Back to Journals » Clinical Ophthalmology » Volume 17
Spotlight on Schlemm’s Canal MicroStent Injection in Patients with Glaucoma
Authors Wagner IV , Ang B, Checo L , Simsek D , Draper C, Dorairaj S
Received 22 March 2023
Accepted for publication 30 May 2023
Published 2 June 2023 Volume 2023:17 Pages 1557—1564
DOI https://doi.org/10.2147/OPTH.S388293
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Isabella V Wagner,1 Bryan Ang,2 Leticia Checo,1 Derya Simsek,3 Christian Draper,1 Syril Dorairaj1
1Department of Ophthalmology, Mayo Clinic, Jacksonville, FL, USA; 2Department of Ophthalmology, Tan Tock Seng Hospital, Singapore; 3Department of Ophthalmology, Baskent University Medical School, Ankara, Turkey
Correspondence: Syril Dorairaj, Department of Ophthalmology, Mayo Clinic, 4500 San Pablo Road, Jacksonville, FL, USA, Tel +1 904-953-2377, Fax +1 904-953-7040, Email [email protected]
Abstract: Minimally invasive glaucoma surgery (MIGS) has revolutionized glaucoma care with its favorable safety profile and ability to delay or minimize the need for traditional, bleb-based procedures. Microstent device implantation is a type of angle-based MIGS, which reduces intraocular pressure (IOP) through bypass of the juxtacanalicular trabecular meshwork (TM) and facilitation of aqueous outflow into the Schlemm’s canal. Although there are limited microstent devices on the market, multiple studies have evaluated the safety and efficacy of iStent® (Glaukos Corp.), iStent Inject® (Glaukos Corp.), and Hydrus® Microstent (Alcon) in the treatment of mild-to-moderate open-angle glaucoma, with and without concurrent phacoemulsification. This review attempts to provide a comprehensive evaluation of injectable angle-based microstent MIGS devices in the treatment of glaucoma.
Keywords: microstent injection, Schlemm’s canal, Hydrus microstent, iStent, glaucoma, aqueous outflow
Introduction
Glaucoma is a progressive degenerative neuropathy, which induces apoptosis of retinal ganglion cells and leads to irreversible vision loss.1,2 Glaucoma is characterized by the anatomy of the anterior chamber angle (ACA) and develops either due to resistance to aqueous outflow despite a visibly unoccluded angle (open-angle glaucoma) or due to obstruction at the drainage angle (angle closure glaucoma), both of which typically cause an increase in intraocular pressure (IOP), resulting in optic nerve damage.1,2 Additionally, glaucoma may develop from the compression of Schlemm’s canal following age-related lens enlargement.3 Glaucoma affects roughly 76 million people worldwide with primary angle glaucoma (POAG), the predominant disease form, occurring in an estimated 57.5 million.4 As the incidence of glaucoma is expected to rise with the aging population, early intervention and appropriate care are critical. Disease progression is slowed by reducing IOP, which may be accomplished through medicinal therapy and/or lasers, and in more advanced or refractory disease, surgical treatment.
Options for surgical management of glaucoma have expanded rapidly within the last decade. While traditional incisional surgeries (trabeculectomy and tube shunt implantation) have been shown to be efficacious in reducing disease progression, their inherent intra-operative and post-operative risks may lead to serious and sight-threatening complications. Minimally invasive glaucoma surgeries (MIGS) have emerged in the recent decade. Having been demonstrated to possess a superior safety profile, are easier to perform and result in a faster patient recovery, MIGS have been employed by a growing number of surgeons in the treatment of glaucoma, with MIGS in combination with cataract surgery providing the greatest IOP-lowering effect.5 Given the variety of ways to target aqueous outflow, the constantly expanding MIGS market consists of multiple devices, which aim to lower IOP by facilitating aqueous drainage into either the suprachoroidal space, subconjunctival space, or the Schlemm’s canal.6
The raised IOP in open-angle glaucoma is believed to be due to increased resistance to aqueous humor drainage at the level of the trabecular meshwork (TM), with an estimated 50–75% of outflow resistance residing within the TM and inner wall of Schlemm’s canal.7–9 MIGS devices that target Schlemm’s canal (or “trabecular bypass” MIGS) divert aqueous outflow directly into Schlemm’s canal, bypassing most of the trabecular resistance. However, residual resistance (25–50%) within Schlemm’s canal and its distal elements (collector channels and episcleral veins) limits the IOP-lowering efficacy of trabecular bypass MIGS devices to the low-teens, making these MIGS more suitable for the treatment of mild-to-moderate POAG.6,9 The five trabecular bypass MIGS devices, which have been implanted over the last few years, inserted into Schlemm’s canal ab-interno and under gonioscopic view, include the iStent® (Glaukos Corp.), iStent inject®, iStent inject W®, iStent infinite®, and Hydrus® Microstent (Alcon).6
iStent
