Back to Journals » Eye and Brain » Volume 15
Spotlight on Trans-Synaptic Degeneration in the Visual Pathway in Multiple Sclerosis
Authors Filippatou AG , Calabresi PA , Saidha S, Murphy OC
Received 8 June 2023
Accepted for publication 15 November 2023
Published 29 December 2023 Volume 2023:15 Pages 153—160
DOI https://doi.org/10.2147/EB.S389632
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Margaret Wong-Riley
Angeliki G Filippatou, Peter A Calabresi, Shiv Saidha, Olwen C Murphy
Division of Neuroimmunology and Neurological Infections, Department of Neurology, Johns Hopkins University, Baltimore, MD, USA
Correspondence: Olwen C Murphy, Department of Neurology, Johns Hopkins Hospital, 600 N. Wolfe St, Baltimore, MD, 21287, USA, Tel +1 410-614-1522, Fax +1410-502-6736, Email [email protected]
Abstract: A putative mechanism of neurodegeneration in multiple sclerosis (MS) is trans-synaptic degeneration (TSD), whereby injury to a neuron leads to degeneration of synaptically connected neurons. The visual system is commonly involved in MS and provides an ideal model to study TSD given its well-defined structure. TSD may occur in an anterograde direction (optic neuropathy causing degeneration in the posterior visual pathway including the optic radiations and occipital gray matter) and/or retrograde direction (posterior visual pathway lesions causing retinal degeneration). In the current review, we discuss evidence supporting the presence of anterograde and retrograde TSD in the visual system in MS.
Keywords: multiple sclerosis, optic neuritis, transsynaptic degeneration, neurodegeneration, optical coherence tomography
Introduction
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disease of the central nervous system (CNS).1 Neurodegeneration occurs throughout the course of the disease and is a key component of disease progression and permanent neurologic disability. A putative mechanism of neurodegeneration is trans-synaptic degeneration (TSD) – degeneration of neurons that synapse with the initially injured neurons. The visual pathway is an ideal model to study TSD due to its well-defined structure. The anterior visual pathway consists of ganglion cell neurons, whose cell bodies are located in the retina and form the ganglion cell layer, while their axons form the optic nerves, optic chiasm and optic tracts and synapse at the lateral geniculate nuclei (LGN) of the thalami. The posterior visual pathway consists of third-order neurons located in the LGN, the axons of which project to form the optic radiations and terminate in the primary visual cortices within the occipital lobes. TSD can occur both in an antero-grade direction (lesions in the anterior visual pathway causing degeneration of third-order neurons projecting from the LGN to the primary visual cortex) and in a retrograde direction (lesions in the posterior visual pathway causing degeneration of retinal ganglion cells (RGCs), or a lesion affecting second-order neurons resulting in degeneration of the first-order neurons – the bipolar cells of the retinal inner nuclear layer)2 (Figure 1). It is also conceivable that multi-chain TSD may occur, where lesions of the third order neurons in the posterior visual pathway may result in retrograde TSD of both second order and first-order neurons sequentially. Experimental evidence from animal models supports the presence of bidirectional neuroaxonal degeneration and TSD after optic nerve or intraretinal axotomy.3,4
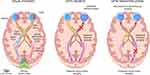 |
Figure 1 Patterns of trans-synaptic degeneration in the afferent visual pathway in multiple sclerosis. The visual pathway is a functionally eloquent sensory pathway made up of 3 neurons, travelling from the retina to the primary visual cortex of the occipital lobe. The axons of the 2nd order neurons (travelling from the retinal ganglion cell layer through the optic nerve and optic tracts to the thalami) are highly organized, with the axons from the nasal half of the retina crossing over to the contralateral cerebral hemisphere within the optic chiasm, while the axons from the temporal half of the retina remain uncrossed and continue their path within the ipsilateral cerebral hemisphere. The crossed and uncrossed nature of the pathway means that each thalamus and visual cortex receives inputs from the right and left eye in a homonymous pattern. In optic neuritis, injury to the axons of the 2nd order neurons (prior to the optic chiasm) can result in anterograde degeneration of affected axons, a process which may then proceed trans-synaptically, resulting in anterograde trans-synaptic degeneration of the 3rd order neurons travelling from both thalami to both primary visual cortices. Neuroaxonal degeneration after optic neuritis may also proceed