Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 14
ST2 Predicts Mortality In Patients With Acute Hypercapnic Respiratory Failure Treated With Noninvasive Positive Pressure Ventilation
Authors Jónsdóttir B , Ziebell Severinsen M, von Wowern F , San Miguel C, Goetze JP, Melander O
Received 8 April 2019
Accepted for publication 16 September 2019
Published 23 October 2019 Volume 2019:14 Pages 2385—2393
DOI https://doi.org/10.2147/COPD.S211448
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Brynja Jónsdóttir,1–3 Marie Ziebell Severinsen,4 Fredrik von Wowern,1,3 Carmen San Miguel,3 Jens P Goetze,4 Olle Melander1,3
1Department of Clinical Sciences Malmo, Faculty of Medicine, Lund University, Lund, Sweden; 2Department of Pulmonary Medicine and Allergology, Skåne University Hospital, Malmö, Sweden; 3Department of Internal Medicine and Emergency Medicine, Skåne University Hospital, Malmö, Sweden; 4Department of Clinical Biochemistry, Copenhagen University Hospital, Rigshospitalet, Copenhagen, Denmark
Correspondence: Brynja Jónsdóttir
Department of Pulmonary Medicine and Allergology, Skånes Universitetssjukhus Malmö, Södra Förstadsgatan, Malmö 20502, Sweden
Email [email protected]
Background: Patients with Acute Hypercapnic Respiratory Failure (AHRF) are often treated with Noninvasive Positive Pressure Ventilation (NPPV). In this heterogeneous patient group, there is a lack of clinical tools for predicting mortality and outcome.
Aims: In order to facilitate the choice of treatment in patients with AHRF we evaluated the protein ST2, an established biomarker for cardiac stress, and its role in predicting mortality in patients with AHRF treated with NPPV. We also examined if ST2 baseline levels and changes during the first 12 hrs of treatment were predictive of treatment outcome.
Methods: The study population consisted of 46 patients treated with NPPV for AHRF. Background data and clinical parameters were obtained and blood samples taken at various time points during the treatment. During the follow-up period of 18 months, the prognostic value of ST2 with regards to mortality was evaluated using Cox proportional hazard model.
Results: Of the 46 patients, there were 3 subgroups in regards to primary diagnosis: Acute Exacerbation of COPD (n=34), Acute Heart Failure (n=8) and Acute Exacerbation in Obesity Hypoventilation Syndrome (n=4). We found that ST2 was an independent predictor of both short-term and long-term mortality during the follow-up period. The Hazard Ratio (HR) per 1-SD increment of ST2 for 28-day mortality was 11.00 (95% CI 1.8–67.2, p 0.009) and for 18-month mortality 2.11 (95% CI 1.4–3.2, p 0.001). The results seem to be driven by the largest subgroup of patients, with Acute Exacerbation of COPD, and deaths within the first 28 days. Furthermore, changes in ST2 values during the first 12 hrs of treatment were not predictive of treatment outcome.
Conclusion: Our results show that ST2 is a target to explore further as a predictor of short-term mortality in patients with AHRF treated with NPPV.