The first-generation iStent was the first ab-interno trabecular bypass implant, which received US FDA approval in 2012. This was an L-shaped, 1-mm long heparin-coated titanium device consisting of a curved convex canal portion (designed to lie across the inner wall of the canal) and a tubular portion facing the anterior chamber (responsible for bypassing most of the outflow resistance).6 The device was implanted with the use of a preloaded injector inserted into the anterior chamber through a clear corneal incision and advanced to the nasal ACA, where the stent was manually inserted through the TM and into the Schlemm’s canal. The iStent demonstrated high safety and long-term efficacy in the treatment of eyes with well-controlled glaucoma on two or fewer medications. Seven-year outcomes of combined phacoemulsification and iStent implantation demonstrated clinically significant reduction in both IOP (−22.9%) and antiglaucoma medications (−27.2%), with an impressive safety profile comparable to that of standalone phacoemulsification.10 A meta-analysis of 13 studies (78 eyes) found there to be benefits in performing standalone iStent surgery, with pooled mean IOP reductions of 31.1% from 6 to 12 months, 30.4% from 36 to 48 months, and 32.9% through 60 months.11 A greater IOP-lowering effect and long-term efficacy has been shown to occur with the placement of multiple stents. In particular, a clinical trial of 29 eyes, with uncontrolled glaucoma receiving implantation of two iStents showed minimal adverse effects and clinically meaningful reductions in both IOP (−40%) and antiglaucoma medications (−30%), with 89.7% of eyes achieving >20% IOP reduction at five-years post-operation.12 Another study of 62 surgery-naïve POAG eyes receiving implantation of two iStents with cataract surgery demonstrated significant reduction in both IOP (−26%) and antiglaucoma medications (−17.9%), with a favorable safety profile (determined by stability of the cup-to-disc ratio, retinal nerve fiber layer thickness, ganglion cell-inner plexiform layer thickness, and best corrected visual acuity), seen through eight-years postoperatively.13 The excellent safety profile and high IOP-lowering effectiveness observed with the placement of multiple stents combined with cataract surgery led to the development of the second-generation iStent inject.
iStent Inject
The iStent inject is similar in design to the first-generation iStent, albeit smaller and conical shaped, and delivered in a pair with the use of a single injector device.6 The device, FDA-approved in 2018, is pre-packaged with two stents, has a symmetrical configuration, and is designed for easier use, with no sideways sliding.14 The device head contains one central outlet and four side outlets to promote multidirectional flow of aqueous humor.15,16 The implantation technique is nearly identical to that of the first-generation iStent, with both stents placed two clock hours away (Figure 1A). The FDA pivotal trial evaluated eyes with mild to moderate POAG, randomized 3:1 to receive iStent inject implantation after cataract surgery (n=387) or standalone cataract surgery (n=118).15 At 24 months, significantly greater IOP and medication reduction was observed in the combined phacoemulsification-iStent inject group, with 75.8% of eyes vs 61.9% of standalone cataract surgery eyes experiencing ≥20% reduction of unmedicated mean diurnal IOP, compared to baseline (MDIOP; P = 0.005).15 An excellent safety profile was seen in both groups, with minimal changes in visual field mean deviation and cup-to-disc ratio and no reports of hypotony or corneal decompensation.15 Although typically used in mild to moderate POAG, the iStent inject has also shown good safety and efficacy when implanted in various glaucoma subtypes and severities. Three-year outcomes of the second generation iStent combined with phacoemulsification in mild to severe normal tension glaucoma (NTG), primary angle closure glaucoma (PACG), and pseudoexfoliative glaucoma showed clinically significant reductions in both IOP (−22%) and antiglaucoma medications (−51%), with a significant percentage of eyes achieving an IOP of < 15 mmHg (80%) and eliminating > 1 medication (76%).16 Several studies have investigated the use of combined phacoemulsification and iStent inject implantation in NTG.17–19 In 62 eyes with mild to severe NTG, Salimi et al observed significant IOP (−22%) and antiglaucoma medication reductions (−70%), with all eyes achieving an IOP <18 mmHg at 12 months.17 In a single-centre study, Ang et al retrospectively analyzed 91 Asian eyes with NTG and found reductions in both IOP and antiglaucoma medications (−80%) at 12 months.18 No vision threatening complications or further glaucoma interventions were reported in either study.17,18 Ang et al additionally conducted a prospective study of 30 Asian eyes with NTG and reported significant reductions in IOP (−8%) and antiglaucoma medications (−77%), with 83% of eyes remaining medication-free at 12 months.19 The most frequent complication observed was stent occlusion by iris (10%; n=3).19 Prospective studies have shown that iStent inject implantation combined with phacoemulsification has a minimal effect on corneal endothelial cell loss (CECL).15,20,21 In the iStent inject pivotal trial, Samuelson et al reported CECL percentages of −13.1% and −12.3% in eyes receiving phacoemulsification-alone and phacoemulsification-iStent inject surgery, respectively, with a comparable CECL >30% observed in both groups at 24 months (10.4% vs 9.5%).15 Likewise, Gillman et al analyzed 54 eyes with mild to moderate open angle glaucoma and found similar CECL percentages in phacoemulsification-alone (−14.4%) and phacoemulsification-iStent inject eyes (−14.6%) at 12 months.21 In a separate study, these same authors found that the positioning of the iStent inject within the anterior chamber may have several implications on the device’s IOP-lowering capabilities.22 In 25 eyes with mild to moderate open angle glaucoma, Gillman et al found an association between the amplitude of device protrusion, Schlemm’s canal diameter, and a postoperative IOP.22 The iStent inject W is a recent advancement of the iStent inject and includes a wider base flange (360 µm vs 230 µm diameter) to increase visibility during surgery and reduce the risk of overimplantation, but is otherwise identical in design (Figure 1B).23