in the retrograde direction, resulting in loss of cell bodies in the ipsilateral retinal ganglion cell layer (GCL), and potentially trans-synaptically to the 1st order neurons contained in the ipsilateral retinal inner nuclear layer (INL). On the other hand, if a demyelinating lesion causes axonal injury to the 3rd order neurons (eg within the optic radiations), anterograde neuroaxonal degeneration may result in atrophy of the ipsilateral visual cortex, while retrograde degeneration may proceed to the ipsilateral thalamus, and potentially trans-synaptically to the highly organized crossed and uncrossed 2nd order neurons, resulting in retrograde trans-synaptic degeneration with a homonymous pattern of atrophy of the retinal ganglion cell layer (and possibly even trans-synaptically again to the 1st order neurons within the retinal inner nuclear layer). Reproduced from Murphy OC, Calabresi PA, Saidha S. Illustrations of the Afferent Visual Pathway and Concepts Surrounding Trans-Synaptic Neuroaxonal Degeneration in the Visual Pathway in Multiple Sclerosis.5 |
In recent years, quantitative retinal and brain imaging techniques have been increasingly employed to interrogate neurodegenerative processes such as TSD in-vivo in people with MS (PwMS). Optical coherence tomography (OCT) allows quantification of individual retinal layer thicknesses and has allowed the characterization of structural changes that occur in the anterior visual pathway in MS.6 The posterior visual pathway can be studied with advanced MRI technology, including volumetric analysis of various brain substructures and diffusion tensor imaging which is sensitive to the microscopic motion of water molecules in brain tissue and can reveal disruptions to axonal and myelin microstructure.7
Optic Neuritis in MS
Optic neuritis (ON) is common in MS, representing the initial manifestation of the disease in approximately 20% of PwMS, and occurring in roughly 50% of PwMS at some point during their disease course. Moreover, optic neuropathy is virtually ubiquitous in MS, with more than 90% of PwMS having evidence of demyelinating lesions in their optic nerves at post-mortem.8 During acute ON, there is damage to the axons of RGCs primarily related to demyelination and/or inflammatory transection resulting in retrograde degeneration of the axons, and subsequently the cell bodies of the neurons from which these axons originate (ie, RGCs). These processes result in thinning of the retinal nerve fiber layer (RNFL – containing the axons of the RGCs which coalesce to make up the optic nerve) and ganglion cell layer (containing the cell bodies of the RGCs). With OCT, the ganglion cell layer is typically measured as a composite with the inner plexiform layer (GCIPL), since these layers are not easily distinguished in OCT images. These dynamic changes typically occur during the first three to six months after ON, with the majority of degeneration occurring rapidly post-ON.9 RNFL and GCIPL thinning, albeit less severe, has also been observed in eyes that have not experienced clinical episodes of ON, and is more commonly referred to as chronic optic neuropathy.6
Anterograde Trans-Synaptic Degeneration in the Visual Pathway in MS
Several studies in MS have attempted to examine whether there is TSD of the third-order neurons of the posterior visual pathway following ON (Table 1). Gabilondo et al evaluated a cohort of 100 PwMS with OCT and MRI and found that PwMS with a prior history of severe ON had lower visual cortex volume compared to those without ON, independently of lesion volume within the optic radiations.10 Balk et al found lower thickness of the visual cortex in 39 PwMS with prior ON compared to 107 PwMS with no history of ON, while the thickness of the whole brain cortex did not differ between the groups.11 While these findings were suggestive of possible anterograde TSD in PwMS with ON, the direction and temporal evolution of the findings could not be determined in the absence of longitudinal data.
 |
Table 1 Summary of Evidence in Support of Anterograde and Retrograde Trans-Synaptic Degeneration in the Visual System in MS |
Our group examined a longitudinal prospective cohort of 49 PwMS or high risk clinically isolated syndrome (CIS) who were recruited within 40 days of acute ON onset and were followed longitudinally with OCT and MRI.12 Over the study period, this cohort had accelerated atrophy of the occipital gray matter, calcarine gray matter and thalami compared to a comparator cohort of PwMS without an episode of ON in the prior 3 years or during follow-up. There were no significant differences in whole brain, white matter, cortical gray-matter, non-occipital cortical or subcortical gray matter or T2 lesion accumulation between the cohorts.