Keywords: acute hypercapnic respiratory failure, noninvasive positive pressure ventilation, chronic obstructive pulmonary disease, heart failure
Introduction
The search for biomarkers as predictors of outcome in various diseases is an ongoing challenge. Patients with acute respiratory failure are targets for this kind of research. In some cases, the results have had extensive effects and the biomarkers researched have become an important part of clinical decision-making.1,2 As the primary cause of dyspnea is not always obvious during the Emergency Care encounter, discovering biomarkers with predictive value for outcome, could be supportive for choosing appropriate treatment and decide level of care before the primary diagnosis has been established.2–4
A substantial part of patients with acute respiratory failure has hypercapnia (Acute Hypercapnic Respiratory Failure, AHRF).5–7 In this patient group, treatment with Noninvasive Positive Pressure Ventilation (NPPV) has been shown to reduce the need of endotracheal intubation, the length of hospital stay and mortality.8–11 Patient selection is important and the treatment is most effective in the early stages of acidosis.7,12–15 The majority of patients with AHRF have Acute Exacerbation of Chronic Obstructive Pulmonary Disease (AECOPD), but other known causes where NPPV treatment is recommended, are Acute Heart Failure (AHF), Pneumonia and other infections in immunocompromised patients and Acute Exacerbation in Obesity Hypoventilation Syndrome (AEOHS).6,8,15–18 Patients with AHRF treated with NPPV are thus a heterogenous group and some patients have multiple factors contributing to the acute deterioration.19
One of the biomarkers that have been evaluated as predictor of outcome in patients with acute dyspnea is called ST2 (Suppression of Tumorigenicity 2), most widely recognized as a predictive biomarker for mortality in cardiac diseases.1,20–22 It exists in a membrane-bound form (ST2L) as a receptor for the pro-inflammatory cytokine IL-33, and a soluble form (sST2) which is measurable in plasma. The soluble form is upregulated in states of mechanical strain in cardiac myocytes, thus signaling myocardial hypertrophy and fibrosis. It is thought to act as a decoy receptor for IL-33 decreasing its cardioprotective effects, as well as having a more complex immunomodulatory role in binding to other receptors. It is also released by vascular and pulmonary endothelial cells, but the major source of ST2 in healthy individuals has not been established.1,20–22 The PRIDE study showed that in patients with dyspnea, with or without heart failure, ST2 was a strong predictor of short- and long-term mortality.23 ST2 is used in clinical practice as an independent predictive biomarker of mortality, alone or in relation with other prognostic biomarkers of heart disease.24–26 Furthermore, monitoring ST2 levels during treatment has been shown to provide additional prognostic information, implying that serial measurements may play an important role in the future biomarker-based evaluation of heart failure.27
In recent years, the potential role of ST2 as a biomarker in both allergic and non-allergic pulmonary diseases has been addressed in several studies.28 ST2 levels have been shown to increase in patients with Acute Exacerbations of Idiopathic Pulmonary Fibrosis, Acute Respiratory Distress Syndrome and Sepsis.28–30 It has even been suggested that the IL-33/ST2 pathway might contribute to the pathogenesis and progression of COPD.31 Although ST2 levels might not be specific for a diagnosis of a distinct cause of acute respiratory failure, its prognostic value regarding mortality seems to be consistent.1,20,23,32
In our current study, the main goal was to investigate if ST2 could be used as a predictor of outcome and mortality, in patients with AHRF of various underlying causes. As a second goal, we repeatedly measured ST2 during the first 12 hrs of treatment, in order to conclude if a change in ST2 could predict treatment outcome. We hypothesized that a decrease in ST2 might indicate a better clinical outcome, and thus could be used as a marker of treatment response.
Materials And Methods
Study Population
The procedure of selection and inclusion was thoroughly described in our previous work.33 In short, during the period January–June 2014 we enrolled adult patients with AHRF treated with NPPV according to local clinical guidelines, in the Intermediate Emergency Care Department at Skane University Hospital in Malmö, Sweden. No power calculation was performed, as no prior suggested effect size for the association between ST2 values and mortality in the patient group researched exists, on which the calculation could be based. Treatment was decided by the attending physicians. Written informed consent was obtained from all patients or their next of kin. Enrollment in the study did not affect the treatment in any way and the study was thus observational and prospective. The study was approved by the Regional Ethics Board of Lund, Sweden and followed the precepts established by the Declaration of Helsinki.
Clinical Parameters And Follow-Up
Vital parameters were obtained before and during the treatment at several previously decided time points (0, 4, and 12 hrs after the start of treatment) and venous blood samples and arterial blood gases (ABG) were taken.33 The ABGs were analyzed immediately on a ABL800 Flex (Radiometer, Copenhagen, Denmark), while the venous blood samples were frozen and stored at −80ºC for later analysis of biomarkers, after having separated serum and plasma.
