Back to Journals » Journal of Asthma and Allergy » Volume 16
Sputum Neurturin Levels in Adult Asthmatic Subjects
Authors Sato S , Suzuki Y, Kikuchi M, Rikimaru M, Saito J, Shibata Y
Received 2 June 2023
Accepted for publication 12 August 2023
Published 31 August 2023 Volume 2023:16 Pages 889—901
DOI https://doi.org/10.2147/JAA.S421742
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Amrita Dosanjh
Suguru Sato, Yasuhito Suzuki, Masami Kikuchi, Mami Rikimaru, Junpei Saito, Yoko Shibata
Department of Pulmonary Medicine, School of Medicine, Fukushima Medical University, Fukushima, Japan
Correspondence: Suguru Sato, Email [email protected]
Background: Neurturin (NRTN) is a neurotrophic factor that was originally identified in the development and maintenance of neural cells. Recent studies involving NRTN knockout mice have reported its anti-inflammatory effects in allergic airway conditions. However, the role of NRTN in human asthma has not yet been identified.
Objective: The purposes of the present study were to confirm the presence of NRTN in the airways and to investigate the clinical and pathogenetic roles of NRTN in asthma.
Methods: The NRTN levels in the induced sputum were measured by enzyme-linked immunosorbent assay (ELISA). Relationships between NRTN and clinical characteristics, asthma control status, and airway inflammation were assessed.
Results: Sixty-four asthmatic subjects were enrolled in the study. All asthmatic subjects had detectable sputum NRTN levels, with a mean (SD) level of 2.03 (1.29) ng/mL. The sputum NRTN levels had significant positive correlations with sputum eosinophil and exhaled nitric oxide levels and were significantly higher in the atopic subjects than in the non-atopic subjects. No significant difference in sputum NRTN levels were observed for asthma control status and asthma exacerbation. In sputum inflammatory analyses, sputum NRTN level was positively correlated with interleukin (IL)-5 and IL-13 levels, and negatively correlated with matrix metalloproteinase (MMP)-9 level.
Conclusion: It is plausible that sputum NRTN could serve as a new marker for Type 2 airway inflammation, implicating its role in the process of airway remodeling in asthma. Future studies should investigate the clinical relevance of sputum NRTN level in prospective analyses.
Keywords: neurturin, asthma, sputum, airway inflammation, type 2 inflammation
Introduction
Neurturin (NRTN) is a member of the glial cell line-derived neurotrophic factor (GDNF) family of ligands (GFLs). GDNF is a protein structurally related to transforming growth factor (TGF)-β and mediates signals through the glycosyl-phosphatidylinositol (GPI)-anchored coreceptors (GFRα1-4), which acts as a ligand binding component.1,2 The neurotrophic factors, comprising GDNF, NRTN, artemin and persephin, are well known for their implication in the growth and survival of the various nerve systems.3 Due to its ability to control functions in neutral cells, NRTN has been assessed in clinical trials as a treatment for neurodegenerative diseases such as Parkinson’s disease.4,5 Moreover, exogenous NRTN rescues neurons in models of Parkinson’s disease, as well as in Hirschsprung disease.6 It has also been reported that the GDNF, NRTN, and their predominant receptors (GFRα1 and GFRα2) mRNA are expressed during embryogenesis, most notably in epithelial–mesenchymal interactions. GDNF and NRTN play critical roles in the outgrowth of epithelial structures.7 In recent years, accumulating evidence suggests that the neuronal and immune systems exhibit bidirectional interactions that play critical roles in tissue homeostasis and inflammation (neuro-immune crosstalk).8 GFLs are expressed during inflammation outside the nerve system in peripheral tissues.7 In addition to their role as a nerve growth factor, GFLs have also been suggested to be involved in inflammation.
Both GDNF and NRTN have been detected in airway smooth muscle cells, while GFRα2, RET, and neural cell adhesion molecules were predominantly found in respiratory epithelial cells.9 NRTN exhibits anti-inflammatory properties by inhibiting the secretion of interleukin (IL)-6 and tumor necrosis factor (TNF)-α from lipopolysaccharide (LPS)-stimulated human peripheral blood mononuclear cells.10 The anti-inflammatory effects in both the acute and chronic phases of allergic airway condition were reported in NRTN knockout (NRTN−/−) mouse models.11,12 However, there are few reports about the role of NRTN in human asthmatic subjects. In the present study, we investigated the clinical role of NRTN in asthma, as well as its relationship with inflammatory pathology, by measuring sputum NRTN levels in adult asthmatic subjects.
