Chinese women. A tale of two cities. Int J Cosmet Sci. 2018;40(5):467–481. doi:10.1111/ics.12487
30. Verdier‐Sevrain S, Bonte R. Skin hydration: a review of its molecular mechanisms. J Cosmet Dermatol. 2007;6(2):
31. Loden M. Role of topical emollients and moisturizers in the treatment of dry skin barrier disorders. Am J Clin Dermatol. 2003;4:771–788.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.