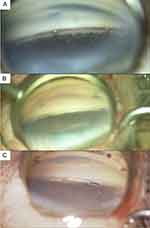 |
Figure 1 Intraoperative photos of: (A) iStent inject (B) iStent inject W (C) Hydrus Microstent, showing positioning of the inlet in the anterior chamber. Courtesy of Bryan Ang. |
iStent vs iStent Inject
Several studies have compared outcomes of combined cataract surgery with the iStent or iStent inject.24–27 Results from each study indicate that implantation of one or two stents is equally safe, with a typically greater IOP-lowering effect observed with the placement of two stents. In a study of 197 eyes with mild to severe open-angle glaucoma, Shalaby et al observed that eyes implanted with the iStent inject achieved lower IOPs at month 6 (−14% vs −8%), with comparable surgical success (>20% IOP reduction from baseline) seen in both groups at 6 and 12 months after surgery.24 Both groups experienced significant medication reductions (−35% vs −18%) and improvement in visual field mean deviation at 12 months.24 Guedes et al compared outcomes in 58 eyes with primarily (96.6%) mild-to-moderate open-angle glaucoma and observed greater efficacy in the iStent inject group, with a significantly higher percentage of iStent inject eyes achieving an IOP <18 mmHg (100% vs 80%) and <15 mmHg (73.9% vs 34.3%) at 12 months.25 Mean medication numbers were also significantly lower in the iStent inject group (0.1 vs 0.5 medications).25 The safety profile was similar in both groups, with no vision-threatening complications observed through 12 months.25 Hooshmand et al evaluated 245 eyes with POAG and observed comparable safety and efficacy in both cohorts, with similar percentages achieving an IOP ≤18 mmHg with zero medications (iStent: 56.0%; iStent inject 51.3%) or with reduced medications (iStent: 63.1%; iStent inject: 57.7%) at 12 months.26 Manning analyzed 137 eyes with mild-to-moderate glaucoma and demonstrated greater IOP (−29.4% vs −22.8%) and medication reductions (−92.3% vs −83.3%) in the iStent inject group, with favorable safety observed in both groups.27 No decreases in visual acuity were seen in any study, suggesting that stent implantation does not negate the visual benefits of cataract surgery.
iStent Infinite
The iStent infinite, a 3-stent device, is the newest development of the iStent series and received FDA clearance in 2022 for standalone use or combined with cataract surgery. Prior to its FDA clearance, beneficial results have been seen with implantation of three stents. A retrospective study comparing implantation of two or three stents (iStent inject + iStent; Multi-Stent group) to trabeculectomy with mitomycin C (Trab group) found there to be successful IOP (−31% vs −43%) and medication reductions (−51% vs −84%) in both groups, with a favorable safety profile seen in the Multi-Stent group, for up to 24 months.28 Subgroup analysis indicated that a higher proportion of 3-stent eyes reached IOP target compared to 2-stent eyes, suggesting an additive effect in the placement of three stents.28 Similarly, a study evaluating clinical outcomes following implantation of three first-generation iStents found there to be an incrementally greater and better sustained IOP and medication reductions when compared to the implantation of one or two stents.29 The iStent infinite contains three preloaded iStent inject W devices designed to be placed two clock hours apart, over the four nasal clock hours of the TM to promote bypass of aqueous humor to Schlemm’s canal and reduce IOP.30 The only present trial of the iStent infinite evaluates 12-month outcomes of standalone implantation in 72 eyes with uncontrolled glaucoma despite prior surgical interventions or maximum tolerated medical therapy.30 Clinically significant results were achieved, with 76.1% of all enrolled eyes achieving ≥20% MDIOP reduction from baseline on the same or fewer antiglaucoma medications.30 Of these eyes, 53% achieved ≥30% MDIOP reduction without the need for surgical reinterventions, with no cases of infection, hypotony, or explantation.30 Additionally, a low target IOP of <15 mmHg was met in 36.4% of eyes.30 The most common complications observed were ocular surface disease (9.7%), visual field loss of >2.5 dB (6.9%), and loss of >2 lines of best corrected visual acuity (8.3%), all of which were considered inherent to disease progression and not related to device implantation.30 While more studies evaluating the iStent infinite are needed, future outcomes are promising and the iStent infinite appears to be a reasonable surgical option in the treatment of severe and refractory glaucoma.