Methods
Study Subjects and Design
This study is a cross-sectional study of adult asthmatic subjects, who were recruited from the outpatient clinic at the Department of Pulmonary Medicine in Fukushima Medical University Hospital between March 2017 and September 2019. We enrolled asthmatic subjects who had received standard asthmatic treatment by their attending physicians. All subjects were diagnosed according to the American Thoracic Society (ATS) criteria13 and they were regularly followed up at our outpatient clinic. Asthma was defined on the basis of recurrent episodes of at least one symptom (cough, wheeze, or dyspnea) associated with reversible airflow limitation (12% and 200 mL variability in forced expiratory volume in one second [FEV1] either spontaneously or with an inhaled short-acting β2-agonist) and/or increased airway responsiveness. Control status and severity of asthma were assessed according to the guidelines of the Japanese Society of Allergology.14 Asthmatic subjects were classified into three groups based on severity: mild asthma (mild intermittent and mild persistent), moderate asthma (moderate persistent), or severe asthma (severe persistent). All subjects provided written informed consent for analysis of their clinical data and an induced sputum was approved by the Ethics Committee of Fukushima Medical University in June 2019 (No.2019-069). This study was conducted in accordance with the Declaration of Helsinki.
To measure sputum NRTN, induced sputum samples were obtained following a fractional exhaled nitric oxide (FeNO) measurement, pulmonary function test, and Asthma Control Test (ACT). These procedures and tests were all performed in a single day when each patient had not had an asthma exacerbation for at least four weeks. In addition, blood samples were obtained from subjects who consented to the measurement of their serum NRTN. Sputum and serum NRTN were measured by enzyme-linked immunosorbent assay (ELISA).
Sputum Induction and Processing
Sputum induction was performed with inhaled nebulized 5.0% NaCl solution.15 Sputum plugs were harvested and processed with 0.1% dithiothreitol (DTT) and phosphate-buffered saline (PBS). The sputum supernatant of 0.8–1.0 mL was aliquoted into the serum tube. The rest of the sputum supernatant was also stored at −80°C for cytokine measurements. Cytospins (Auto Smear CF-120: Sakura Finetek Japan Co. Ltd., Tokyo, Japan) were prepared for differential cell counts. Each subject was assigned to only one of four groups on the basis of sputum eosinophil and neutrophil percentages. Cutoffs of 2% and 50% were used to define eosinophilic and neutrophilic asthma, respectively.16 Four groups were eosinophilic asthma (eosinophils ≥ 2% and neutrophils < 50%), mixed-granulocytic asthma (eosinophils ≥ 2% and neutrophils ≥ 50%), neutrophilic asthma (eosinophils < 2% and neutrophils ≥ 50%), and pautigranulocytic asthma (eosinophils < 2% and neutrophils < 50%), respectively.
Measurement of Sputum and Serum NRTN Levels
ELISA was performed using a commercially available sandwich ELISA kit according to the manufacturer’s instructions (LifeSpan BioSciences, Seattle, WA, USA) for the detection of sputum NRTN (measuring range: 0.313–20 ng/mL, sensitivity 0.114 ng/mL). Liu et al suggested that serum NRTN level may be outside the measuring range for detection of the sputum NRTN ELISA kit.17 Hence, another ELISA kit (RayBiotech, Norcross, GA, USA) was used to measure serum NRTN (measuring range: 0.036 ng/mL–10 ng/mL, sensitivity 0.036 ng/mL).
Measurement of Inflammatory Cytokines and Matrix Metalloproteinase
We measured IL-4, IL-5, and IL-13 in the induced sputum supernatant as indicators of Type 2 inflammation, IL-6 level as an indicator of proinflammatory cytokine, and interferon (IFN)-γ level as an indicator of Type 1 inflammation. In addition, matrix metalloproteinase (MMP)-2 and MMP-9 were also measured. Measurements were performed using commercially available sandwich ELISA kits (IL-4, IL-5 and IL-13: Invitrogen, Waltham, MA, USA; IL-6 and MMP-9: R&D Systems, Minneapolis, MN, USA; MMP-2; Proteintech, Rosemont, IL, USA; IFN-γ: Immuno-Biological Laboratories Co., Ltd., Gunma, Japan).