All patients received Bilevel NPPV treatment with Trilogy100 and a suitable NPPV mask (Respironics, Murrysville, Pennsylvania/USA), using S/T mode with volume-controlled autoregulated ventilation.33 Oxygen was applied as needed with a goal SpO2 of 88–90%.The patients were monitored closely during the treatment. The attending physicians decided if the treatment was discontinued. Details about treatment length and installations were obtained from the Directview Program (Respironics, Murrysville, Pennsylvania/USA).
The patients consented to have their medical history and current medication obtained through the journal database of the hospital, and to allow the retrieval of information on mortality and readmissions for the follow-up period of 2014–2016. Before analysis of data, the medical records were examined by an internist and a primary discharge diagnosis was made.
Biomarker Measurement
92 biomarkers were measured in frozen plasma samples using the Proseek Multiplex CVD 1 biomarker panel (Olink Bioscience, Uppsala, Sweden). The method is a multiplex immunoassay based on a Proximity Extension Assay. The unit of measurement is NPX (normalized protein expression) that gives a relative quantification and values can be compared only for the same protein across the samples analyzed.34 All assay characteristics including detection limits and measurements of assay performance and validations are available from the manufacturer’s webpage.35
ST2 was additionally measured with the Critical Diagnostics Presage ST2 clinical assay (Sopachem Diagnostics, Copenhagen, Denmark) in the baseline samples. The method is an in vitro diagnostic assay that quantitatively measures ST2 in serum or plasma by enzyme-linked immunosorbent assay (ELISA) in a microtiter plate format. All assay characteristics are available from the manufacturer´s webpage.36
Endpoint
The primary endpoint in the study was defined as mortality, with a follow-up period of, respectively, 28 days and 18 months after admission. Mortality and date of death were confirmed using the Swedish National civil registry. The secondary endpoint was clinical factors related to improvement in the Acute Hypercapnic Respiratory Failure, acquired during the hospital stay with a follow-up time of 12 hrs.
Statistical Analysis
All statistical analyses were performed with IBM SPSS statistics version 21 (SPSS Inc., Chicago, IL, USA). In univariate analyses, we used Kruskal–Wallis test to analyze continuous variables and expressed data as medians and interquartile ranges. For categorical variables, Fishers exact test was used and data were expressed as numbers and percentages. We used Cox proportional hazards model to relate baseline variables to risk of death during the timepoints of follow-up. Both types of ST2 levels measured were transformed with the natural logarithm and expressed as hazard ratios (95% confidence interval) on a standardized scale (per 1 standard deviation increment). We adjusted for age and gender (model 1) and age, gender and C-reactive protein (CRP) (model 2). The ST2 levels were divided into tertiles, with the lowest tertile as the reference group, and crude Kaplan-Meier plots for tertiles of ST2 levels were plotted. To evaluate the correlation between the two methods of ST2 measurement, we used Pearson´s correlation coefficient test. Wilcoxon paired rank test was used to evaluate if changes of ST2, pCO2 and pH between baseline and the first 4 or 12 hrs of treatment were significant. Subsequently, we used linear regression models to relate baseline ST2 levels to change in pCO2, pH and Respiratory Rate (RR) during the first 4 and 12 hrs of treatment. Same model was used to relate changes in ST2 levels during the first 4 and 12 hrs of treatment with simultaneous change in pCO2, pH and RR. Both baseline and delta values were log transformed before being entered in the linear regression analyses. All tests were two-sided and a p-value of <0.05 was considered statistically significant.
Results
Patient Characteristics
Fifty-one patients were enrolled in the study but five patients were excluded because of withdrawal of consent (n=3), presence of neurological disease (n=1) and sepsis (n=1) as the main underlying causes of AHRF, leaving forty-six patients in the analysis. Demographic characteristics of the study group are described in detail in our previous work33 and are summarized in Table 1.
 |
Table 1 Characteristics Of The Patients, As A Whole Group And Divided Into Subgroups |
All patients were evaluated by attending physician and in thirty-three patients (72%), the “Do Not Resuscitate” order was made and recorded in the medical journal, according to local guidelines. Twenty-four patients (52%) were evaluated not to be eligible for Intensive Care Unit in case of worsening. No patient was intubated during the hospital stay. Thirteen patients died within 28 days after admission (28%), of whom ten patients died during the hospital stay. During the long-term follow-up a total of thirty patients had died after 18 months (65%).