Additional studies have utilized advanced MRI techniques, including diffusion-weighted MRI measures to detect microstructural damage in the posterior visual pathway following ON. A cross-sectional study by Pawlitzki et al showed decreased optic radiations fractional anisotropy (FA) and increased radial diffusivity (RD), axial diffusivity (AD) and mean diffusivity (MD) in 17 PwMS with history of ON compared to 11 PwMS without a history of ON.13 Decreased FA and increased diffusivity are suggestive of an increase in the ability of water molecules to diffuse, which can be seen in demyelination or axonal loss.14 Balk et al examined 38 PwMS with bilateral ON and found decreased FA and increased MD compared to PwMS without a history of ON and healthy controls (HC).15 Thicknesses of the retinal layers (GCIPL and peripapillary RNFL [pRNFL]) were positively associated with primary visual cortex thickness (V1) and optic radiations FA and negatively associated with optic radiations MD in PwMS, however most of these associations were not statistically significant. Rocca et al examined 102 PwMS and found that optic radiations diffusivity abnormalities were more severe in PwMS with prior ON history compared to PwMS without prior ON history, and mainly located in the anterior and middle portions of the optic radiations.16 Additional studies in isolated ON by Kolbe et al found abnormal FA and diffusivity in the optic radiations.17,18
Diffusion-weighted MRI measures have also been studied longitudinally in MS. Tur et al studied a cohort of 28 PwMS after ON and 8 HC longitudinally with serial MRI scans.19 Over one year following ON, the FA in the optic radiations decreased and the RD increased in PwMS, whereas no significant longitudinal changes occurred in HC. These results were similar when adjusting for lesion load in the optic radiations. Additionally, affected optic nerves with lower cross-sectional areas (ie, thinner optic nerves) at three months after ON were associated with lower FA and higher RD at one year in the optic radiations. This study suggests the presence of microstructural damage in the optic radiations after ON, independent of optic radiation lesion load and most pronounced in PwMS with optic nerve atrophy on orbital scan MRIs, further supporting the notion of anterograde TSD after ON. You et al studied people with glaucoma and PwMS with a history of ON and no optic radiation lesions.20 Both cohorts had anisotropic increase of water diffusion detected in the optic radiations, characterized by changes in RD that extended more posteriorly than AD. It is thought that RD is more reflective of myelin integrity, whereas AD is more reflective of axonal integrity, therefore this finding suggested that myelin pathology might start earlier in the process of anterograde TSD.14 The PwMS were followed for three years longitudinally and were found to have an increase in RD (mainly observed in the anterior segments of the optic radiations) that correlated with GCIPL retinal thinning. The authors then used an animal model of optic nerve injury (unilateral optic nerve axonotomy) to characterize changes in the posterior visual pathway and observed early glial activation and demyelination in the posterior visual projections that occurred prior to axonal loss.20
Retrograde Trans-Synaptic Degeneration in the Visual Pathway in MS
Post-chiasmal pathology in the visual pathway has the potential to affect the second-order neurons of the optic nerve and retina through retrograde TSD. Given the retinotopic organization of the visual system, the pattern of atrophy that would be expected at the optic disc is temporal thinning in the ipsilateral eye and a bow-tie pattern of atrophy in the contralateral eye, whereas the macula would be expected to show a homonymous hemi-macular pattern of atrophy (temporal hemi-atrophy in the ipsilateral eye and nasal hemi-atrophy in the contralateral eye).21 These findings can be caused by a lesion in the optic radiations and are reflected in the pattern of peripapillary RNFL and GCIPL loss, respectively, as measured by OCT (Figure 2).
