Back to Journals » Journal of Asthma and Allergy » Volume 13
Sputum IL-26 Is Overexpressed in Severe Asthma and Induces Proinflammatory Cytokine Production and Th17 Cell Generation: A Case–Control Study of Women
Authors Louhaichi S , Mlika M , Hamdi B , Hamzaoui K , Hamzaoui A
Received 2 September 2019
Accepted for publication 12 January 2020
Published 3 February 2020 Volume 2020:13 Pages 95—107
DOI https://doi.org/10.2147/JAA.S229522
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Amrita Dosanjh
Sabrine Louhaichi, 1–3 Mona Mlika, 2, 4 Besma Hamdi, 1–3 Kamel Hamzaoui, 1, 2 Agnès Hamzaoui 1–3
1Research Laboratory 19SP02 “Chronic Pulmonary Pathologies: From Genome to Management”, Abderrahman Mami Hospital, Ariana, Tunisia; 2Medicine Faculty of Tunis, Department of Basic Sciences, Tunis El Manar University, Tunis, Tunisia; 3Department of Paediatric and Respiratory Diseases, Abderrahman Mami Hospital, Pavillon B, Ariana, Tunisia; 4Pathology Department, Abderrahman Mami Hospital, Ariana, Tunisia
Correspondence: Agnès Hamzaoui
Department of Paediatric and Respiratory Diseases, A. Mami Hospital, Pavillon B, Ariana, Tunisia
Email [email protected]
Objective: Asthma inflammation is a complex pathway involving numerous mediators. Interleukin-26 (IL-26), a member of the IL-10 cytokine family, is abundant in human airways and induces the production of proinflammatory cytokines. Our aim was to investigate the possible role of IL-26 in severe asthma. We analysed the expression of IL-26 in severe asthma both in peripheral blood and induced sputum.
Patients and Methods: A total of 50 adult women with severe asthma were recruited and compared to 30 healthy controls (HC). Serum and sputum fluid (SF) levels of IL-26 and IL-17 were defined by ELISA. IL-26 mRNA expression and IL-26 protein were analysed using RT-PCR and Western blot. In vitro, we studied the effect of recombinant IL-26 (rIL-26) and SF-IL-26 on cultured CD4+ T cells and monocytes, comparing patients and controls.
Results: Concentrations of IL-26 are higher in serum and induced sputum of asthmatic patients than in HC. Moreover, IL-26 protein and mRNA expression were significantly elevated in asthma sputum cells compared to PBMCs. We observed a positive correlation between body mass index (BMI) and sputum fluid IL-26, while the correlation between IL-26 and lung function tests (FEV1% and FEV1/FVC ratio) was negative. IL-17A was highly expressed in SF and correlated positively with IL-26. In patients’ sputum IL-26 and IL-17A were significantly associated with neutrophils. Stimulation of cultured CD4+ T cells with monocytes by recombinant IL-26 promoted the generation of RORγt+ Th17+ cells inducing the production of IL-17A, IL-1β, IL-6 and TNF-α cytokines. IL-26 expressed in SF was biologically active and induced IL-17 secretion in the presence of IL-1β and IL-6 cytokines.
Conclusion: These findings show that IL-26 is highly produced in asthmatic sputum, induces pro-inflammatory cytokine secretion by monocytes/macrophages, and favours Th17 cell generation. IL-26 thereby appears as a novel pro-inflammatory cytokine, produced locally in the airways that may constitute a promising target to treat asthma inflammatory process.
Keywords: asthma, induced sputum, IL-26, IL-17A, IL-6, IL-1β
Introduction
Asthma is a heterogeneous disease characterized by inflammation, reversible airway obstruction, airway hyperresponsiveness (AHR) and tissue remodeling. Recent progresses in molecular biology suggest complex cytokines interactions between innate and adaptive immune cells and resident cells.1 Genome-wide associations studies (GWAS) have well-associated numerous single nucleotide polymorphisms (SNPs) of cytokines genes with asthma.2 The level of gene expression varies according to asthma phenotypes.
Although asthma has been first considered as a Th2-mediated disease, the pro-inflammatory Th17 cells (the major source of IL-17A) have recently focused attention.3,4 Th17 and regulatory T cells (Treg) are inversely modulated in asthma, as Th17 cells are increased and Treg cells decreased both in the peripheral circulation and in induced sputum (IS).5 Through IL-17A, Th17 cells contribute to pulmonary recruitment and accumulation of neutrophils and macrophages.4 However, anti-TNF-α and anti-IL-17A failed to show efficacy in asthma patients6 emphasizing the need to establish the role of other Th17 cytokines such as IL-26.7 An up-regulation of IL-26 has been previously reported in paediatric asthma.8
IL-26 is a member of the IL-10 cytokine family that includes IL-10, interferon (IFN)-λ (IL-28A/B and IL-29), and the IL-20 subfamily (IL-19, IL-20, IL-22, IL-24, and IL-26).9 The expression of IL-26 has been observed in T cells, natural killer (NK) cell subsets,10 macrophages and epithelial cells.11 IL-26 has been reported to signal via the IL-10R2/IL-20R1 heterodimeric receptor.12 Of interest, whereas IL-10R2 is broadly expressed, IL-20R1 expression is restricted to epithelial cells.13
Innate immunity is gaining interest in asthma, particularly in severe neutrophilic phenotypes. The control of innate immunity in human lungs is complex, probably because of its fundamental importance for host survival.14 IL-26 may act both as a driver and an effector of inflammation, leading to the establishment of a deleterious amplification loop and ultimately, sustained inflammation as reported by Larochette et al.15 IL-26 is involved in the immune response to bacterial endotoxin in the airways of healthy human subjects.16 Experimental studies demonstrated in vivo a direct anti-microbial effect of IL-26 against extracellular bacteria.17 Human alveolar macrophages and bronchial epithelial cells are able to produce IL-26, acting then as a critical immune barrier.18 Moreover, IL-26 enhances the chemotactic response of human neutrophils to bacterial and inflammatory stimuli.12 In this way, Che et al19 found increased extracellular IL-26 protein in the airways of long-term smokers in vivo. Recombinant human IL-26 increased gene expression of NF-κB and pro-inflammatory cytokines.19 Innate lymphoid cells (ILCs) are part of the innate immune system.20 ILC3s, the most implicated in asthma can play a role in the recruitment of neutrophils to the lung via the cytokine IL-17A eventually leading to neutrophilic inflammation.21 Hekking et al recently reported that a series of sputum ILC3-specific genes were upregulated in adult-onset severe asthma patients suggesting a role for ILC3 in this asthma subtype.22
We have investigated in this study the expression of IL-26 in severe asthma and its role in inducing inflammatory cytokines production. We report that protein and mRNA IL-26 expression are elevated in asthmatic patients and associated with neutrophilic inflammation. We also show that IL-26 triggers the production of pro-inflammatory cytokines by cultured macrophages and memory CD4+ T cells inducing the generation of RORγt+ Th17+ cells. IL-26 represents a new immunological parameter that must be taken into account in the inflammatory process in severe asthma.