Blood Tests
Blood tests included peripheral blood eosinophil count, serum non-specific IgE and antigen-specific IgE. A radioallergosorbent test for antigen-specific IgE was performed for weeds, mites, house dust, cats, dogs, cedar, cypress, orchard grass, moths, Aspergillus, Candida, and Alternaria. Non-specific IgE was measured by fluorescence enzyme immunoassay (UniCAP; Pharmacia & Upjohn, Uppsala, Sweden). Atopy was defined as either a non-specific IgE level of ≥250 IU/mL or any positive antigen-specific IgE (≥0.70 UA/mL).
Asthma Control Assessment
Pulmonary function testing was performed using rolling seal spirometers (Chestac-11 Cyber S-type; Chest M.I., Inc., Tokyo, Japan) to measure forced vital capacity (FVC) and FEV1. The tests were performed by experienced respiratory technicians according to ATS guidelines.18 FVC and FEV1 were expressed as percent predicted values. FeNO measurements were taken according to the ATS and European Respiratory Society recommendations19 using a chemiluminescence analyzer NA623N® (Kimoto Electronic Co., Ltd., Osaka, Japan). Measurements were performed, as described previously,20 with the patient in a sitting position and without wearing a nose clip. From total lung capacity without holding their breath, the patient exhaled at a constant flow of 50 mL/s. FeNO was measured three times, with differences in measured values within 10%. The mean value of which two measurements were used as data for statistical analysis. FeNO values were expressed as parts per billion (ppb). The Asthma Control Test (ACT) was used for subjective assessment of asthma control.21 An ACT score of ≥20 points indicates well-controlled asthma.
Statistical Analyses
As a preliminary study of the present study, the relationship between sputum NRTN and serum NRTN levels was assessed. In a previous study about serum biomarker analyses in patients with ovarian insufficiency, serum NRTN levels were measured using a high-sensitivity ELISA kit.17 First, sputum NRTN levels and asthma-related parameters (characteristics, atopic status, and asthma severity) were assessed. The relationships between sputum NRTN levels and asthma control parameters (FeNO, pulmonary function, and ACT score) or sputum inflammatory cell counts were also assessed. For the secondary assessment, the relationships between sputum NRTN and airway inflammation were assessed. We measured inflammatory cytokine and metalloproteinase concentrations in the collected induced sputum supernatant and examined their relationships with NRTN in sputum.
All values are expressed as mean ± standard deviation (SD) of the mean unless otherwise specified. Comparisons of sputum NRTN levels between asthmatic subjects with various conditions were performed by Mann–Whitney U-tests. The associations between the sputum NRTN level and characteristics of asthmatic subjects, asthma-related parameters, sputum inflammatory cell counts, and inflammatory molecules (cytokines and MMPs) were assessed using Spearman’s rank correlation analysis. Comparisons of sputum NRTN levels between various asthma severities and different asthma inflammatory phenotypes based on sputum inflammatory cell counts were performed using analysis of variance followed by Tukey’s post hoc test. P values of less than 0.05 were considered statistically significant. Statistical analysis was performed with SPSS Statistics Base for Windows (version 28.0.0.0; IBM, Armonk, NY, USA).
Results
In the study period, a total of 65 adult asthmatic subjects were included in the study. One subject was excluded from the study due to lack of clinical data. The characteristics of the remaining 64 subjects (32 males and 32 females) are shown in Table 1. The treatment contents and control parameters of the subjects at sputum collection are shown in Table 2.
 |
Table 1 Characteristics of Asthmatic Subjects |
 |
Table 2 Summary of Asthma Control-Related Parameters |
Relationship Between Sputum NRTN and Serum NRTN Levels
The mean (SD) sputum NRTN level was 2.03 (1.29) ng/mL. As the serum NRTN levels were below the measurement sensitivity in four samples, the mean (SD) NRTN level in 11 asthmatic subjects was 0.12 (0.10) ng/mL. The amount of Serum NRTN was detected at approximately one-tenth that in the sputum. There was no significant correlation between the sputum NRTN and serum NRTN levels (r = 0.136, p = 0.628) (Figure 1).