Al-Louzi et al reported a case series of six PwMS with posterior visual pathway lesions (four with lesions in the occipital white matter and two with lesions in the thalamus) who were found to have homonymous, hemi-macular pattern of GCIPL thinning on OCT, three of which also had corresponding homonymous hemi-macular microcystic macular pathology (MMP) of the inner nuclear layer (INL) in the same distribution as the hemi-macular GCIPL thinning.22 An index has been proposed to quantify hemi-macular retinal thickness; the proposed index represents a ratio comparing ganglion cell thickness in nasal and temporal hemi-macular sectors across both eyes.23
Balk et al examined 103 PwMS with no prior history of ON and found that increased lesion volume in the optic radiations was associated with lower pRNFL and GCIPL thicknesses, while higher GCIPL thickness and, to a lesser extent, higher pRNFL thickness, were associated with higher optic radiations FA and lower MD.15 Reich et al studied 36 PwMS and found moderate correlations of pRNFL thickness with FA and RD (but not AD or MD) along the optic radiations.24 Sinnecker et al studied 30 PwMS with OCT and 7 T MRI and found an inverse correlation between optic radiations lesion volume and RNFL thickness, including in PwMS without previous ON.25 Gabilondo et al evaluated a cohort of 100 PwMS and found that lower RNFL thickness was associated with lower visual cortex volume, higher lesion volume within the optic radiations and lower N-acetyl aspartate in visual cortex, a potential surrogate marker of axonal damage measured with magnetic resonance spectroscopy, independent of ON history.10 The analysis was repeated with data obtained after one year of follow-up and similar associations were observed, however the study was not powered to allow a reliable longitudinal analysis. Since these studies had a cross-sectional design, the direction of these findings could not be corroborated.
Klistorner et al studied 53 PwMS and 50 HC cross-sectionally and found that temporal RNFL thickness was reduced in MS eyes with no prior history of ON and there was a correlation between thinning of the temporal RNFL and optic radiations lesion volume, as well as optic radiations diffusion tensor imaging indices (FA demonstrated a positive correlation with temporal RNFL thickness, while all diffusivity measures were inversely associated with temporal RNFL).26 Klistorner et al subsequently performed a 3-year longitudinal study, during which 55 PwMS were followed with annual MRI and OCT.27 The authors found that PwMS with new lesions involving the optic radiations had greater temporal RNFL thinning compared to PwMS with new lesions outside the optic radiations. This study supports the notion that retrograde TSD caused by optic radiations lesions might play a role in progressive retinal nerve fiber layer loss.
There is also interest in how the deeper layers of the retina are affected in MS and whether retrograde TSD occurs within the retina after ON, with degeneration of the retinal ganglion cell potentially followed by trans-synaptic changes in the bipolar cells which are located in the inner nuclear layer (INL). Balk et al examined 144 MS ON eyes and 279 MS non-ON eyes and found sparing of the INL and outer retinal layers in ON eyes, despite significant thinning of the inner retinal layers (RNFL and GCIPL).11 This led to the hypothesis that the INL may act as a physiological barrier against retrograde TSD after ON within the retina. However, in the previously mentioned study by our group, during which 49 PwMS or high-risk CIS who were recruited within 40 days of acute ON onset and were followed longitudinally, ON eyes showed reductions in all retinal layer thicknesses over the first year post-ON.12 Reductions in pRNFL, GCIPL, and INL thicknesses over the first year post-ON were significantly greater in ON eyes than in their fellow eyes, possibly supporting the occurrence of retrograde TSD extending to the bipolar cells in the INL. Notably, changes seen in the INL after ON in this study were of a much smaller magnitude (and therefore more difficult to detect) than changes in the GCIPL/pRNFL, which may explain the discrepancy between these results and prior reports in the literature. Furthermore, the transient swelling of the INL that is known to occur in both the affected and unaffected eye after ON means that the measurement and interpretation of longitudinal changes and inter-eye differences in this layer must be carefully considered in the context of ON.28
Importantly, both anterograde and retrograde TSD are proposed to occur through synapses in the LGN, however there are technical difficulties in studying the LGN due to its small size that renders accurate volumetric measurements challenging. Papadopoulou et al proposed an atlas-based automated LGN segmentation method and studied a cohort of 34 PwMS.29 LGN volume was associated with GCIPL thickness (temporal in the ipsilateral and nasal in the contralateral eye) in PwMS with a history of ON. LGN volume was also associated with optic radiations lesion volume independently of GCIPL, suggesting a bidirectional effect of both anterior and posterior visual pathway damage on LGN volume.