Materials and Methods
Patients
Fifty severe asthmatic patients were recruited for this study (Department of Paediatric and Respiratory Diseases; A. Mami Hospital of lung diseases, Ariana, Tunisia). The diagnosis of severe asthma was established according to the ATS/ERS criteria.23 Patients with respiratory co-morbidities (COPD, Bronchiectasis or lung interstitial disease) or heart failure were excluded. Due to the specificity of our department, all patients were female. The control group (all females, aged 45–58 years) comprised 30 healthy volunteers with no history of obstructive lung disease. All subjects were nonsmokers. Patients produced samples of sufficient quality for subsequent inflammatory cell counts (Table 1). Twenty-seven patients were considered atopic with a positive skin prick test to at least one inhalant allergen.
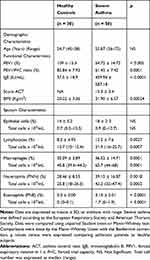 |
Table 1 Demographic and Inflammatory Characteristics of Patients and Controls |
The study protocols were reviewed and approved by the ethics committees of our hospital (Abderrahmen Mami Hospital, Ariana, Tunisia), and informed written consent was obtained from all participating subjects. This study has been conducted in accordance with the Declaration of Helsinki.
Patients with asthma were treated with regular high dose inhaled glucocorticoids (ICS) (daily ICS dose ranged 1000–2000 µg beclomethasone). Asthma control was evaluated using the “asthma control test” (ACT). Table 1 describes the characteristics of the asthmatic patients and the controls.
Lung Function Parameters
Spirometry was performed according to the American Thoracic Society and European Respiratory Society guidelines.24 Spirometry was carried out in the patient and the control group. Forced expiratory volume in 1s (FEV1) and Forced vital Capacity (FVC) were assessed by using a spirolab II and were expressed as a percent of the predicted value.
Sputum Induction and Processing
Patients were allowed to continue their own anti-asthma medication before sputum induction and all procedures were conducted in the morning. The procedure of sputum induction was the same in control and asthma group and was preceded by inhalation of 400 μg of salbutamol and subsequent pulmonary function testing. Patients with a post-bronchodilator FEV1< 50% of predicted and <1.0 L were excluded from the induction procedure. All patients were pre-treated with two puffs of salbutamol (400 μg) and inhaled a nebulized solution of normal saline followed by increasing concentrations (3%, 4% and 5%) of hypertonic saline using an ultrasonic nebulizer (ULTRA-NEBTM 2000, DeVilbiss, Somerset, PA, USA). Patients were asked to rinse their mouth and were then instructed to cough sputum into a sterilized sputum container. These procedures were repeated for up to a total of 7 min of inhalation sequentially with each concentration until an adequate sputum sample (one mL) was obtained.
Sputum Processing
Induced sputum was processed immediately on receipt as described by Fahy et al and Rosewich et al.25,26 The volume of the sputum was measured and the plugs were separated and weighed. A freshly prepared 0.1% solution of dithiothreitol (DTT, Sigma Aldrich, St. Louis, MO) in sterile phosphate-buffered saline (PBS) was added in a volume equal to fourth the weight of the sputum, and the mixture was shaken for 15 min. Subsequently, a double volume of sterile PBS was added and the mixture was vortexed briefly. After filtration through 70-mm nylon cell strainer (Falcon, Corning Brand, NY, Columbia, USA) the sputum was centrifuged for 10 min at 1800 g. The cells were counted in Neubauer hemocytometer, percentage of dead cells and epithelial cells (squamous and ciliated) was assessed.27 Total number of immunological cells (x 106) and total cell count (106 cells/g sputum) were calculated. Smears from each patient were performed: four smears were stored at – 80° C for further investigation, two smears from each subject were stained with May-Gr€unwald-Giemsa method to assess the percentage of immunological cells, based on the morphology of 300 cells from various fields. The criteria for appropriate IS quality were as follows:28 less than 50% epithelial cells, and more than 300 non-epithelial cells on one slide. Samples with a contamination of >80% of squamous cells were excluded from analyses. Eosinophilic phenotype was defined as >3% sputum eosinophil counts while neutrophilic phenotype required a neutrophil count >61%.
Isolation of Peripheral Blood and Sputum Fluid Mononuclear Cells
Mononuclear cells were isolated from the peripheral blood mononuclear cells (PBMCs) of severe asthmatic patients and healthy donors and from induced sputum, by density centrifugation using lymphocyte separation medium (PAA Laboratories). Then, T cell and non-T cell fractions were purified by positive or negative selection, respectively, using the CD3+ T cell isolation kit (Miltenyi Biotec), and were stimulated or not with 1 μg/mL anti-CD3 plus anti-CD28 mAbs or 10 μg/mL recombinant soluble CD40L (R&D Systems), respectively, in order to analyze IL-26 mRNA expression by reverse transcription (RT)-quantitative PCR (RT-qPCR) and IL-26 production (ELISA). T cell and non-T mononuclear cell purity, assessed by flow cytometry on a FACS Calibur (BD Biosciences), was >99%. Memory (CD4+ CD45RO+) T cells were purified by negative selection (Miltenyi Biotec); purity, assessed by flow cytometry, was >99%.