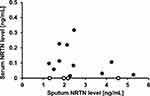 |
Figure 1 Relationship between sputum NRTN levels and serum NRTN levels in asthmatic subjects (n = 15). Open circles indicate levels below the lower limit of the measurement range. |
Relationships Between Sputum NRTN Level and Asthma Related Parameters
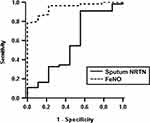
The sputum analyses data in 64 asthmatic subjects are shown in Table 3. There were no significant correlations between sputum NRTN level and the characteristics of the asthmatic subjects in Table 1 except atopic status. The mean sputum NRTN level was significantly higher in the atopic subjects than in the non-atopic subjects (mean ± SD; 2.19 ± 1.34 ng/mL vs 1.53 ± 1.0 ng/mL; p = 0.046), although no significant difference was observed regarding asthma severity (Figure 2A and B). The sputum NRTN level appeared lower in the controlled subjects with an ACT score ≥20 points compared to the uncontrolled subjects, although the trend was not statistically significant (mean ± SD; 1.81 ± 1.15 vs 2.25 ± 1.40; p = 0.096) (Figure 2C). In addition, there were no significant differences in sputum NRTN levels between the asthmatic subjects with or without a fixed airway obstruction and those with or without asthma exacerbations in the previous year (Figure 2D–F). There was a significant positive correlation between sputum NRTN and FeNO levels (r = 0.364, p = 0.003), and no significant correlations were observed between sputum NRTN level and asthma symptoms (ACT score) or pulmonary function (% predicted FEV1) (Figure 3A–C). The Global Initiative for Asthma (GINA) uses a lower cut-off of 20 ppb in the classification of asthma with Type 2 inflammation.22 No significant difference was observed in sputum NRTN levels between the asthmatic subjects with FeNO <20 ppb and those with FeNO ≥20 ppb (Figure 3D). We constructed receiver operating characteristics (ROC) curves using sputum NRTN and FeNO levels as predictors of sputum eosinophilia (eosinophils ≥ 2%) (Figure 4). The area under the curves for sputum NRTN and FeNO were 0.578 and 0.945, respectively.
 |
Table 3 Summary of Sputum Analyses Data |
Relationships Between Sputum NRTN Level and Airway Inflammation Parameters
Figure 5 shows the relationships between sputum inflammatory cell counts and sputum NRTN level. There was a significant positive correlation between sputum NRTN level and proportion of sputum eosinophils (r = 0.348, p = 0.005), whereas there was a significant negative correlation between sputum NRTN level and sputum neutrophils (r = −0.458, p < 0.001). Sputum NRTN level was not significantly correlated with sputum macrophages (r = 0.022, p = 0.864) and sputum lymphocytes (r = 0.114, p = 0.371). In the classification of airway inflammation by sputum inflammatory cell fraction, 27 cases were eosinophilic, 28 cases were mixed granulocytic, seven cases were neutrophilic, and two cases were paucigranulocytic. In the eosinophilic group, the sputum NRTN levels were significantly higher than those in the mixed granulocytic (mean ± SD; 2.56 ± 1.57 ng/mL vs 1.58 ± 0.78 ng/mL; p = 0.022) (Figure 6).
 |
Figure 5 Correlations between sputum NRTN level and sputum eosinophils (A), sputum neutrophils (B), sputum macrophages (C), and sputum lymphocytes (D) in asthmatic subjects. |
 |
Figure 6 Comparisons of sputum NRTN levels between asthmatic subjects by airway inflammatory phenotypes. *p < 0.05. |
To investigate the relationship between sputum NRTN level and the pathology of airway inflammation, we performed a molecular search using the collected sputum supernatant. The cytokine and MMPs levels in the sputum supernatant are shown in Table 3. Due to the insufficient amounts of collected sputum supernatant, it was difficult to examine all inflammatory molecules of all the subjects. IL-4 and IL-5 were measured in 44 cases, IL-13 in 51 cases, IL-6 in 36 cases, respectively, IFN-γ in 38 cases, MMP-2 in 33 cases, and MMP-9 in 37 cases. Figure 7 shows the relationships between sputum NRTN level and inflammatory molecules. In terms of Type 2 cytokines, sputum NRTN level was significantly positively correlated with IL-5 level (r = 0.315, p = 0.037) and IL-13 level (r = 0.374, p = 0.007) in sputum, whereas no significant correlation was observed between sputum NRTN level and IL-4 level (r = 0.107, p = 0.488). In Type 1 and proinflammatory cytokines, no significant correlations were observed between the sputum NRTN level and cytokines (IFN-γ and IL-6) levels in sputum (r = −0.132, p = 0.431 and r = −0.207, p = 0.226, respectively). In the analyses of MMP levels in the sputum supernatant, sputum NRTN level was significantly negatively correlated with MMP-9 level (r = −0.478, p = 0.009), whereas no significant correlation was observed between sputum NRTN level and sputum MMP-2 level (r = 0.019, p = 0.918).
