Back to Journals » Journal of Pain Research » Volume 12
Spinal matrix metalloproteinase 8 regulates pain after peripheral trauma
Authors Tajerian M, Clark JD
Received 11 December 2018
Accepted for publication 27 February 2019
Published 1 April 2019 Volume 2019:12 Pages 1133—1138
DOI https://doi.org/10.2147/JPR.S197761
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Katherine Hanlon
Maral Tajerian,1 J David Clark2–4
1Department of Biology, Queens College, City University of New York, Queens, NY 11367, USA; 2Veterans Affairs Palo Alto Health Care System, Palo Alto, CA 94304, USA; 3Department of Anesthesiology, Stanford University School of Medicine, Stanford, CA 94305, USA; 4Palo Alto Veterans Institute for Research, Palo Alto, CA 94304, USA
Abstract: It is well documented that pain chronification requires a host of plastic mechanisms at the spinal cord (SC) level, including alterations in neuronal and glial structure and function. Such cellular plasticity necessitates the existence of a plastic extracellular matrix (ECM). Here, we describe a key role for ECM remodeling in the regulation of chronic pain following peripheral injury. Three weeks following tibia fracture in mice, we show increased levels of MMP8 in the SC. Furthermore, we show that the pharmacological or genetic downregulation of MMP8 ameliorates the pain phenotype observed after injury. These results delineate an extracellular mechanism for pain chronification, thereby improving our mechanistic understanding of pain and providing novel therapeutic venues that go beyond targeting individual cell types.
Keywords: spinal cord, chronic pain, matrix metalloproteinase 8, mouse model, mechanical allodynia, shRNA
Introduction
The sensation of pain carries out a vital protective role against tissue damage. In many cases, however, the pain outlasts the original injury and itself becomes a pathology that is typically resistant to classical forms of treatment. This concept of pain chronification refers to sensory events that parallel peripheral trauma and gradually alter the central nervous system (CNS), thereby augmenting injury-associated pain or resulting in the experience of pain in the absence of any injury.1 These CNS alterations encompass aberrant activity in the spinal cord (SC) dorsal horn neurons as well as hyperactive glial cells, which contribute to the pathologic somatosensory signals that are conveyed to the brain.2
While most studies have focused on cellular plasticity and chronic pain, we have recently described a key role for the hippocampal extracellular matrix (ECM) in regulating pain and memory function following peripheral injury in mice.3 In particular, our biophysical analyses showed decreased ECM rigidity and dysregulated microarchitecture that were paralleled by biochemical changes in various ECM components and enzymes, including increased levels of matrix metalloproteinase 8 (MMP8), also known as collagenase-2 or neutrophil collagenase. Furthermore, we showed that normalizing MMP8 upregulation is sufficient in reversing the behavioral and cellular alterations observed after injury.
The current manuscript aims at translating these findings to spinal tissues, a direction that stems from well-characterized SC plasticity in pain. In our rodent model of chronic pain due to distal tibia fracture, we have observed neuronal and glial parallels of central sensitization. In particular, we have reported biochemical and molecular correlates of allodynia, hyperalgesia, and latent sensitization in terms of altered glutamatergic tone in the SC4,5 as well as neuroinflammation and glial activation.6 Furthermore, we have described broad spinal transcriptomic changes as the animals progress from an acute to a more chronic stage of pain.7 These observations have prompted us to look at the ECM where these cells function, with the supposition that SC cellular plasticity requires a plastic ECM niche.8
Our central hypothesis is that chronic pain is associated with disturbed spinal ECM homeostasis. In the current study, we took aim at examining the role of specific SC matrix remodeling enzymes following peripheral injury. Using biochemical, behavioral, and molecular tools, we demonstrate a key role for spinal MMP8 in regulating pain after peripheral trauma.
Methods
All testing and analysis was blinded to the identity and experimental condition of the animal/tissue.