Monocytes from PBMCs were isolated by negative selection, using the EasySep human monocyte Enrichment kit (Stemcell). Cell purity, determined by flow cytometry, was routinely >95%. Monocytes were differentiated into macrophages by culture at 1 x 106 cells/mL for 10 days in complete medium (consisting of RPMI 1640 medium [GE Healthcare Life Sciences, Vélizy-Villacoublay, France] supplemented with 10% fetal calf serum [Biowest; Germany], 2 mM L-glutamine, 1 mM sodium pyruvate, 0.1 mM non-essential amino acids, 10 mM HEPES, 100 U/mL penicillin, and 100 μg/mL streptomycin [all from Lonza]) supplemented with 20 ng/mL GM-CSF or with 20 ng/mL GM-CSF plus 50 ng/mL IL-4 (both from Cellgenix; Freiburg, Germany).
Effect of Recombinant IL-26 on IL-17 Modulation
We evaluated whether IL-26-treated CD4+ T cell and autologous monocytes from asthmatic patients and healthy controls may modulate IL-17, IL-4, IL-10, TNF-α, IL-1β and IL-6 secretion in vitro. To determine the required dose of recombinant IL-26 (rIL-26: Abnova; Catalog #: H00055801-Q01) we performed a test in vitro on control PBMCs (n = 10). Purified CD4+ T cell (memory CD4+ T cells: CD4+CD45RO+) fractions and monocytes, isolated from PBMCs were cultured in complete RPMI 1640 medium [supplemented with 2% FCS (GE Healthcare Life Sciences, Vélizy-Villacoublay, France), 2 mM glutamine, 1 mM sodium pyruvate, 0.1 mM non-essential amino acids, 10 mM HEPES, 100 U/mL penicillin, and 100 μg/mL streptomycin] (GE Healthcare Life Sciences, Vélizy-Villacoublay, France).
CD4+ T cells (1 × 105) and autologous monocytes (5× 103) were cultured in complete medium in 96-well U-bottom plate, stimulated with an immobilized anti-CD3 monoclonal antibody, with or without rIL-26 at different concentrations (0 ng/mL; 20 ng/mL; 40 ng/mL; 50 ng/mL; 100 ng/mL; 150 ng/mL and 200 ng/mL). After 7 days of incubation, culture supernatant cells were tested for IL-17 levels by ELISA. The minimal concentration of rIL-26 inducing measurable levels of IL-17 was 100 ng/mL. It was used in the subsequent experiments. CD4+ T cells with autologous monocytes from 10 healthy controls and 12 severe asthmatic patients were cultured in the same conditions with or without rIL-26. Cytokines production was measured on day 7.
Activity of Sputum IL-26 in Asthmatics
IL-26 present in asthmatic sputum fluid was tested for its activity in inducing IL-17 in cultured sputum CD4+ and macrophages. Sputum CD4+ T cells co-cultures with autologous macrophages in RPMI 1640 medium (supplemented with 2mM glutamine, 1% (vol/vol) non-essential amino acids, 1% (vol/vol) sodium pyruvate, penicillin (50 U/mL) and streptomycin (50 mg/mL) and containing 10% (vol/vol) FCS (Gibco BRL)). Cells were stimulated for 7 days in the presence of SF, depleted or not in IL-26. Sputum memory CD4+ T cells (2 x 102 cells/mL) and macrophages (1 x 102 cells/mL) were stimulated with an anti-CD3 mAb supplemented by 10% sputum fluid of asthmatic patients, depleted or not in IL-26. After 7 days, IL-17A was quantified by ELISA in cultures supernatants. The addition of anti-TNFα (4 pg/mL), anti-IL-1β (2 pg/mL) and anti-IL-6 (2 pg/mL) were tested in regulating IL-17 secretion. The origins of the non-labeled mAbs were from R&D Systems (R&D Systems, Abingdon, UK).
IL-26 Quantification by ELISA
Approximately 5 mL of fasting venous blood was collected in a tube with Ethylene diamine tetra-acetic acid for each participant. On the same day of taking blood samples, the samples were centrifuged at 1500 rpm for 10 mins at room temperature, and then the serum samples were isolated, aliquoted and stored at −80°C until analysis.
IL-26 was quantified in serum, and sputum fluid as recently reported using commercially available ELISA kits (LS-F4914, LifeSpan) tested for non-specific binding as previously described.29 Samples were blocked for heterophilic antibodies with bovine, murine, and rabbit immunoglobulin G (Jackson Immuno Research). ELISA-Amplification System (ELAST) (NEP116001EA, PerkinElmer) was applied before adding 3,3′,5,5′-tetramethylbenzidine (TMB); otherwise, the assays were performed according to the manufacturer’s protocol. Samples were analyzed in duplicates, and values below the detection limit were assigned the same value as the detection limit, which in this case was 15.63 pg/mL. Optical density (OD) was measured at 450 nm with a reference value of 570 nm (Thermo Scientific, Multiskan GO). Optical densities were converted to concentrations using a four-parametric logistic regression.
IL-1β, TNFα, IL-17 were measured by “Ready-Set-Go” Elisa (eBioscience, UK). Lower levels of quantification (LLOQ) for all assays were 4 pg/mL. IL-6 was measured by Duo set ELISA (R&D Systems, Abingdon, UK). LLOQ for all assays were 9.4 pg/mL.