Hydrus MicroStent
The Hydrus Microstent is a flexible, 8-mm-long device that received US FDA approval in 2018. The device is implanted ab internally through a clear corneal incision, with the device placed in Schlemm’s canal and its inlet remaining in the anterior chamber (Figure 1C).31 The Hydrus device dilates Schlemm’s canal up to five times its cross-sectional area to scaffold its lumen and provide access for aqueous outflow to distal collector channels.31–33 The device is composed of nitinol, which has several applications in medical devices and has previously demonstrated intraocular biocompatibility.31,34,35 Hydrus received FDA clearance following the results of the HORIZON clinical trial by Samuelson et al, which evaluated 556 POAG eyes randomized 2:1 following phacoemulsification to receive implantation of the Hydrus Microstent (HMS group; n=369) or to receive no microstent (NMS group; n=187).36 At 24 months, eyes in the HMS group experienced a greater reduction in mean washed-out MDIOP (−31.8% vs −24.4%), with 77.3% of HMS eyes meeting ≥20% washed-out MDIOP reduction, compared to 57.8% in the NMS group.36 The HMS group also experienced a significantly higher reduction in medication burden, with 78% of HMS eyes being medication-free at 24 months, compared to 48% of eyes in the NMS group.36 No significant differences in CECL were found between groups, and no device-related vision-threatening complications were observed in the HMS group.36 The most common complications observed were hyphema and focal peripheral anterior synechiae (PAS), which did not produce a significant difference in IOP.36 Three- and five-year results of the HORIZON trial have also been published and show similar results, with a lower cumulative probability of incisional surgery found in the HMS group (3 years: 0.6% vs 3.9%; P = 0.020; 5 years: 2.4% vs 6.2%; P = 0.027).37,38 Three-year outcomes demonstrated a relatively higher percentage of CECL in HMS patients compared to NMS patients (14.2 vs 10%; P=0.239); however, statistical significance was not reached.20,37 Of note, five-year outcomes showed a significantly lower rate of visual field progression in the HMS group compared to the NMS group (−0.26 dB/year vs −0.49 dB/year; P = 0.0138), demonstrating the efficacy of phacoemulsification-HMS implantation in preserving peripheral vision and slowing glaucoma progression compared to phacoemulsification-alone.39 Studies outside of a randomized clinical trial setting have also shown excellent results. Three-year outcomes of concomitant phacoemulsification and Hydrus microstent implantation in 106 eyes with open-angle glaucoma were reported by Salimi et al and demonstrated significant reductions in both IOP (−26.5%) and antiglaucoma medications (−33%), with a preserved visual acuity and stable structural and functional disease parameters (cup-to-disc ratio, retinal nerve fiber layer thickness, ganglion cell-inner plexiform layer thickness, and visual field mean defects).40 Similarly, Fea et al evaluated clinical outcomes of combined phacoemulsification and Hydrus implantation in 92 eyes with POAG and observed safe and effective IOP (−19.1%) and medication reductions (−66.7%), with 64% of patients being medication-free at 24 months.41 The main complications observed were intraoperative microstent repositioning (n=2) and device obstruction (n=1).41 While the Hydrus is indicated for use in mild-to-moderate glaucoma, results following implantation in 11 pseudophakic eyes with refractory glaucoma have also demonstrated excellent IOP (−34.1%) and medication reductions (−75.6%) at 24 months, suggesting the device may also be an effective treatment for advanced glaucoma.42
Hydrus vs iStent
With both microstents demonstrating long-term safety and efficacy, the challenge for surgeons lies in deciding which device to use. Studies within the literature have compared Hydrus and iStent implantation as standalone procedures43 or when combined with phacoemulsification.44 The COMPARE study, performed by Ahmed et al, is a prospective, multicenter clinical trial, which randomized 152 eyes with open-angle glaucoma to receive standalone implantation of Hydrus or two first-generation iStents.43 At 12 months, there was no significant difference in IOP reduction between both groups (Hydrus: −8.9%, iStent: −5.2%; P = 0.3), although a greater percentage of Hydrus eyes achieved an IOP <21 mmHg.43 Hydrus eyes experienced a greater reduction in medication burden (Hydrus: −1.6 ± 1.2 medications, iStent: −1.0 ± 1.2 medications; P = 0.004), with 22.6% more patients in the Hydrus group being medication-free.43 Both groups had a similar safety profile, but complete surgical success (IOP ≤18 on no antiglaucoma medications and with no secondary glaucoma surgical reinterventions) was higher in Hydrus eyes (Hydrus: 35.6%, iStent: 10.5%; P = 0.001) at 12 months.43 These