Discussion
In the present study, we measured NRTN levels in sputum from adult asthmatic subjects, and investigated the relationships between clinical features, asthma control, inflammatory cells, cytokines, and MMPs in sputum. Sputum NRTN was measured by ELISA in all cases. Furthermore, no correlation was observed between sputum and serum NRTN levels which were collected on the same day. In the characteristics of the study subjects, sputum NRTN levels were significantly higher in the atopic asthmatic subjects than in the non-atopic asthmatic subjects. On the other hand, sputum NRTN level did not have a clear relationship with current asthma symptoms, asthma severity, fixed airway obstructions and asthma exacerbations, which were assessed retrospectively. The sputum NRTN level was positively correlated with FeNO, sputum eosinophils, IL-5, and IL-13 in induced sputum supernatant and negatively correlated with neutrophils and MMP-9. These results suggest that sputum NRTN level is associated with Type 2 airway inflammation in adult asthmatic subjects.
Although there have been reports on the detection of NRTN in several peripheral tissues in vivo,10,23 there are few reports on quantitative evaluation. NRTN has been detected in human serum and in premature ovarian failure and is downregulated in the serum of patients with premature ovarian insufficiency compared to healthy fertile and menopausal groups.17 In the present study, NRTN in sputum was detected and quantified by the ELISA method in all adult asthmatic subjects. Furthermore, the level in sputum was about 10 times higher than that in the serum. Although immunohistological analyses of lung tissue were not performed in the present study, NRTN detected in induced sputum appears to have been derived from airway epithelial cells, based on past reports.23,24 Our results support the presence of NRTN in the airway, and suggest that it may be present at higher levels than previously measured in serum.
Several studies have investigated the role of NRTN in allergic airway inflammation. Previous reports on airway inflammation induced by ovalbumin (OVA) and house dust in NRTN−/− mice models revealed that the absence of NRTN is correlated with an increased number of eosinophils and Type 2 immune responses in lung tissue and lung draining lymph node cells.11,12 In the chronic inflammatory phase, NRTN−/− mice displayed increased markers of airway remodeling like collagen deposition, higher levels of neutrophils, MMP-9, TNF-α, and IL-6. Moreover, administration of exogenous NRTN before challenge with OVA reduce remodeling. According to the NRTN−/− mice studies, NRTN might be produced by airway epithelial cells as a response to stimulation from the allergen. It may be able to modulate epithelial and immune cells like dendritic cells (DCs) through the regulation of acute airway inflammation. In the chronic inflammatory phase, NRTN might protect the airway remodeling by regulating the expression of TNF-α. In the present study, sputum NRTN level was significantly positively correlated with FeNO level and sputum eosinophils, and this relationship was also supported by analyses of Type 2 cytokines (IL-5 and IL-13) level. Moreover, sputum NRTN levels in asthmatic subjects with atopy were significantly higher than those without atopy. Similar to sputum NRTN, sputum eosinophils and FeNO levels were significantly higher in the atopic asthma subjects than in the non-atopic asthma subjects. On the other hand, no significant differences were found in the peripheral blood eosinophils between the two groups. The difference in sputum NRTN levels by atopic status may be influenced by Type 2 airway inflammation in asthma. These results are paradoxically relevant compared to previous studies with NRTN−/− mice,12 suggesting the possibility that NRTN is elevated as negative feedback against strong Type 2 immune responses in asthmatic airways. However, it was not clear whether the airway NRTN alleviates strong Type 2 inflammation and clinically suppresses the disease. In this retrospective study, there was no clear difference in asthma exacerbation or airway obstruction between high and low sputum NRTN levels. Moreover, we analyzed whether sputum NRTN is a marker that reflects eosinophilic airway inflammation using ROC curves. However, it was not concluded that sputum NRTN is a sensitive marker of eosinophilic airway inflammation like FeNO. Comparison of the time course of NRTN levels over a few years may reveal the role of NRTN in the airway and further study is needed.