RNA Extraction and Real-Time Polymerase Chain Reaction (RT-PCR)
The determination of mRNA expression was performed by the real-time quantitative reverse transcription-polymerase chain reaction (qRT-PCR) as we previously reported.29 The mRNA was extracted from sputum mononuclear cells and peripheral blood mononuclear cells (PBMNCs) using TRIZOLR reagent (Invitrogen) according to the manufacturer’s instructions. Complementary DNA (cDNA) samples were synthesized using random hexamer primers and RNase H-reverse transcriptase (Fermentas). The qRT-PCR was done in a 25 ll reaction using an Applied Biosystems system (AB, Foster City, CA). The reaction systems include 15 μg/ll (2.5 μL) of cDNA template, 10 μM of each forward and reverse primers set and the appropriate dilution of SYBR Green mix (QIAGEN, Germany), and the adequate volume of distilled water (Gibco). The β-actin mRNA (internal control) was quantified in the same way as the IL-26 mRNA, using the forward and reverse primers: β-actin, 5ʹ-ATGACTTCCAAGCTGGCCGT-3ʹ and 5ʹ-CCTCTTCAAAAACTTCTCCACACC-3ʹ; IL-26, 5ʹ-GCTGTTAGTCACT CTGTCTCTTG-3ʹ and anti-sense 5ʹ-GGACAAT GTTCCCCTTGGGTA-3ʹ; IL-17,5ʹ-CCCGGACTGTGATG GTCAAC-3ʹ anti-sense: 5ʹ-GCACTTTGCCTCCCAGATCA3ʹ RORγt;5′-AATCTCATCCTCGGAAAAGTG-3′ and anti-sense reverse primer 5′-TCTCAA AGCAGGAGCAATGGA-3′. The qRT-PCR detection system was used for amplification and the employed cycling program was as follows: initial denaturation step at 94°C for 2 min; then 40 cycles of a second denaturation step at 94°C for 15 s; an annealing step at 59°C for 45 s, and an extension step at 72°C for 45 s; after that, a final extension at 72°C for 10 mins was carried out.
Immunofluorescent Staining and FACS Analysis
To measure intracellular CD4+ IL-17+ in the presence of rIL-26 or none, cultured CD4+ cells were stained with FITC-conjugated anti-CD4 (BioLegend, USA), before incubating in permeabilizing and fixation buffers (BioLegend, USA) for 30 mins at 4°C. Cells were washed with PBS, treated with fixation and permeabilization buffers, and subsequently washed and resuspended in 100 μL of permeabilization buffer for 30 mins at 4°C in the dark, with monoclonal antibodies (Abs) against the following antibodies: PE-conjugated anti-IL-17. To assess cytokine production, the cells were gated on forward scatter (FSC) and side scatter (SSC) dot plot and lymphocytes were gated to analyze the percentage of CD4+IL-17+ cells. The events were acquired by flow cytometry (FC500, Beckman Coulter, USA) and analyzed by CXP software.
Western Blotting Analysis
Protein expression of IL-26, IL-17A and RORγt were determined by Western blotting. SDS-PAGE was performed on 12.5% gels using a Mini-Protean Electrophoresis system (Bio-Rad, Switzerland) and blotted onto a hybond-P polyvinylidene difluoride (PVDF) membrane (GE Healthcare, Glattbrugg, Switzerland) in the same device. Membranes were blocked with 5% milk powder in TBS-T (50 mM Tris, Calbiochem, pH 7.6; 150 mM NaCl, 0.1% Tween-20; Sigma-Aldrich) and incubated overnight at 4°C with mouse anti-bodies, anti-IL-26 (KU32-52; BioLegend), anti-1L17A (Abcam), anti-RORγt (Abcam) or β-Actin antibodies (Sigma) in 10% FCS in TBS-T. HRP-conjugated secondary antibodies (anti-mouse; Cell Signaling, Danvers, MA, USA) were used. The ECL chemiluminescence reagent was used to detect the signal bands as and semi-quantitative analyses using densitometry were performed using Image J version 1.48v (National Institutes of Health, Bethesda, MD).
Statistical Analysis
The Statistical Package for Social Sciences (SPSS) version 19 (LEAD Technology Inc., Charlotte, NC, USA) was used to analyze the data. Continuous variables were summarized through mean ± standard deviation (SD). The difference in the average between the study groups was examined by Mann–Whitney test. Spearman’s rank correlation was used to examine the relationship between two continuous variables.
Results
Patients Characteristics
Baseline patient characteristics and sputum in asthmatics and controls are shown in Table 1. Asthmatic patients are hospitalized in a women’s respiratory ward (Pavillion B, A. Mami Hospital). The screening cohort consisted of 30 healthy subjects and 50 patients with severe asthma. The mean age was 55.87 (36–75) years for asthmatics and 54.7 (45–58) years for controls. Within these asthma and control groups, 12 patients and 10 healthy persons were investigated for their in vitro stimulated CD4+ T cells and macrophages producing inflammatory mediators. Lung function parameters such as FEV1 and FEV1/FVC in asthmatic patients were significantly lower than those in healthy controls (p < 0.0001).
IL-26 Is Overexpressed in Severe Asthmatic Patients
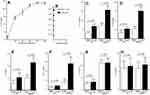
Serum IL-26 concentrations and IL-26 mRNA expression were studied in 38 asthmatic patients and 20 non-asthmatic controls. Serum IL-26 concentrations (1.10 ± 0.39 ng/mL) were increased compared to healthy subjects (0.55 ± 0.25 ng/mL; p < 0.0001) (Figure 1A). As asthma is characterized by lung inflammation, IL-26 was also quantified in sputum fluid (SF). IL-26 in asthmatic patients was highly elevated (2.48 ± 0.94 ng/mL) compared to healthy controls (0.74 ± 0.26 ng/mL; p < 0.0001).
As expected, qRT-PCR revealed that PBMCs from severe asthmatic patients showed increased IL-26 mRNA expression (2.134 ± 0.632; p = 0.0012) comparatively to HC (1.61 ± 0.33). Sputum asthmatics cells exhibited an important increase of mRNA expression (7.49 ± 2.86; p < 0.0001) compared to HC (2.39 ± 0.81) (Figure 1B). The increased IL-26 mRNA expressed was confirmed by Western blot analysis (Figure 1C). Sputum fluid IL-26 level was negatively correlated with FEV1 (r = - 0.502; p = 0.0043) and FEV1/FVC (r = - 0.416; p = 0.0092) (Figure 1D and E)
Sputum fluid IL-26 concentrations in asthma were highly expressed comparatively to their serum values (p < 0.0001). These data were corroborated and confirmed by the results at the mRNA and western blot in sputum cells (p < 0.0001).