Before analysis of data, the medical records were examined by an internist and a primary discharge diagnosis was made. There were three subgroups of patients with regards to primary diagnosis: Acute Exacerbation of COPD (AECOPD, n=34), Acute Heart Failure (AHF, n=8) and Acute Exacerbation in OHS (AEOHS, n=4). To evaluate if there was difference between the subgroups, clinical characteristics were compared between the groups, with regards to basic characteristics as well as variables related to AHRF and thus determining the NPPV treatment (Table 1). There were significant subgroup differences in age, BMI and smoking status. A significant difference was found in only one of the AHRF related variables, Base Excess (BE). Nine patients in the AECOPD subgroup (26%) received long-term oxygen therapy (LTOT) at home before hospitalization, and three patients received long-term NPPV treatment at home (9%). No patient in the other subgroups received LTOT, while one patient in the AEOHS subgroup had long-term NPPV treatment (25%). Analyses were made on the whole group as well as on the largest subgroup of patients, i.e. that with AECOPD. There was no significant difference between ST2 values in the different subgroups (Kruskal Wallis test, p = 0.22).
ST2 As Predictor Of Short-Term And Long-Term Mortality
In order to evaluate the predictive value of ST2 in terms of mortality, ST2 was measured both as a part of the biomarker panel and with the Presage ST2 clinical assay. The correlation coefficient between the two measurements was r = 0.95 (Pearson´s correlation coefficient test, p = <0.001), as seen in Figure 1.
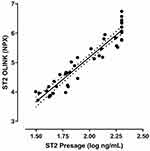 |
Figure 1 Correlation between ST2 values measured with Olink biomarker panel and Presage ST2 clinical assay. |
We analyzed ST2 levels (measured with Presage ST2 clinical assay) at admission before treatment was started and evaluated its correlation with short-term mortality. Each 1 SD increment of ST2 was associated with eleven – twelvefold-increased risk of 28 days mortality (Table 2). Regarding long-term mortality, this association remained significant with about twofold-increased risk of 18 months mortality (Table 2).
 |
Table 2 Relationship Between ST2 And Risk Of 28 Days And 18 Months Mortality |
To analyse this correlation further, ST2 values were divided into tertiles and then entered into Model 1 and 2. Patients in the highest tertile showed a significant increased risk of both short-term and long-term mortality compared with the lowest tertile, as shown in Table 2 and with a Kaplan–Meier curve in Figure 2.
 |
Figure 2 Kaplan–Meier plot showing cumulative mortality during 18 months follow-up period. Tertile 1 denotes the lowest values of ST2, and Tertile 3 the highest values. |
Finally, we tested ST2 levels and correlation to mortality in the largest subgroup of patients with AECOPD as a primary diagnosis. Each 1 SD increment of ST2 was associated with more than sevenfold increased risk of 28 days mortality (HR 7.11, 95% CI 1.3 −38.1, p = 0.022). Regarding long-term mortality, this association remained significant with a twofold-increased risk of 18 months mortality (HR 2.07, 95% CI 1.3–3.3, p = 0.003).
The ST2 correlation for both short-term and long-term mortality was independent of blood gas results and lactate, as well as BMI (data not shown).