results indicate that while both devices are safe and effective, standalone implantation of Hydrus appears to result in greater surgical success and fewer medications through 12 months. Holmes et al retrospectively analyzed 344 eyes with mild-to-moderate open-angle glaucoma receiving phacoemulsification combined with the Hydrus Microstent (n=120) or iStent inject (n=224).44 Following propensity matching, no significant difference was found in mean IOP (Hydrus: −12.8%, iStent inject: −13.3%; P = 0.372) and medication reduction (Hydrus: −38.1%, iStent inject: −46.7%; P = 0.615) between both groups, at 24 months.44 A similar percentage of eyes within both groups achieved >20% IOP reduction with a final IOP of <18 mmHg (Hydrus: 38.3%, iStent inject: 37.9%; P = 1.000) or <15 mmHg (Hydrus: 31.7%, iStent inject: 32.6; P = 0.957).44 A good safety profile was observed in both groups, with early hypotony (3.3% vs 0.4%) and hyphema with loss of ≥2 lines of visual acuity (2.5% vs 0%) occurring more commonly in Hydrus eyes, though study values were too small to compare.44 These results seem to suggest that both devices have comparable safety and efficacy with a small additional medication reduction effect in eyes receiving the iStent inject. These results differ from the COMPARE study, which suggested a higher surgical success following Hydrus implantation. However, in the real-world setting, stent implantation in combination with cataract surgery, and use of the iStent inject over the first-generation iStent within Holmes et al’s study must be considered.44 Results of a meta-analysis of six prospective randomised clinical trials (1397 patients) concluded findings similar to the COMPARE study, with rank probability analysis revealing phacoemulsification combined with Hydrus implantation may provide better IOP control compared to implantation of one or two iStents, albeit with a higher incidence of focal PAS.45 This analysis also determined that the implantation of Hydrus and two iStents enacted a greater probability of reaching medication-free status when compared to one iStent and standalone phacoemulsification.45 When compared to the implantation of two iStents in ex-vivo studies, the Hydrus microstent has been suggested to provide additional benefits in reducing aqueous outflow resistance.46 In a study of 12 pairs of cadaveric eyes, Hays et al observed a greater increase in outflow facility (73% vs 34%) across a greater range of perfusion pressures (20–50 mmHg vs 40 mmHg only) with the Hydrus microstent compared to two iStents, likely due to greater collector channel access.46 However, more studies, both in-vivo and ex-vivo, are needed to more conclusively demonstrate any difference in outflow facility increase and IOP-lowering between both devices.
Conclusions
Trabecular bypass microstent devices combined with cataract surgery represent an important advancement in the realm of MIGS. Advantages of Microstent devices lie in their conjunctival preservation, ease of implantation, quick operation time, and high safety profile. Both the Hydrus Microstent and iStent series have produced comparable safety profiles and IOP-lowering efficacies, with the implantation of multiple iStent microstent devices demonstrating an additive IOP-lowering effect. Microstent implantation is an effective option in the treatment of mild-to-moderate glaucoma. Further randomized controlled comparative trials are needed to better assess and compare clinical outcomes of these devices along with their effectiveness in the treatment of advanced glaucoma.
Literature Search
A literature search of PUBMED for all literature from 1 January 1960 to 1 April 2023 was performed. The search was carried out with a combination of the following key terms: “MicroStent Injection,” “Schlemm’s canal inject,” “Hydrus MicroStent,” “iStent,” and “minimally invasive glaucoma surgery.” All accessible article types (clinical studies, randomized controlled trials, review articles, case series, and case reports) were included. A total of 546 articles were found. All articles and their references were scrutinized, and 46 articles were deemed relevant for the purpose of this review.
Consent for Publication
No personal identifying information is contained within this review article. All images have been deidentified.
Acknowledgments
The authors acknowledge Bryan Ang, FRCOphth for his intraoperative photos of the iStent inject (Figure 1A), iStent inject W (Figure 1B), and Hydrus Microstent (Figure 1C).
Author Contributions
All authors made a significant contribution to the work reported (conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas); took part in drafting, revising, or critically reviewing this article; gave final approval of the version to be published and agreed on the journal the article was submitted to; and agree to be accountable for all aspects of the work.