IL-17A Expression in Severe Asmathic Patients
Serum IL-17A was highly expressed in severe asthmatics (340.39 ± 104.06 pg/mL) compared to controls (160.75 ± 39.27 pg/mL; p <0.0001). In the same way, IL-17A level in sputum fluid was increased in asthmatics (521.65 ± 113.76 pg/mL) compared to non-asthmatic controls (102.55 ± 15.38 pg/mL; p < 0.0001) (Figure 2A).
A significant correlation was observed between IL-26 and IL-17 expression in sputum fluid of severe asthmatics (r = 0.427; p = 0.0077) (Figure 2B). However, no correlation was observed in HC. PNN showed significant association with sputum IL-26 (r = 0.681, p < 0.0001) and sputum IL-17 (r = 0.543, p < 0.0001) (Figure 3A and B).
According to previous results, sputum in asthmatics is characterized by an increase in IL-26 expression, correlated with the expression of IL-17 cytokine. The question that might arise is about the direct effect of rIL-26 on the production of associated cytokines to Th2 (IL-4), Treg (IL-10), and Th17 (IL-17) cells in vitro after LPS stimulation of CD4+ T cells and autologous monocytes in the PBMCs. We measured the presence in culture supernatants of some key mediators of inflammation such as IL-1β, IL-6 and TNF-α, after rIL-26 stimulation.
However, we first defined in non-asthmatic controls the dose of rIL-26 capable of inducing a measurable concentration of IL-26.
Recombinant IL-26 Inducing IL-17, IL-1β, TNF-α, IL-10, IL- 4 in vitro
Asthma is characterized by excessive proinflammatory Th2 and Th17 responses.30 We therefore evaluated whether rIL-26-treated monocytes may modulate CD4+ T cell polarization. Purified CD4+ T cells were cultured with autologous monocytes and anti-CD3 mAb, in the presence of rIL-26. This in vitro study was performed at PBMCs level in 10 non-asthmatic controls.
Different doses of rIL-26 were tested (20 ng/mL; 50 ng/mL; 100 ng/mL; 150 ng/mL and 200 ng/mL) to optimize the concentration allowing a significant increase in IL-17 secretion. IL-26 upregulated IL-17A secretion by CD4+T cells and autologous monocytes (Figure 4A), in a dose-dependent manner. The selected concentration of rIL-26 was 100 ng/mL inducing a measurable IL-17 release in cell culture supernatants from healthy controls.
The culture control of separately stimulating CD4+ cells alone and monocytes alone from asthmatic patients in the presence of rIL-26 does not show any secretion of IL-17, IL-4, IL-10, IL-1β, TNF-α or IL-6 (Figure 4B).
In a second step, CD4+ T cells and autologous monocytes from 12 asthmatic patients were investigated by adding or not rIL-26 (100 ng/mL) to test the modulation of the increase of IL-4, IL-6, TNFα, IL-10, IL-1β and IL-17A cytokines secretion after anti-CD3 and LPS stimulation.
Table 2 reports the cytokine values. rIL-26 upregulated IL-17A (Figure 4C), IL-1β, IL-6 and TNF-α (Figure 4D–F) while IL-4, and IL-10 production was not modified (Figure 4G and H). rIL-26 had no effect on the production of Th2 and Treg cytokines by CD4+ T cells associated with autologous monocytes, both in asthmatic patients and in HC. The increased secretion of IL-17A, IL-6, TNF-α and IL-1β was more important in asthmatic patients compared to non-asthmatic controls.
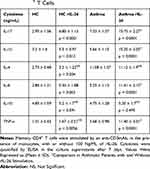 |
Table 2 IL-26 Upregulates IL-17A, IL-β and TNF-α Secretion by Memory CD4+ T Cells. |
Interestingly, rIL-26-stimulated monocytes and memory CD4+ T cells promote the generation of Th17. rIL-26 induced the expression of the mRNA encoding the Th17 associated molecules IL-17A and RORγt+ in memory CD4+T cells (Figure 5A). Western blot analysis and cytometry showed increased expression of CD4+ IL-17+ (Figures 5B–D) after rIL-26 stimulation.
Correlations of IL-26 and IL-17 with Obesity and Menopausal Status
Of the patients studied, only 10/38 patients with asthma are not menopausal. Observed IL-26 values were not significantly different between post-menopausal women (1.35 ± 0.36 ng/mL) and pre-menopausal women (1.078 ± 0.33 ng/mL) (p = 0.053). Similarly, no association was observed in serum IL-17 levels between menopausal (357.56 ± 97.6 ng/mL) and non-menopausal status (361.36 ± 105.7 ng/mL; p = 0.067). A more judicious analysis would be to increase and balance the number of patients in both categories.
Obesity was defined as a body mass index (BMI) of more than or equal to 30 kg/m2, overweight was defined as BMI between 25 and 29.9 kg/m2, and lean weight was defined as BMI < 25 kg/m2. In our study we found that IL-26 mRNA (r = 0.407; p = 0.0124) and IL-17 mRNA (r = 0.455; p = 0.0015) were significantly correlated with asthmatic patients having a body mass > to 30 kg/m2. Our analysis shows that inflammatory cytokines IL-26 and IL-17 are associated with the effects of obesity in asthma.
Sputum IL-26 in Severe Asthmatic Triggers Proinflammatory Cytokine
We examined whether IL-26 present in asthmatic sputum fluid is biologically active. We evaluated the capacity of IL-26 present in asthma sputum fluid to induce IL-17A. Sputum CD4+ T cells co-cultures with autologous macrophages were stimulated for 7 days in the presence of sputum fluid, depleted or not in IL-26. In these conditions, asthmatic patients exhibited increased IL-17 production (14.91 ± 2.93 ng/mL; p < 0.0001) compared to HC (6.55 ± 1.70 ng/mL) (Figure 6A).