Serial ST2 Measurements And Correlation To Treatment Response
ST2 levels (Olink biomarker panel) before the start of treatment and after 4 and 12 hrs of treatment were compared with changes in pH and pCO2, which were chosen to represent changes in status of AHRF and thus treatment response (Table 3). Linear regression analysis did not indicate a predictive correlation between ST2 levels before treatment was started, and the subsequent changes in pH and pCO2 (data not shown). Median levels of ST2 increased during the follow-up time of 12 hrs. The amount of increase was positively related to change of pH between baseline and 4 hrs post start of treatment (beta 0.21, 95% CI 0.07 to 0.34, p 0.004) as well as between baseline and 12 hrs post start of treatment (beta 0.23, 95% CI 0.12 to 0.34, p <0.001). On the other hand, the amount of increase in ST levels was negatively related to change of pCO2 between baseline and 4 hrs post start of treatment (beta – 0.21, 95% CI – 0.36 to – 0.06, p 0.008) as well as between baseline and 12 hrs post start of treatment (beta – 0.29, 95% CI – 0.46 to – 0.14, p <0.001). These were missing values in the repetitive ST2 measurements (at admission n=46, after 4 hrs n=41, after 12 hrs n=36).
 |
Table 3 ST2, pCO2 And pH Values Before Start Of Treatment, And After 4 And 12 hrs |
Discussion
Our findings suggest that the protein ST2, a known prognostic biomarker for mortality in patients with cardiovascular diseases and pulmonary diseases,20,28 is a strong predictor of short-term mortality in patients with AHRF treated with NPPV. Moreover, our hypothesis that decreasing ST2 values during the first 12 hrs of treatment predicted improved outcome was not supported.
Patients with AHRF of various underlying causes are often treated with NPPV in addition to standard medical treatment.7 Patients with underlying AECOPD have a well-established treatment indication, but as the use of NPPV treatment has increased, further research has supported the benefits of NPPV in non-COPD patients with AHRF.7,11 Moreover, the exact cause of the clinical illness is not always obvious at the Emergency Room encounter and many patients have multiple clinical conditions contributing to AHRF.1,3,5,15 Thus, the search for factors that help predict treatment outcome regardless of the underlying cause, could be an important additional piece in the puzzle of clinical decision-making during the first hours of admission, before more targeted disease-specific treatment can be initiated.
To our knowledge, no study has been published regarding ST2 in patients with AHRF. Considering existing knowledge about ST2 and its prognostic value for mortality in both cardiac-related and non-cardiac related acute respiratory failure,20,28 our results might not seem surprising. However, they are valuable in regards to strengthening the value of ST2 as a prognostic factor for mortality, even in an unselected group of patients with AHRF. Further research on a larger group of patients with better-defined phenotypes of AHRF are needed to confirm our results.
The long-term predictive value of ST2 in regards to mortality in the patient group is more uncertain, since the results seem mostly dependent on the strong association with 28 days mortality (Table 2 and Figure 2). We conclude that in this patient group, the prognostic value of ST2 is most informative for short-term mortality and likely dependent on the acute illness rather than the underlying chronic conditions.
ST2 measurements in patients with Acute Heart Failure (AHF) have been suggested to be of even greater value when measured repeatedly, as an indicator of treatment outcome.27 Manzano-Fernandez et al, measured ST2 at presentation and after four days of treatment in patients with AHF, and found that in the acute setting medical treatment resulted in a significant decrease in ST2 levels, and that serial ST2 measurements resulted in an improvement in predicting long-term mortality.37 Breidthardt et al, came to similar conclusions when measuring ST2 before the start of treatment and after 48 hrs, also in patients with AHF.38 To our knowledge, no study has been published regarding serial measurements of ST2 within the first day of admission for AHF.
Our study on unselected patients with AHRF treated with NPPV, suggests that increasing ST2 levels during the first 12 hrs of treatment was related to changes in variables related to AHRF representing improving clinical state (increase in pH and decrease in pCO2). The initial hypothesis that decreasing ST2 values during the first 12 hrs of treatment predicted improved outcome, was therefore not supported. The missing ST2 values in the repetitive measurements might represent the patients dying in the very first hours after admission or where blood samples were non-retrievable for other reasons. This is a possible source of bias, as this might be the patients presenting a worsening clinical state.
Our results might suggest that it takes time for ST2 concentrations in serum to decrease, or maybe even that a maximal concentration develops shortly after treatment is started. In this matter, no conclusions can be reached at this point because of the small size and heterogeneity of the patient group and further studies are needed.