Funding
No funding or grant support was obtained for this study.
Disclosure
Dr Bryan Ang is a consultant for and reports research and speaker’s honorariums from Glaukos Corporation and Alcon, Inc., outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Stein JD, Khawaja AP, Weizer JS. Glaucoma in adults-screening, diagnosis, and management: a review. JAMA. 2021;325(2):164–174. PMID: 33433580. doi:10.1001/jama.2020.21899
2. Wagner IV, Stewart MW, Dorairaj SK. Updates on the diagnosis and management of glaucoma. Mayo Clin Proc Innov Qual Outcomes. 2022;6(6):618–635. PMID: 36405987; PMCID: PMC9673042. doi:10.1016/j.mayocpiqo.2022.09.007
3. Zhao Z, Zhu X, He W, Jiang C, Lu Y. Schlemm’s canal expansion after uncomplicated phacoemulsification surgery: an optical coherence tomography study. Invest Ophthalmol Vis Sci. 2016;57(15):6507–6512. PMID:27918824. doi:10.1167/iovs.16-20583
4. Allison K, Patel D, Alabi O. Epidemiology of Glaucoma: the Past, Present, and Predictions for the Future. Cureus. 2020;12(11):e11686. PMID: 33391921; PMCID: PMC7769798. doi:10.7759/cureus.11686
5. Mathew DJ, Buys YM. Minimally invasive glaucoma surgery: a critical appraisal of the literature. Annu Rev Vis Sci. 2020;6:47–89. PMID: 32936738. doi:10.1146/annurev-vision-121219-081737
6. Pereira ICF, van de Wijdeven R, Wyss HM, Beckers HJM, den Toonder JMJ. Conventional glaucoma implants and the new MIGS devices: a comprehensive review of current options and future directions. Eye. 2021;35(12):3202–3221. PMID: 34127842; PMCID: PMC8602385. doi:10.1038/s41433-021-01595-x
7. Grant WM. Experimental aqueous perfusion in enucleated human eyes. Arch Ophthalmol. 1963;69:783–801. PMID: 13949877. doi:10.1001/archopht.1963.00960040789022
8. Rosenquist R, Epstein D, Melamed S, Johnson M, Grant WM. Outflow resistance of enucleated human eyes at two different perfusion pressures and different extents of trabeculotomy. Curr Eye Res. 1989;8(12):1233–1240. PMID: 2627793. doi:10.3109/02713688909013902
9. Mäepea O, Bill A. Pressures in the juxtacanalicular tissue and Schlemm’s canal in monkeys. Exp Eye Res. 1992;54(6):879–883. PMID: 1521580. doi:10.1016/0014-4835(92)90151-h
10. Ziaei H, Au L. Manchester iStent study: long-term 7-year outcomes. Eye. 2021;35(8):2277–2282. PMID: 33139875; PMCID: PMC8302602. doi:10.1038/s41433-020-01255-6
11. Healey PR, Clement CI, Kerr NM, Tilden D, Aghajanian L. Standalone iStent trabecular micro-bypass glaucoma surgery: a systematic review and meta-analysis. J Glaucoma. 2021;30(7):606–620. PMID: 33596009. doi:10.1097/IJG.0000000000001805
12. Saheb H, Donnenfeld ED, Solomon KD, et al. Five-year outcomes prospective study of two first-generation trabecular micro-bypass stents (iStent®) in open-angle glaucoma. Curr Eye Res. 2021;46(2):224–231. PMID: 32715828. doi:10.1080/02713683.2020.1795881
13. Salimi A, Watt H, Harasymowycz P. Long-term outcomes of two first-generation trabecular micro-bypass stents (iStent) with phacoemulsification in primary open-angle glaucoma: eight-year results. Eye Vis. 2021;8(1):43. PMID: 34782017; PMCID: PMC8594216. doi:10.1186/s40662-021-00263-1
14. Shalaby WS, Jia J, Katz LJ, Lee D. iStent inject: comprehensive review. J Cataract Refract Surg. 2021;47(3):385–399. PMID: 32842078. doi:10.1097/j.jcrs.0000000000000325
15. Samuelson TW, Sarkisian SR, Lubeck DM, et al. Prospective, randomized, controlled pivotal trial of an Ab interno implanted trabecular micro-bypass in primary open-angle glaucoma and cataract: two-year results. Ophthalmology. 2019;126(6):811–821. PMID: 30880108. doi:10.1016/j.ophtha.2019.03.006
16. Salimi A, Watt H, Harasymowycz P. Three-year outcomes of second-generation trabecular micro-bypass stents (iStent inject) with phacoemulsification in various glaucoma subtypes and severities. J Glaucoma. 2021;30(3):266–275. PMID: 33105306. doi:10.1097/IJG.0000000000001716