The sputum NRTN levels were significantly lower in the mixed granulocytic group than in the eosinophilic group. It was reported that subjects with sputum eosinophils of >2% in combination with sputum neutrophils of >50% showed greater loss of pulmonary function, whereas those with highly variable sputum eosinophils had greater healthcare use.16,25 The results of the present study suggest that low NRTN concentrations may also be a risk of airway remodeling in mixed granulocytic asthma. Moreover, sputum NRTN level was significantly negatively correlated with the MMP-9 level of the sputum supernatant. MMP-9 is the predominant MMP found in blood, bronchoalveolar lavage fluid, sputum, and transbronchial biopsy specimens of individuals with asthma, and the increase of MMP-9 activity in the tissues is related to the increase of collagen deposition in the airway walls.26 In the present study, a fixed airway obstruction (FEV1 / FVC < 70%) and % predicted FEV1 were used as indices of airway remodeling during the stable period of asthma. We found no significant difference between the two indices due to sputum NRTN level and we found no association between the sputum NRTN level and IL-6, which is a proinflammatory cytokine related to airway remodeling in asthma. Future prospective studies are needed to clarify whether airway NRTN suppresses airway remodeling in asthmatic conditions. It is necessary to consider how much NRTN level in the airway should be increased to suppress the airway remodeling. In NRTN−/− mice study, to suppress the immune response from bone marrow-derived DCs and spleen CD4+ T cells of OVA-induced asthma model mice, simultaneous additions of NRTN > 10 ng/mL were required.12
GFRα2 is a predominant receptor of NRTN, and it has been identified as a marker for the human intermediate monocyte subset.27 In respiratory viral infection, toll-like receptor (TLR) activation of airway epithelial cells enhances the production of NRTN, which binds to constitutively expressed GFRα2 on the surface of lung macrophages. Viral TLR activation induces RET translocation to the cytoplasm and binding to GFRα2. These pathways stimulate the production of MMP-2, which dampens the pro-inflammatory cytokine response.24 It was also reported that MMP-2 has beneficial effects in a mouse model of asthma, where this MMP facilitates the resolution of allergic inflammation by allowing cells to egress to the lung.28 We investigated whether the increased production of MMP-2 induced by NRTN in the airway was evident in the pathogenesis of asthma, but no clear relationship was observed. Although the airway macrophages, which are the main source of MMP-2 production, may be related to airway MMP-2 level, no clear correlation was observed between the sputum NRTN and sputum macrophage.
There were no significant differences between the sputum NRTN levels and asthma severities, asthma control parameters or airflow obstruction in the present study. Future studies may be conducted to increase airway NRTN levels for their inhibitory effects on Type 2 inflammation and airway remodeling. However, in a previous study on human airway smooth muscle (ASM) cells, brief exposure to NRTN resulted in intracellular Ca2+ level responses and enhancement of bronchoconstrictor responses.29 In contrast to previous data in NRTN−/− mice, the result of above-mentioned study indicated that NRTN appeared to be detrimental rather than protective at the level of ASM. Further studies are needed to clarify the possibility of the biphasic effect of NRTN in the airway.
The present study has some methodological limitations. First, clinical information at the time of sputum collection and one year before collection was used to assess the clinical impact of sputum NRTN level on asthmatic subjects. However, prospective analyses may be needed to investigate the usefulness of sputum NRTN-level measurements as a predictor of asthma exacerbation and airway remodeling. In order to prospectively assess the clinical role of airway NRTN with various degrees of severity and treatment content, a trial needs to be designed with a larger sample size than that of the present study. Second, the mean age of the asthmatic subjects in the present study was relatively high compared to those in past studies of asthma.30 Therefore, it may be necessary to consider the effects of aging on the pathology of sputum inflammatory cells in the present study. It is known that the mixed granulocytic pattern, in which neutrophils and eosinophils are increased in sputum, increases with aging.31 Although it is unknown how asthma is divided into each inflammatory type, the significant difference in the sputum NRTN level between the eosinophilic pattern and the mixed granulocytic pattern may be related to the difference in clinical characteristics and prognosis of these inflammatory patterns. Third, inflammatory cytokines and metalloproteinase levels in the induced sputum supernatant could not be measured for all the cases due to insufficient amounts of sputum supernatant. However, even in cases where a sufficient amount of sputum supernatant was secured, the relationship between sputum NRTN levels and inflammatory cell counts in induced sputum showed similar results compared to all cases (data not shown).