Our findings do not at this point call for the regular use of ST2 measurements in clinical practice in patients with AHRF, but in combination with other data, there are indications that further research in the field is desirable.20 The limitations of the study are outlined in detail in our previous work, with the main one being the relatively small patient group.33 In comparison with some other studies in the field, the patients may be considered as more seriously ill, in terms of high short-term mortality (28 days mortality 28%) and high percentage of the “Do Not Resuscitate” order (72%). This is a limitation in terms of comparing different results. On the other hand, research in this particular group of seriously ill patients is difficult to perform despite its important value. Since another possible limitation of the study was that the ST2 values were not measured in a quantitative manner, it was decided to repeat measurements with the Presage 2 clinical assay. The high correlation between both the measurement methods strengthens the results of our study. It is also of interest to evaluate if repetitive ST2 measurements over a longer period of time could help in predicting prognosis in patients in Chronic Hypercapnic Respiratory Failure.
Conclusion
Our findings suggest that ST2 is a target to explore further as a clinically useful predictor of short-term mortality, in patients with AHRF treated with NPPV, regardless of the underlying causes. The data do on the other hand not imply that serial ST2 measurements in the first hours of treatment can aid in predicting immediate clinical outcome. Further research with larger group of patients is needed.
Abbreviations
ABG, arterial blood gas; AECOPD, Acute Exacerbation of Chronic Obstructive Pulmonary Disease; AEOHS, Acute Exacerbation of Obesity Hypoventilation Syndrome; AHF, Acute Heart Failure; AHRF, Acute Hypercapnic Respiratory Failure; BE, Base Excess; BMI, body mass index; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; ELISA, enzyme-linked immunosorbent assay; FEV1, forced expiratory volume in 1 second; HR, hazard ratio; IL-33, interleukin-33; IQR, interquartile range; NPPV, Noninvasive Positive Pressure Ventilation; NPX, normalized protein expression; pCO2, partial pressure of CO2; pO2, partial pressure of O2; SD, standard deviation; ST2, suppression of tumorigenicity 2; ST2L, suppression of tumorigenicity 2 ligand; sST2, soluble suppression of tumorigenicity 2.
Ethics Statement
The study was approved by the Regional Ethics Board of Lund, Sweden and followed the precepts established by the Declaration of Helsinki. Written informed consent was obtained from all patients or their next of kin. Enrollment in the study did not affect the treatment in any way and the study was thus observational and prospective.
Data Sharing Statement
Data cannot be shared publicly at this time because our research group does not have an online-based database. Data are available from the corresponding author Dr. Brynja Jónsdóttir, for potential Editors or reviewers at request, and can be sent via e-mail as a SPSS file.
Acknowledgments
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Stokes NR, Dietz BW, Liang JJ. Cardiopulmonary laboratory biomarkers in the evaluation of acute dyspnea. Open Access Emerg Med. 2016;8:35–45. doi:10.2147/OAEM.S71446
2. Dieplinger B, Gegenhuber A, Kaar G, Poelz W, Haltmayer M, Mueller T. Prognostic value of established and novel biomarkers in patients with shortness of breath attending an emergency department. Clin Biochem. 2010;43(9):714–719. doi:10.1016/j.clinbiochem.2010.02.002
3. DeVos E, Jacobson L. Approach to adult patients with acute dyspnea. Emerg Med Clin North Am. 2016;34(1):129–149. doi:10.1016/j.emc.2015.08.008