17. Salimi A, Clement C, Shiu M, Harasymowycz P. Second-generation trabecular micro-bypass (iStent inject) with cataract surgery in eyes with normal-tension glaucoma: one-year outcomes of a multi-centre study. Ophthalmol Ther. 2020;9(3):585–596. PMID: 32613589; PMCID: PMC7406634. doi:10.1007/s40123-020-00266-6
18. Ang BCH, Tecson ICRO, Hu JY, Kan JTC, Yip LWL. 12-month outcomes of combined phacoemulsification and iStent Inject in Asian eyes with normal tension glaucoma: a single-centre experience. Int Ophthalmol. 2022;42(2):611–620. PMID: 34652545. doi:10.1007/s10792-021-02033-3
19. Ang BCH, Chiew W, Yip VCH, et al. Prospective 12-month outcomes of combined iStent inject implantation and phacoemulsification in Asian eyes with normal tension glaucoma. Eye Vis. 2022;9(1):27. PMID: 35794666; PMCID: PMC9258099. doi:10.1186/s40662-022-00294-2
20. Obuchowska I, Konopińska J. Corneal endothelial cell loss in patients after minimally invasive glaucoma surgery: current perspectives. Clin Ophthalmol. 2022;16:1589–1600. PMID: 35642179; PMCID: PMC9148582. doi:10.2147/OPTH.S359305
21. Gillmann K, Mansouri K, Ambresin A, Bravetti GE, Mermoud A. A prospective analysis of istent inject microstent implantation: surgical outcomes, endothelial cell density, and device position at 12 months. J Glaucoma. 2020;29(8):639–647. PMID: 32433094. doi:10.1097/IJG.0000000000001546
22. Gillmann K, Bravetti GE, Mermoud A, Mansouri K. A prospective analysis of istent inject microstent positioning: schlemm canal dilatation and intraocular pressure correlations. J Glaucoma. 2019;28(7):613–621. PMID: 31058666. doi:10.1097/IJG.0000000000001273
23. Hangerer FH, John T. Istent inject W offers better results than earlier-generation devices. Healio Ophthalmology. 2021;79(1):464–490.
24. Shalaby WS, Lam SS, Arbabi A, et al. iStent versus iStent inject implantation combined with phacoemulsification in open angle glaucoma. Indian J Ophthalmol. 2021;69(9):2488–2495. PMID: 34427250; PMCID: PMC8544096. doi:10.4103/ijo.IJO_308_21
25. Guedes RAP, Gravina DM, Lake JC, Guedes VMP, Chaoubah A. One-year comparative evaluation of istent or istent inject implantation combined with cataract surgery in a single center. Adv Ther. 2019;36(10):2797–2810. PMID: 31440981; PMCID: PMC6822971. doi:10.1007/s12325-019-01067-5
26. Hooshmand J, Rothschild P, Allen P, Kerr NM, Vote BJ, Toh T. Minimally invasive glaucoma surgery: comparison of iStent with iStent inject in primary open angle glaucoma. Clin Exp Ophthalmol. 2019;47(7):898–903. PMID: 31034687. doi:10.1111/ceo.13526
27. Manning D. Real-world case series of istent or istent inject trabecular micro-bypass stents combined with cataract surgery. Ophthalmol Ther. 2019;8(4):549–561. PMID: 31422555; PMCID: PMC6858412. doi:10.1007/s40123-019-00208-x
28. Paletta Guedes RA, Gravina DM, Paletta Guedes VM, Chaoubah A. Standalone Implantation of 2–3 trabecular micro-bypass stents (iStent inject ± iStent) as an alternative to trabeculectomy for moderate-to-severe glaucoma. Ophthalmol Ther. 2022;11(1):271–292. PMID: 34825352; PMCID: PMC8770764. doi:10.1007/s40123-021-00424-4
29. Katz LJ, Erb C, Carceller Guillamet A, et al. Long-term titrated IOP control with one, two, or three trabecular micro-bypass stents in open-angle glaucoma subjects on topical hypotensive medication: 42-month outcomes. Clin Ophthalmol. 2018;12:255–262. PMID: 29440867; PMCID: PMC5798569. doi:10.2147/OPTH.S152268
30. Sarkisian SR, Grover DS, Gallardo MJ, et al.; iStent infinite Study Group. Effectiveness and safety of istent infinite trabecular micro-bypass for uncontrolled glaucoma. J Glaucoma. 2023;32(1):9–18. PMID: 36260288; PMCID: PMC9722368. doi:10.1097/IJG.0000000000002141
31. Samet S, Ong JA, Ahmed IIK. Hydrus microstent implantation for surgical management of glaucoma: a review of design, efficacy and safety. Eye Vis. 2019;6:32. PMID: 31660323; PMCID: PMC6805473. doi:10.1186/s40662-019-0157-y