The IL-17 secretion was significantly reduced after IL-26 depletion in asthmatic patients (5.0 ± 1.27 ng/mL; p < 0.003) and in HC (4.21 ± 1.81ng/mL) (Figure 6A), but not totally prevented, supporting that other factors (such as TNF α, IL-1β and IL-6) may also favor Th17 cell generation. The addition of anti-TNFα did not interfere with IL-17 secretion. Indeed, the addition of anti-IL-6 and anti-IL-1β monoclonal antibodies (MoAb) largely abolished the secretion of IL-17 in asthmatics and in HC. The addition of anti-IL-6, anti-TNFα and anti-IL-1β to the cultures from asthmatic patients abrogated IL-17 production in the same manner as the presence of anti-IL-6 and anti-IL-1β (Figure 6B).
Collectively, these results suggest that IL-26 in sputum fluid from severe asthmatic patients may promote locally IL-17 secretion in the presence of IL-6 and IL-1β.
Discussion
Innate immunity is actually considered as a major player in asthma pathogenesis. Non-specific immunologic pathways have been particularly involved in severe asthma resistant to corticosteroid therapy.31 Several biologics targeting key Th2 cytokines IL-4, IL-5 and IL-13 have been tested and approved to treat severe asthma patients, mainly eosinophilic, allowing a decrease in corticosteroids doses.32,33 However, therapies targeting neutrophilic inflammation such as anti-TNF-α, and anti-IL-17RA failed to show efficacy in patients with non-eosinophilic asthma.6 We report here that IL-26, a neutrophil-mobilizing cytokine is overexpressed and produced in airways of severe asthma patients, with higher levels in sputum fluid compared to serum. IL-26 induces the secretion of proinflammatory non-Th2 cytokines (IL-17, IL-1β, TNF-α and IL-6) and favors the generation and local recruitment of Th17 cells.34 IL-26 located upstream of the local proinflammatory cascade thereby appears as a pivotal cytokine that may constitute a promising therapeutic target.
Several resident and inflammatory pulmonary cells express and release IL-26.14,18 A local production has been demonstrated by bronchial and lung biopsies with immunostaining of bronchial epithelial cells and macrophages/monocytes.35 Lung fibroblasts participate as well to IL26 production.36 Our data show an increased concentration of IL-26 in sputum fluid from severe asthma patients compared to healthy controls. Moreover, we report higher levels of mRNA in patients than in controls suggesting the involvement of IL-26 in inflammatory pathways of severe asthma. In a similar way, uncontrolled asthma, in children and adults was characterized by an increase in IL-26 levels compared to controlled cases.8,35 Further, we observed a negative correlation between IL-26 levels and FEV1, confirming previous studies.35 However, our results contrast with data on IL-26 in BAL in adult patients that were lower than those observed in healthy controls, despite a positive correlation between IL-26 and FeNO.35 This may be linked to the degree of asthma severity as in the study of Tufvesson et al, asthma was reported as mild or moderate.35 The lowest level observed in controlled asthma compared to uncontrolled and severe asthma was associated with a clinical response to corticosteroid. In vitro, hydrocortisone, a corticosteroid, inhibited IL-26 production from lung fibroblasts.36 Severe patients as such are characterized by a resistance to steroid therapy that could favor IL-26 production. The difference could also be due to the difference in the sites sampled, BAL reflecting distal airways and alveolar compartment, whereas sputum is mainly representative of central airways.
We made in the second step in vitro functional experiments to evaluate the effect of rIL-26 on inflammatory cytokines production from co-cultured monocytes/macrophages and CD4+ T cells. Higher levels of IL-17A, IL-6, IL-1β and TNF-α were measured in stimulated cells culture supernatants from patients than those from healthy controls. In the same way, IL-17A mRNA and RORγt were highly expressed after rIL-26 stimulation, reflecting Th17 generation.34 Furthermore, the same experiments repeated with asthma sputum fluid-induced similar results, confirming that IL-26 intrinsically present in patients sputum was biologically active. Our data showed that IL-26 favored the generation of Th17 cells mainly through the induction of TNF-α, IL-1β and IL-6 production by sputum immune cells. Studies in mice have identified transforming growth factor-β (TGF-β) and IL-6 as the critical cytokines driving the Th-17 differentiation of naïve T cells.37,38 In addition, proinflammatory cytokines such as IL-1β and TNF-α can increase the efficiency of Th17 differentiation.39 The identification of IL-26 associated with IL-17 generation is thus crucial to eventually design future therapeutic strategies to reduce the proinflammatory cytokines expression in asthma. The presence of IL-26 in sputum from asthmatic patients suggests its involvement in the pro-inflammatory microenvironment associated with Th17 differentiation. Of notice, IL-4 (Th2) and IL-10 (Treg) cytokines release was not modified by rIL-26.
IL-26 is a main actor in antibacterial defence17 and its production in severe asthma could be linked to modifications of the airways microbiota. In mice, exposure to endotoxin enhances the production of IL-26, IL-6 and IL-8 in the airways.40 We observed a positive correlation between IL-26 and sputum neutrophils, suggesting an enhanced recruitment of these cells in severe asthma with a possible role of microbial agents. Virus are reported as a major factor of asthma exacerbation, inducing non-specific innate immunity activation involving Th17 cytokines.41 Viral stimulation of bronchial epithelial cells induces IL-26 production that is enhanced by IL-17, suggesting a role to IL-26 in asthma attacks.18 In the same way, modifications of airways microbiota have been reported in severe asthma, together with a possible role of bacteria in the severity of asthma.42,43 The role of macrophages, which are a prominent source of IL-26 during activation of anti-bacterial host defense, should be more deeply investigated.44,45 Otherwise, neutrophilic inflammation in asthma has also been associated with obesity.46 The positive correlation that we observed between IL-26 and IL-17A expression and BMI in our patients is consistent with a role of IL-26 and innate immunity in particular severe phenotypes.