4. Parshall MB, Schwartzstein RM, Adams L, et al. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185(4):435–452. doi:10.1164/rccm.201111-2042ST
5. Roberts CM, Stone RA, Buckingham RJ, Pursey NA, Lowe D. Acidosis, non-invasive ventilation and mortality in hospitalised COPD exacerbations. Thorax. 2011;66(1):43–48. doi:10.1136/thx.2010.153114
6. Schnell D, Timsit JF, Darmon M, et al. Noninvasive mechanical ventilation in acute respiratory failure: trends in use and outcomes. Intensive Care Med. 2014;40(4):582–591. doi:10.1007/s00134-014-3222-y
7. Bello G, De Pascale G, Antonelli M. Noninvasive ventilation. Clin Chest Med. 2016;37(4):711–721. doi:10.1016/j.ccm.2016.07.011
8. Cabrini L, Landoni G, Oriani A, et al. Noninvasive ventilation and survival in acute care settings: a comprehensive systematic review and metaanalysis of randomized controlled trials. Crit Care Med. 2015;43(4):880–888. doi:10.1097/CCM.0000000000000819
9. Roberts CM, Brown JL, Reinhardt AK, et al. Non-invasive ventilation in chronic obstructive pulmonary disease: management of acute type 2 respiratory failure. Clin Med. 2008;8(5):517–521. doi:10.7861/clinmedicine.8-5-517
10. Celikel T, Sungur M, Ceyhan B, Karakurt S. Comparison of noninvasive positive pressure ventilation with standard medical therapy in hypercapnic acute respiratory failure. Chest. 1998;114(6):1636–1642. doi:10.1378/chest.114.6.1636
11. Osadnik CR, Tee VS, Carson-Chahhoud KV, Picot J, Wedzicha JA, Smith BJ. Non-invasive ventilation for the management of acute hypercapnic respiratory failure due to exacerbation of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017;7:Cd004104. doi:10.1002/14651858.CD003881.pub4
12. Lightowler JV, Wedzicha JA, Elliott MW, Ram FS. Non-invasive positive pressure ventilation to treat respiratory failure resulting from exacerbations of chronic obstructive pulmonary disease: cochrane systematic review and meta-analysis. BMJ (clinical Research Ed). 2003;326(7382):185. doi:10.1136/bmj.326.7402.1329-b
13. Plant PK, Owen JL, Elliott MW. Non-invasive ventilation in acute exacerbations of chronic obstructive pulmonary disease: long term survival and predictors of in-hospital outcome. Thorax. 2001;56(9):708–712. doi:10.1136/thorax.56.9.708
14. Mas A, Masip J. Noninvasive ventilation in acute respiratory failure. Int J Chron Obstruct Pulmon Dis. 2014;9:837–852. doi:10.2147/COPD.S42664
15. Davidson AC, Banham S, Elliott M, et al. BTS/ICS guideline for the ventilatory management of acute hypercapnic respiratory failure in adults. Thorax. 2016;71(Suppl 2):ii1–i35. doi:10.1136/thoraxjnl-2015-208209
16. Confalonieri M, Potena A, Carbone G, Porta RD, Tolley EA, Umberto Meduri G. Acute respiratory failure in patients with severe community-acquired pneumonia. A prospective randomized evaluation of noninvasive ventilation. Am J Respir Crit Care Med. 1999;160(5 Pt 1):1585–1591. doi:10.1164/ajrccm.160.5.9903015
17. Bello G, De Pascale G, Antonelli M. Noninvasive ventilation for the immunocompromised patient: always appropriate? Curr Opin Crit Care. 2012;18(1):54–60. doi:10.1097/MCC.0b013e32834e7c21
18. Vital FM, Ladeira MT, Atallah AN. Non-invasive positive pressure ventilation (CPAP or bilevel NPPV) for cardiogenic pulmonary oedema. Cochrane Database Syst Rev. 2013;31(5):Cd005351.
19. Adler D, Pepin JL, Dupuis-Lozeron E, et al. Comorbidities and subgroups of patients surviving severe acute hypercapnic respiratory failure in the intensive care unit. Am J Respir Crit Care Med. 2017;196(2):200–207. doi:10.1164/rccm.201608-1666OC
20. Ciccone MM, Cortese F, Gesualdo M, et al. A novel cardiac bio-marker: ST2: a review. Molecules. 2013;18(12):15314–15328. doi:10.3390/molecules181215314
21. Januzzi JL
22. Mueller T, Dieplinger B. Soluble ST2 and galectin-3: what we know and don’t know analytically. Ejifcc. 2016;27(3):224–237.