Significance of Trans-Synaptic Degeneration in MS
There is accumulating evidence to suggest the presence of both anterograde and retrograde TSD in the visual system in MS after inflammatory activity (Table 1). This process may have broader implications in MS pathophysiology, as it may indicate more aggressive disease with greater tissue damage. It is conceivable that TSD may be a key driver of progressive neurodegeneration, CNS atrophy and disability accumulation in MS. Studies utilizing OCT and MRI have allowed in-vivo investigation and quantification of TSD in people with MS. An imaging modality that quantifies TSD may serve as outcome measures in clinical trials, particularly for putative therapeutic agents that may target remyelination, neuroprotection or neurorestoration within the visual system. For example, future work can evaluate whether novel therapeutics can halt damage to the posterior visual pathway after ON.
Future studies are needed to enhance our understanding of the pathophysiology of TSD, which could inform more broadly mechanisms of neurodegeneration in MS. The majority of existing studies have a cross-sectional design, hence the direction and temporal evolution of the findings cannot be determined. It is currently unclear how quickly TSD occurs, if at all, after an inflammatory demyelinating event and how much of the global burden of neurodegeneration in MS can be attributed to TSD. While the severity of injury conferred by a focal lesion to the posterior visual pathways could be the driver of TSD, there are substantial data supporting variable synaptic pathology in MS brain tissues.30,31 The mechanisms by which C1q and C3 opsonize synapses, which are then stripped by microglia may vary within individuals due to genetic variation within reactive glia.32,33 With regard to MS therapeutics, a crucial outstanding question is whether current MS disease-modifying treatments can affect TSD and whether future neuroprotective therapies could prevent TSD and impact disease progression in MS. An additional limitation is the challenge of quantifying TSD on an individual patient level within the visual system, given the difficulty and lack of standardization in employing quantitative imaging in everyday clinical practice.
Conclusion
TSD is of interest, as it may be a key mechanism underlying neurodegeneration, progressive disability and brain atrophy in MS.34 Based on accumulated evidence from quantitative imaging studies in recent years, there is compelling support for the occurrence of both anterograde and retrograde TSD within the visual system in PwMS. Additional research is needed to determine whether the extent of TSD is a marker of more aggressive disease with greater tissue destruction and poorer tissue repair. Better understanding of these mechanisms may enhance our understanding of pathobiologic mechanisms of neurodegeneration in MS and facilitate the development and/or study of neuroprotective therapies.
Acknowledgments
Figure 2 is created by Omar Ezzedin, MD, Department of Neurology, Johns Hopkins University School of Medicine.
Funding
This work was funded by the National MS Society (RG-1606-08768 & RG-1907-34405 to S.S), Race to Erase MS (to O.C.M. and S.S.), and NIH/NINDS (R01NS082347 to P.A.C.).
Disclosure
Olwen Murphy receives funding from the Race to Erase MS Foundation during the conduct of the study.
Peter A Calabresi has received consulting fees from Disarm, Nervgen, and Biogen; personal fees from Lilly, Idorsia, Novartis; grants from Myelin Repair Foundation and Genentech and is PI on grants to JHU from Biogen, Genentech, Principia, and Annexon.
Shiv Saidha has received consulting fees from Medical Logix for the development of CME programs in neurology and has served on scientific advisory boards for Biogen, Novartis, Genentech Corporation, TG therapeutics & Bristol Myers Squibb. He has performed consulting for Novartis, Genentech Corporation, JuneBrain LLC, and Lapix therapeutics. He is the PI of investigator-initiated studies funded by Genentech Corporation and Biogen. He previously received support from the Race to Erase MS foundation. He has received equity compensation for consulting from JuneBrain LLC and Lapix therapeutics. He was also the site investigator of trials sponsored by MedDay Pharmaceuticals, Clene Pharmaceuticals, and is the site investigator of a trial sponsored by Novartis.