Our study has some limitations as our patients were exclusively women, not allowing us to extend our conclusions to severe asthma in general. However, we did not observe differences depending on menopausal status suggesting no effect of hormones. Our samples were obtained by induced sputum a less aggressive technique than BAL, decreasing the risk in these severe patients but reducing the number of collected cells. We will have though to expand our population to be able to explore more deeply the relationships between IL-26, cellular populations, clinical phenotypes and inhaled treatment, as IL-26 production has been reported to be modulated in vitro by asthma medications.36
IL-10R2/IL-20R1 the heterodimeric receptor of IL-26 is expressed on the surface of epithelial bronchial cells13 suggesting a possible activation of these cells by IL-26 following non-specific epithelium aggression, as these cells produce important levels of IL-33 and TSLP in asthmatic patients.47,48 The addition of IL-17 and IL-26 to alarmin and TSLP could play an active role in the pathogenesis of asthma by stimulating mononuclear cells in establishing a pro-inflammatory micro-environment.
Abbreviations
ELISA, enzyme-linked immunosorbent assay; FEV1, Forced expiratory volume in one second % predicted; FVC, forced vital capacity; IL, interleukin; SF, sputum fluid; rIL-26, recombinant human IL-26; PBMCs, peripheral blood mononuclear cells; RT-PCR, real-time polymerase chain reaction; NS, not significant.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2015;16(1):45–56. doi:10.1038/ni.3049
2. Forno E, Wang T, Yan Q, et al. A multi-omics approach to identify genes associated with childhood asthma risk and morbidity. Am J Respir Cell Mol Biol. 2017;57(4):439–447. doi:10.1165/rcmb.2017-0002OC
3. Silva MJ, de Santana MBR, Tosta BR, et al. Variants in the IL17 pathway genes are associated with atopic asthma and atopy makers in a South American population. Allergy Asthma Clin Immunol. 2019;15:28. doi:10.1186/s13223-019-0340-7
4. Whitehead GS, Kang HS, Thomas SY, et al. Therapeutic suppression of pulmonary neutrophilia and allergic airway hyperresponsiveness by a RORγt inverse agonist. JCI Insight. 2019. doi:10.1172/jci.insight.125528
5. Charrad R, Berraïes A, Hamdi B, et al. Anti inflammatory activity of IL-37 in asthmatic children: correlation with inflammatory cytokines TNF-α, IL-β, IL-6 and IL-17A. Immunobiology. 2016;221(2):182–187. doi:10.1016/j.imbio.2015.09.009
6. Corren J, Parnes JR, Wang L, et al. Tezepelumab in adults with uncontrolled asthma. N Engl J Med. 2017;377:936–946. doi:10.1056/NEJMoa1704064
7. Donnelly RP, Sheikh F, Dickensheets H, et al. Interleukin-26: an il-10-related cytokine produced by th17 cells. Cytokine Growth Factor Rev. 2010;21:393–401. doi:10.1016/j.cytogfr.2010.09.001
8. Konradsen JR, Nordlund B, Levänen B, et al. The cytokine interleukin-26 as a biomarker in pediatric asthma. Respir Res. 2016;17:32. doi:10.1186/s12931-016-0351-6
9. Ouyang W, Rutz S, Crellin NK, et al. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol. 2011;29:71–109. doi:10.1146/annurev-immunol-031210-101312
10. Cella M, Fuchs A, Vermi W, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457(7230):722–725. doi:10.1038/nature07537
11. Fujii M, Nishida A, Imaeda H, et al. Expression of Interleukin-26 is upregulated in inflammatory bowel disease. World J Gastroenterol. 2017;23(30):5519–5529. doi:10.3748/wjg.v23.i30.5519
12. Che KF, Tengvall S, Levänen B, et al. Interleukin-26 in antibacterial host defense of human lungs: effects on neutrophil mobilization. Am J Respir Crit Care Med. 2014;190(9):1022–1031. doi:10.1164/rccm.201404-0689OC
13. Hor S, Pirzer H, Dumoutier L, et al. The T-cell lymphokine interleukin-26 targets epithelial cells through the interleukin-20 receptor 1 and interleukin-10 receptor 2 chains. J Biol Chem. 2004;279:33343–33351. doi:10.1074/jbc.M405000200
14. Bals R, Hiemstra PS. Innate immunity in the lung: how epithelial cells fight against respiratory pathogens. Eur Respir J. 2004;23:327–333. doi:10.1183/09031936.03.00098803
15. Larochette V, Miot C, Poli C, et al. IL-26, a cytokine with roles in extracellular DNA-induced inflammation and microbial defense. Front Immunol. 2019;10:204. doi:10.3389/fimmu.2019.00204
16. Griffiths KL, Khader SA. Bringing in the cavalry: IL-26 mediates neutrophil recruitment to the lungs. Am J Respir Crit Care Med. 2014;190:1079–1080. doi:10.1164/rccm.201410-1870ED
17. Meller S, Di Domizio J, Voo KS, et al. T(H)17 cells promote microbial killing and innate immune sensing of DNA via interleukin 26. Nat Immunol. 2015;16:970–979. doi:10.1038/ni.3211
18. Che KF, Kaarteenaho R, Lappi-Blanco E, et al. Interleukin-26 production in human primary bronchial epithelial cells in response to viral stimulation: modulation by Th17 cytokines. Mol Med. 2017;23:247–257. doi:10.2119/molmed.2016.00064
19. Che KF, Tufvesson E, Tengvall S, et al. The neutrophil-mobilizing cytokine interleukin-26 in the airways of long-term tobacco smokers. Clin Sci (Lond). 2018;132:959–983. doi:10.1042/CS20180057
20. Kortekaas Krohn I, Shikhagaie MM, Golebski K, et al. Emerging roles of innate lymphoid cells in inflammatory diseases: clinical implications. Allergy. 2017;73:837–850. doi:10.1111/all.13340
21. Rajput C, Han M, Bentley JK, et al. Enterovirus D68 infection induces IL-17-dependent neutrophilic airway inflammation and hyperresponsiveness. JCI Insight. 2018;23:3.