32. Gulati V, Fan S, Hays CL, Samuelson TW, Ahmed II, Toris CB. A novel 8-mm Schlemm’s canal scaffold reduces outflow resistance in a human anterior segment perfusion model. Invest Ophthalmol Vis Sci. 2013;54(3):1698–1704. doi:10.1167/iovs.12-11373
33. Battista SA, Lu Z, Hofmann S, Freddo T, Overby DR, Gong H. Reduction of the available area for aqueous humor outflow and increase in meshwork herniations into collector channels following acute IOP elevation in bovine eyes. Invest Ophthalmol Vis Sci. 2008;49(12):5346–5352. doi:10.1167/iovs.08-1707
34. Castleman LS, Motzkin SM, Alicandri FP, Bonawit VL, Johnson AA. Biocompatibility of nitinol alloy as an implant material. J Biomed Mater Res. 1976;10(5):695–731. doi:10.1002/jbm.820100505
35. Olson JL, Velez-Montoya R, Erlanger M. Ocular biocompatibility of nitinol intraocular clips. Invest Ophthalmol Vis Sci. 2012;53(1):354–360. doi:10.1167/iovs.11-8496
36. Samuelson TW, Chang DF, Marquis R, et al. HORIZON investigators. A schlemm canal microstent for intraocular pressure reduction in primary open-angle glaucoma and cataract: the HORIZON study. Ophthalmology. 2019;126(1):29–37. PMID: 29945799. doi:10.1016/j.ophtha.2018.05.012
37. Ahmed IIK, Rhee DJ, Jones J, et al. HORIZON Investigators. Three-year findings of the HORIZON trial: a schlemm canal microstent for pressure reduction in primary open-angle glaucoma and cataract. Ophthalmology. 2021;128(6):857–865. PMID: 33166551. doi:10.1016/j.ophtha.2020.11.004
38. Ahmed IIK, De Francesco T, Rhee D, et al. HORIZON investigators. long-term outcomes from the HORIZON randomized trial for a schlemm’s canal microstent in combination cataract and glaucoma surgery. Ophthalmology. 2022;129(7):742–751. PMID: 35218867. doi:10.1016/j.ophtha.2022.02.021
39. Montesano G, Ometto G, Ahmed IIK, et al. Five-Year Visual Field Outcomes of the HORIZON Trial. Am J Ophthalmol. 2023;251:143–155. PMID: 36813144. doi:10.1016/j.ajo.2023.02.008
40. Salimi A, Kassem R, Santhakumaran S, Harasymowycz P. Three-year outcomes of a schlemm canal microstent (Hydrus microstent) with concomitant phacoemulsification in open-angle glaucoma. Ophthalmol Glaucoma. 2022;S2589–4196(22):168. PMID: 36038108. doi:10.1016/j.ogla.2022.08.012
41. Fea AM, Rekas M, Au L. Evaluation of a Schlemm canal scaffold microstent combined with phacoemulsification in routine clinical practice: two-year multicenter study. J Cataract Refract Surg. 2017;43(7):886–891. PMID: 28823433. doi:10.1016/j.jcrs.2017.04.039
42. Coventon J, Cronin B. The Hydrus microstent in pseudophakic patients with medically refractory open-angle glaucoma. J Glaucoma. 2021;30(2):192–194. PMID: 33955947. doi:10.1097/IJG.0000000000001694
43. Ahmed IIK, Fea A, Au L, et al. COMPARE Investigators. A prospective randomized trial comparing Hydrus and istent microinvasive glaucoma surgery implants for standalone treatment of open-angle glaucoma: the COMPARE study. Ophthalmology. 2020;127(1):52–61. PMID: 31034856. doi:10.1016/j.ophtha.2019.04.034
44. Holmes DP, Clement CI, Nguyen V, et al. Comparative study of 2-year outcomes for Hydrus or iStent inject microinvasive glaucoma surgery implants with cataract surgery. Clin Exp Ophthalmol. 2022;50(3):303–311. PMID: 35077009. doi:10.1111/ceo.14048
45. Hu R, Guo D, Hong N, Xuan X, Wang X. Comparison of Hydrus and iStent microinvasive glaucoma surgery implants in combination with phacoemulsification for treatment of open-angle glaucoma: systematic review and network meta-analysis. BMJ Open. 2022;12(6):e051496. PMID: 35705355; PMCID: PMC9204447. doi:10.1136/bmjopen-2021-051496
46. Hays CL, Gulati V, Fan S, Samuelson TW, Ahmed II, Toris CB. Improvement in outflow facility by two novel microinvasive glaucoma surgery implants. Invest Ophthalmol Vis Sci. 2014;55(3):1893–1900. PMID: 24550367; PMCID: PMC3973188. doi:10.1167/iovs.13-13353
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