The authors report no other conflicts of interest in this work.
References
1. Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N Engl J Med. 2018;378:169–180. doi:10.1056/NEJMra1401483
2. Costello F. The afferent visual pathway: designing a structural-functional paradigm of multiple sclerosis. ISRN Neurol. 2013;2013:134858. doi:10.1155/2013/134858
3. You Y, Gupta VK, Graham SL, et al. Anterograde degeneration along the visual pathway after optic nerve injury. PLoS One. 2012;7:e52061. doi:10.1371/journal.pone.0052061
4. Kanamori A, Catrinescu M, Belisle JM, et al. Retrograde and Wallerian axonal degeneration occur synchronously after retinal ganglion cell axotomy. Am J Pathol. 2012;181:62–73. doi:10.1016/j.ajpath.2012.03.030
5. Olwen CM, Peter AC, Shiv S. Illustrations of the afferent visual pathway and concepts surrounding trans-synaptic neuroaxonal degeneration in the visual pathway in multiple sclerosis. Available from: https://collections.lib.utah.edu/ark:/87278/s6ty03rf.
6. Petzold A, Balcer L, Balcer LJ, et al. Retinal layer segmentation in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurology. 2017;16:797–812. doi:10.1016/S1474-4422(17)30278-8
7. Granziera C, Wuerfel J, Barkhof F, et al. Quantitative magnetic resonance imaging towards clinical application in multiple sclerosis. Brain. 2021;144:1296–1311. doi:10.1093/brain/awab029
8. Green AJ, McQuaid S, Hauser SL, et al. Ocular pathology in multiple sclerosis: retinal atrophy and inflammation irrespective of disease duration. Brain. 2010;133:1591–1601. doi:10.1093/brain/awq080
9. Syc SB, Saidha S, Newsome SD, et al. Optical coherence tomography segmentation reveals ganglion cell layer pathology after optic neuritis. Brain. 2012;135:521–533. doi:10.1093/brain/awr264
10. Gabilondo I, Martínez-Lapiscina EH, Martínez-Heras E, et al. Trans-synaptic axonal degeneration in the visual pathway in multiple sclerosis. Ann Neurol. 2014;75(1):98–107. doi:10.1002/ana.24030
11. Balk LJ, Twisk JWR, Steenwijk MD, et al. A dam for retrograde axonal degeneration in multiple sclerosis? J Neurol Neurosurg Psychiatry. 2014;85:782–789. doi:10.1136/jnnp-2013-306902
12. Murphy OC, Sotirchos ES, Kalaitzidis G, et al. Trans-synaptic degeneration following acute optic neuritis in multiple sclerosis. Ann Neurol. 2023;93:76–87. doi:10.1002/ana.26529
13. Pawlitzki M, Horbrügger M, Loewe K, et al. MS optic neuritis-induced long-term structural changes within the visual pathway. Neurol Neuroimmunol Neuroinflamm. 2020;7:e665. doi:10.1212/NXI.0000000000000665
14. Klawiter EC, Schmidt RE, Trinkaus K, et al. Radial diffusivity predicts demyelination in ex-vivo multiple sclerosis spinal cords. Neuroimage. 2011;55(4):1454–1460. doi:10.1016/j.neuroimage.2011.01.007
15. Balk LJ, Steenwijk MD, Tewarie P, et al. Bidirectional trans-synaptic axonal degeneration in the visual pathway in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2015;86:419–424. doi:10.1136/jnnp-2014-308189
16. Rocca MA, Mesaros S, Preziosa P, et al. Wallerian and trans-synaptic degeneration contribute to optic radiation damage in multiple sclerosis: a diffusion tensor MRI study. Mult Scler. 2013;19:1610–1617. doi:10.1177/1352458513485146
17. Kolbe SC, van der Walt A, Butzkueven H, et al. Serial diffusion tensor imaging of the optic radiations after acute optic neuritis. J Ophthalmol. 2016;2016:2764538. doi:10.1155/2016/2764538