In conclusion, NRTN was detected in the induced sputum of all adult asthmatic subjects, and the sputum NRTN level was significantly positively correlated with eosinophils and Type 2 cytokines level, and significantly negatively correlated with the MMP-9 level. On the other hand, the present study did not conclusively establish the clinical utility of measuring sputum NRTN levels in human airway. It is plausible that sputum NRTN could serve as a new marker for Type 2 airway inflammation, implicating its role in the process of airway remodeling in asthma. The influence of NRTN on the pathogenesis of asthma remains to be explored.
Abbreviations
NRTN, neurturin; GFR, glycosyl-phosphatidylinositol-anchored coreceptor; FeNO, fractional exhaled nitric oxide; ACT, asthma control test; FEV1, forced expiratory volume in one second; FEV1/FVC, forced expiratory volume in one second percent; ELISA; enzyme-linked immunosorbent assay; IL, interleukin; MMP, matrix metalloproteinase.
Acknowledgments
We would like to acknowledge the late Dr Mitsuru Munakata (an Emeritus Professor of Fukushima Medical University) for his valuable comments and warm guidance. We would also like to acknowledge the members of Pulmonary Medicine Department of Fukushima Medical University for their continuous support, as well as the Scientific English Editing Section of Fukushima Medical University for their linguistic assistance in proofreading the manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Japan Society for the Promotion of Science KAKENHI Grant-in-Aid for Scientific Research (C) (Grant Number JP19K08607).
Disclosure
The authors have no conflicts of interest to disclose for this work.
References
1. Heuckeroth RO, Enomoto H, Grider JR, et al. Gene targeting reveals a critical role for neurturin in the development and maintenance of enteric, sensory, and parasympathetic neurons. Neuron. 1999;22(2):253–263. doi:10.1016/S0896-6273(00)81087-9
2. Pulleyn LJ, Newton R, Adcock IM, Barnes PJ. TGFbeta1 allele association with asthma severity. Hum Genet. 2001;109(6):623–627. doi:10.1007/s00439-001-0617-y
3. Horger BA, Nishimura MC, Armanini MP, et al. Neurturin exerts potent actions on survival and function of midbrain dopaminergic neurons. J Neurosci. 1998;18(13):4929–4937. doi:10.1523/JNEUROSCI.18-13-04929.1998
4. Fjord-Larsen L, Johansen JL, Kusk P, et al. Efficient in vivo protection of nigral dopaminergic neurons by lentiviral gene transfer of a modified neurturin construct. Exp Neurol. 2005;195(1):49–60. doi:10.1016/j.expneurol.2005.03.006
5. Bartus RT, Kordower JH, Johnson EM Jr, et al. Post-mortem assessment of the short and long-term effects of the trophic factor neurturin in patients with alpha-synucleinopathies. Neurobiol Dis. 2015;78:162–171. doi:10.1016/j.nbd.2015.03.023
6. Ruiz-Ferrer M, Torroglosa A, Luzon-Toro B, et al. Novel mutations at RET ligand genes preventing receptor activation are associated to Hirschsprung’s disease. J Mol Med. 2011;89(5):471–480. doi:10.1007/s00109-010-0714-2
7. Widenfalk J, Nosrat C, Tomac A, Westphal H, Hoffer B, Olson L. Neurturin and glial cell line-derived neurotrophic factor receptor-beta (GDNFR-beta), novel proteins related to GDNF and GDNFR-alpha with specific cellular patterns of expression suggesting roles in the developing and adult nervous system and in peripheral organs. J Neurosci. 1997;17(21):8506–8519. doi:10.1523/JNEUROSCI.17-21-08506.1997
8. Kabata H, Artis D. Neuro-immune crosstalk and allergic inflammation. J Clin Invest. 2019;129(4):1475–1482. doi:10.1172/JCI124609
9. Lieu T, Kollarik M, Myers AC, Undem BJ. Neurotrophin and GDNF family ligand receptor expression in vagal sensory nerve subtypes innervating the adult Guinea pig respiratory tract. Am J Physiol Lung Cell Mol Physiol. 2011;300(5):L790–L798. doi:10.1152/ajplung.00449.2010
10. Vargas-Leal V, Bruno R, Derfuss T, Krumbholz M, Hohlfeld R, Meinl E. Expression and function of glial cell line-derived neurotrophic factor family ligands and their receptors on human immune cells. J Immunol. 2005;175(4):2301–2308. doi:10.4049/jimmunol.175.4.2301