22. Hekking PP, Loza MJ, Pavlidis S, et al. Study group. Pathway discovery using transcriptomic profiles in adult-onset severe asthma. J Allergy Clin Immunol. 2018;141(4):1280–1290. doi:10.1016/j.jaci.2017.06.037
23. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373. doi:10.1183/09031936.00202013
24. Brusasco V, Crapo R, Viegi G. Coming together: the ATS/ERS consensus on clinical pulmonary function testing. Eur Respir J. 2005;26(1):1–2. doi:10.1183/09031936.05.00034205
25. Fahy JV, Liu J, Wong H, Boushey HA. Cellular and biochemical analysis of induced sputum from asthmatic and from healthy subjects. Am Rev Respir Dis. 1993;147(5):1126–1131. doi:10.1164/ajrccm/147.5.1126
26. Rosewich M, Zissler UM, Kheiri T, et al. Airway inflammation in children and adolescents with bronchiolitis obliterans. Cytokine. 2015;73(1):156–162. doi:10.1016/j.cyto.2014.10.026
27. Gunawardhana LP, Gibson PG, Simpson JL, et al. Activity and expression of histone acetylases and deacetylases in inflammatory phenotypes of asthma. Clin Exp Allergy. 2014;44:47–57. doi:10.1111/cea.12168
28. Sikkeland LI, Kongerud J, Stangeland AM, et al. Macrophage enrichment from induced sputum. Thorax. 2007;62(6):558–559. doi:10.1136/thx.2006.073544
29. Kaabachi W, Bouali E, Berraïes A, et al. Interleukin-26 is overexpressed in Behçet’s disease and enhances Th17 related -cytokines. Immunol Lett. 2017;190:177–184. doi:10.1016/j.imlet.2017.08.008
30. Corren J. New Targeted Therapies for uncontrolled asthma. J Allergy Clin Immunol Pract. 2019;7(5):1394–1403. doi:10.1016/j.jaip.2019.03.022
31. Pavord ID. Oral corticosteroid-dependent asthma. Curr Opin Pulm Med. 2019;25:51–58. doi:10.1097/MCP.0000000000000541
32. Fahy JV. Type 2 inflammation in asthma–present in most, absent in many. Nat Rev Immunol. 2015;15:57–65. doi:10.1038/nri3786
33. Busse WW, Holgate S, Kerwin E, et al. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med. 2013;188:1294–1302. doi:10.1164/rccm.201212-2318OC
34. Corvaisier M, Delneste Y, Jeanvoine H, et al. IL-26 is overexpressed in rheumatoid arthritis and induces proinflammatory cytokine production and Th17 cell generation. PLoS Biol. 2012;10(9):e1001395. doi:10.1371/journal.pbio.1001395
35. Tufvesson E, Jogdand P, Che KF, et al. Enhanced local production of IL-26 in uncontrolled compared with controlled adult asthma. J Allergy Clin Immunol. 2019;144(4):1134–1136.e10. doi:10.1016/j.jaci.2019.06.035
36. Che KF, Sun J, Linden A. Pharmacological modulation of endotoxin-induced release of IL-26 in human primary lung fibroblasts. Front Pharmacol. 2019;10:956. doi:10.3389/fphar.2019.00956
37. Veldhoen M, Hocking RJ, Atkins CJ, et al. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi:10.1016/j.immuni.2006.01.001
38. Mangan PR, Harrington LE, O’Quinn DB, et al. Transforming growth factor-b induces development of the TH17 lineage. Nature. 2006;441:231–234. doi:10.1038/nature04754
39. Cho ML, Kang JW, Moon YM, et al. STAT3 and NF-kB signal pathway is required for IL-23-mediated IL-17 production in spontaneous arthritis animal model IL-1 receptor antagonist-deficient mice. J Immunol. 2006;176:5652–5661. doi:10.4049/jimmunol.176.9.5652
40. Bao A, Che KF, Bozinovski S, et al. Recombinant human IL-26 facilitates the innate immune response to endotoxin in the bronchoalveolar space of mice in vivo. PLoS One. 2017;12:e0188909. doi:10.1371/journal.pone.0188909
41. Duenas Meza E, Jaramillo CA, Correa E, et al. Virus and mycoplasma pneumoniae prevalence in a selected pediatric population with acute asthma exacerbation. J Asthma. 2016;53(3):253–260. doi:10.3109/02770903.2015.1075548
42. Li N, Qiu R, Yang Z, et al. Sputum microbiota in severe asthma patients: relationship to eosinophilic inflammation. Respir Med. 2017;131:192–198. doi:10.1016/j.rmed.2017.08.016
43. Webley WC, Hahn DL. Infection-mediated asthma: etiology, mechanisms and treatment options, with focus on Chlamydia pneumoniae and macrolides. Respir Res. 2017;18(1):98. doi:10.1186/s12931-017-0584-z
44. Gon Y, Hashimoto S. Role of airway epithelial barrier dysfunction in pathogenesis of asthma. Allergol Int. 2018;67(1):12–17. doi:10.1016/j.alit.2017.08.011
45. Wilson NJ, Boniface K, Chan JR, et al. Development cytokine profile and function of human interleukin 17–producing helper T cells. Nat Immunol. 2007;8(9):950–957. doi:10.1038/ni1497
46. Chang HS, Lee TH, Jun JA, et al. Neutrophilic inflammation in asthma: mechanisms and therapeutic considerations. Expert Rev Respir Med. 2017;11(1):29–40. doi:10.1080/17476348.2017.1268919
47. Berraïes A, Hamdi B, Ammar J, Hamzaoui K, Hamzaoui A. Increased expression of thymic stromal lymphopoietin in induced sputum from asthmatic children. Immunol Lett. 2016;178:85–91. doi:10.1016/j.imlet.2016.08.004
48. Paplińska-Goryca M, Nejman-Gryz P, Proboszcz M, et al. Expression of TSLP and IL-33 receptors on sputum macrophages of asthma patients and healthy subjects. J Asthma. 2018;27:1–10.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.