18. Kolbe S, Bajraszewski C, Chapman C, et al. Diffusion tensor imaging of the optic radiations after optic neuritis. Hum Brain Mapp. 2012;33:2047–2061. doi:10.1002/hbm.21343
19. Tur C, Goodkin O, Altmann DR, et al. Longitudinal evidence for anterograde trans-synaptic degeneration after optic neuritis. Brain. 2016;139:816–828. doi:10.1093/brain/awv396
20. You Y, Joseph C, Wang C, et al. Demyelination precedes axonal loss in the transneuronal spread of human neurodegenerative disease. Brain. 2019;142:426–442. doi:10.1093/brain/awy338
21. Ilardi M, Nolan-Kenney R, Fatterpekar G, et al. Role for OCT in detecting hemi-macular ganglion cell layer thinning in patients with multiple sclerosis and related demyelinating diseases. J Neurol Sci. 2020;419:117159. doi:10.1016/j.jns.2020.117159
22. Al-Louzi O, Button J, Newsome SD, et al. Retrograde trans-synaptic visual pathway degeneration in multiple sclerosis: a case series. Mult Scler. 2017;23:1035–1039. doi:10.1177/1352458516679035
23. Mitchell JR, Oliveira C, Tsiouris AJ, et al. Corresponding Ganglion cell atrophy in patients with postgeniculate homonymous visual field loss. J Neuroophthalmol. 2015;35:353–359. doi:10.1097/WNO.0000000000000268
24. Reich DS, Smith SA, Gordon-Lipkin EM, et al. Damage to the optic radiation in multiple sclerosis is associated with retinal injury and visual disability. Arch Neurol. 2009;66:998–1006. doi:10.1001/archneurol.2009.107
25. Sinnecker T, Oberwahrenbrock T, Metz I, et al. Optic radiation damage in multiple sclerosis is associated with visual dysfunction and retinal thinning--an ultrahigh-field MR pilot study. Eur Radiol. 2015;25:122–131. doi:10.1007/s00330-014-3358-8
26. Klistorner A, Sriram P, Vootakuru N, et al. Axonal loss of retinal neurons in multiple sclerosis associated with optic radiation lesions. Neurology. 2014;82:2165–2172.
27. Klistorner A, Graham EC, Yiannikas C, et al. Progression of retinal ganglion cell loss in multiple sclerosis is associated with new lesions in the optic radiations. Eur J Neurol. 2017;24:1392–1398. doi:10.1111/ene.13404
28. Al-Louzi OA, Bhargava P, Newsome SD, et al. Outer retinal changes following acute optic neuritis. Mult Scler. 2016;22:362–372. doi:10.1177/1352458515590646
29. Papadopoulou A, Gaetano L, Pfister A, et al. Damage of the lateral geniculate nucleus in MS: assessing the missing node of the visual pathway. Neurology. 2019;92:e2240–e2249. doi:10.1212/WNL.0000000000007450
30. Gharagozloo M, Smith MD, Jin J, et al. Complement component 3 from astrocytes mediates retinal ganglion cell loss during neuroinflammation. Acta Neuropathol. 2021;142:899–915. doi:10.1007/s00401-021-02366-4
31. Werneburg S, Jung J, Kunjamma RB, et al. Targeted complement inhibition at synapses prevents microglial synaptic engulfment and synapse loss in demyelinating disease. Immunity. 2020;52:167–182.e7. doi:10.1016/j.immuni.2019.12.004
32. Hammond TR, Marsh SE, Stevens B. Immune signaling in neurodegeneration. Immunity. 2019;50:955–974. doi:10.1016/j.immuni.2019.03.016
33. Fitzgerald KC, Kim K, Smith MD, et al. Early complement genes are associated with visual system degeneration in multiple sclerosis. Brain. 2019;142:2722–2736. doi:10.1093/brain/awz188
34. Bermel RA, Villoslada P. Retrograde trans-synaptic degeneration in MS: a missing link? Neurology. 2014;82:2152–2153. doi:10.1212/WNL.0000000000000532
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.