23. Januzzi JL
24. Pascual-Figal DA, Manzano-Fernandez S, Boronat M, et al. Soluble ST2, high-sensitivity troponin T- and N-terminal pro-B-type natriuretic peptide: complementary role for risk stratification in acutely decompensated heart failure. Eur J Heart Fail. 2011;13(7):718–725. doi:10.1093/eurjhf/hfr047
25. Wang J, Tan GJ, Han LN, Bai YY, He M, Liu HB. Novel biomarkers for cardiovascular risk prediction. J Geriatr Cardiol. 2017;14(2):135–150. doi:10.11909/j.issn.1671-5411.2017.02.008
26. Ghashghaei R, Arbit B, Maisel AS. Current and novel biomarkers in heart failure: bench to bedside. Curr Opin Cardiol. 2016;31(2):191–195. doi:10.1097/HCO.0000000000000254
27. Maisel AS, Richards AM, Pascual-Figal D, Mueller C. Serial ST2 testing in hospitalized patients with acute heart failure. Am J Cardiol. 2015;115(7 Suppl):32b–37b. doi:10.1016/j.amjcard.2015.01.038
28. Bajwa EK, Mebazaa A, Januzzi JL. ST2 in pulmonary disease. Am J Cardiol. 2015;115(7 Suppl):44b–47b. doi:10.1016/j.amjcard.2015.01.040
29. Tajima S, Oshikawa K, Tominaga S, Sugiyama Y. The increase in serum soluble ST2 protein upon acute exacerbation of idiopathic pulmonary fibrosis. Chest. 2003;124(4):1206–1214. doi:10.1378/chest.124.4.1206
30. Hoogerwerf JJ, Tanck MW, van Zoelen MA, Wittebole X, Laterre PF, van der Poll T. Soluble ST2 plasma concentrations predict mortality in severe sepsis. Intensive Care Med. 2010;36(4):630–637. doi:10.1007/s00134-010-1773-0
31. Xia J, Zhao J, Shang J, et al. Increased IL-33 expression in chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol. 2015;308(7):L619–L627. doi:10.1152/ajplung.00305.2014
32. Mueller T, Leitner I, Egger M, Haltmayer M, Dieplinger B. Association of the biomarkers soluble ST2, galectin-3 and growth-differentiation factor-15 with heart failure and other non-cardiac diseases. Clin Chim Acta. 2015;445:155–160. doi:10.1016/j.cca.2015.03.033
33. Jonsdottir B, Jaworowski A, San Miguel C, Melander O. IL-8 predicts early mortality in patients with acute hypercapnic respiratory failure treated with noninvasive positive pressure ventilation. BMC Pulm Med. 2017;17(1):35. doi:10.1186/s12890-017-0500-9
34. Lundberg M, Eriksson A, Tran B, Assarsson E, Fredriksson S. Homogeneous antibody-based proximity extension assays provide sensitive and specific detection of low-abundant proteins in human blood. Nucleic Acids Res. 2011;39(15):e102. doi:10.1093/nar/gkr424
35. Proseek Multiplex. A Precision proteomics solution for targeted protein biomarker discovery; 2016. Available from: http://www.olink.com/products/proseek-multiplex/.
36. Presage® ST2 Assay. [Webpage]; 2018. Available from: http://www.sopachem.com/diagnostics/portfolio/presage-st2-assay/.
37. Manzano-Fernandez S, Januzzi JL, Pastor-Perez FJ, et al. Serial monitoring of soluble interleukin family member ST2 in patients with acutely decompensated heart failure. Cardiology. 2012;122(3):158–166. doi:10.1159/000338800
38. Breidthardt T, Balmelli C, Twerenbold R, et al. Heart failure therapy-induced early ST2 changes may offer long-term therapy guidance. J Card Fail. 2013;19(12):821–828. doi:10.1016/j.cardfail.2013.11.003
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