11. Michel T, Theresine M, Poli A, et al. Increased Th2 cytokine secretion, eosinophilic airway inflammation, and airway hyperresponsiveness in neurturin-deficient mice. J Immunol. 2011;186(11):6497–6504. doi:10.4049/jimmunol.1001673
12. Mauffray M, Domingues O, Hentges F, Zimmer J, Hanau D, Michel T. Neurturin influences inflammatory responses and airway remodeling in different mouse asthma models. J Immunol. 2015;194(4):1423–1433. doi:10.4049/jimmunol.1402496
13. American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, November 1986. Am Rev Respir Dis. 1987;136(1):225–244. doi:10.1164/ajrccm/136.1.225
14. Ichinose M, Sugiura H, Nagase H, et al. Japanese guidelines for adult asthma 2017. Allergol Int. 2017;66(2):163–189. doi:10.1016/j.alit.2016.12.005
15. Pizzichini E, Pizzichini MM, Efthimiadis A, et al. Indices of airway inflammation in induced sputum: reproducibility and validity of cell and fluid-phase measurements. Am J Respir Crit Care Med. 1996;154(2 Pt 1):308–317. doi:10.1164/ajrccm.154.2.8756799
16. Hastie AT, Mauger DT, Denlinger LC, et al. Mixed sputum granulocyte longitudinal impact on lung function in the severe asthma research program. Am J Respir Crit Care Med. 2021;203(7):882–892. doi:10.1164/rccm.202009-3713OC
17. Liu J, Huang X, Cao X, Feng X, Wang X. Serum biomarker analysis in patients with premature ovarian insufficiency. Cytokine. 2020;126:154876. doi:10.1016/j.cyto.2019.154876
18. Crapo RO, Hankinson JL, Irvin C, et al. Standardization of spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152(3):1107–1136. doi:10.1164/ajrccm.152.3.7663792
19. Dweik RA, Boggs PB, Erzurum SC, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602–615. doi:10.1164/rccm.9120-11ST
20. Sato S, Saito J, Sato Y, et al. Clinical usefulness of fractional exhaled nitric oxide for diagnosing prolonged cough. Respir Med. 2008;102(10):1452–1459. doi:10.1016/j.rmed.2008.04.018
21. Nathan RA, Sorkness CA, Kosinski M, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113(1):59–65. doi:10.1016/j.jaci.2003.09.008
22. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2023. Global Initiative for Asthma; 2023.
23. Morel L, Domingues O, Zimmer J, Michel T. Revisiting the role of neurotrophic factors in inflammation. Cells. 2020;9(4):865. doi:10.3390/cells9040865
24. Connolly E, Morgan DJ, Franklin M, et al. Neurturin regulates the lung-resident macrophage inflammatory response to viral infection. Life Sci Alliance. 2020;3(12):e202000780. doi:10.26508/lsa.202000780
25. Abdo M, Pedersen F, Kirsten AM, et al. Longitudinal impact of sputum inflammatory phenotypes on small airway dysfunction and disease outcomes in asthma. J Allergy Clin Immunol Pract. 2022;10(6):1545–1553 e2. doi:10.1016/j.jaip.2022.02.020
26. Atkinson JJ, Senior RM. Matrix metalloproteinase-9 in lung remodeling. Am J Respir Cell Mol Biol. 2003;28(1):12–24. doi:10.1165/rcmb.2002-0166TR
27. Wong KL, Tai JJ, Wong WC, et al. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood. 2011;118(5):e16–e31. doi:10.1182/blood-2010-12-326355
28. Corry DB, Kiss A, Song LZ, et al. Overlapping and independent contributions of MMP2 and MMP9 to lung allergic inflammatory cell egression through decreased CC chemokines. FASEB J. 2004;18(9):995–997. doi:10.1096/fj.03-1412fje
29. Bhallamudi S, Roos BB, Teske JJ, et al. Glial-derived neurotrophic factor in human airway smooth muscle. J Cell Physiol. 2021;236(12):8184–8196. doi:10.1002/jcp.30489
30. Teague WG, Phillips BR, Fahy JV, et al. Baseline features of the Severe Asthma Research Program (SARP III) cohort: differences with age. J Allergy Clin Immunol Pract. 2018;6(2):545–554 e4. doi:10.1016/j.jaip.2017.05.032
31. Soma T, Nagata M. Immunosenescence, inflammaging, and lung senescence in asthma in the elderly. Biomolecules. 2022;12(10):1456. doi:10.3390/biom12101456
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.