Animals
Male C57BL/6J mice aged 12–14 weeks were purchased from a commercial supplier (Jackson Labs, Sacramento, CA, USA) and were allowed to habituate to the animal facility for a minimum of 10 days prior to the experiments. Mice were housed in groups of 4 on a 12-hr light/dark cycle and an ambient temperature of 22±3 °C, with food and water available ad libitum. All animal procedures and experimental designs were approved by the Veterans Affairs Palo Alto Health Care System Institutional Animal Care and Use Committee (Palo Alto, CA, USA) and followed the “animal subjects” guidelines of the International Association for the Study of Pain.
Tibia fracture and cast immobilization
Following the random allocation to the control or the fracture/cast group, mice were anesthetized with 1.5% isoflurane and underwent a distal tibial fracture in the right leg followed by cast immobilization as previously described.4 Casts were removed 3 weeks after surgery under brief isoflurane anesthesia.
Behavioral testing of tactile allodynia
Mice were habituated to handling by the experimenter for a few minutes each day for 7 days before initiation of the behavioral tests. Calibrated monofilaments (Stoelting Co., Wood Dale, IL, USA) were applied to the plantar surface of the hind paw and the 50% threshold to withdraw (grams) was calculated as previously described.9
Biochemical analysis
Mice were euthanized under isoflurane anesthesia, and ipsilateral lumbar SCs were quickly isolated. Tissues were homogenized using T-PER Protein Extraction Reagent (cat. # 87793; Thermo Scientific, Waltham, MA, USA) in the presence of proteinase and phosphatase inhibitors (cat. # 04906837001; Roche Applied Science, Palo Alto, CA, USA) and centrifuged at 12,000 g for 4 mins at 4°C. Supernatant fractions were then frozen at 80°C until use. An aliquot was subjected to protein assay (cat. # 500-0001; Bio-Rad, Hercules, CA, USA) to normalize protein levels. The MMP8 ELISA kit was used as per manufacturer’s instructions (cat. # ab206982; abcam, USA).
MMP8 downregulation
Pharmacological inhibition of MMP8
Mice were randomized to receive either intraperitoneal injections of vehicle (saline +1%DMSO) or the specific MMP8 inhibitor, M8I (3R)-(+)-[2-(4-methoxybenzenesulfonyl)-1,2,3,4-tetrahydroisoquinoline-3-hydroxamate, dose=1mg/kg] (cat. # CAS 236403-25-1; Calbiochem, San Diego, CA, USA). Daily injections of vehicle or MMP8 inhibitor spanned 14 days, starting at 5 weeks post-injury. CNS penetration of the compound was verified by western blot analysis, demonstrating a decreased level of MMP8 in CNS tissues (data not shown).
Lentiviral delivery of shRNA
We manipulated MMP generation capacity in the SC using short hairpin RNA (shRNA) administration through lentiviral delivery. Lentiviral particles for mouse MMP8-specific shRNA (MMP8 Mission® shRNA; 1 × 106 TU/mL; pLKO.1 vector) or nontarget shRNA control (Mission® pLKO.1 puro non-target shRNA control) were purchased commercially (Sigma Aldrich, St Louis, MO, USA). The sequence for MMP8-specific shRNA is: CCGGGCCTTGATGTACCCAAACTATCTCGAGATAGTTTGGGTACATCAAGGCTTTTTG. Three weeks after injury, mice were allocated to random groups to receive a single intrathecal injection (isoflurane anesthesia, injection volume =5 µl) of lentivirus delivering MMP8-specific shRNA or nontarget shRNA control. This method of CNS MMP8 downregulation has been previously validated by our group.3
Lentiviral delivery was confirmed via visual inspection following the spinal administration of a pLKO.1-CMVtGFP vector with a nontarget SHC016 shRNA sequence (Sigma Aldrich) which was used as described above.
Statistical analysis
The data for biochemical measurements were analyzed by unpaired 2-tailed t-tests. F tests were carried out to measure differences of variance between groups. Behavioral data were analyzed by two-way ANOVA followed by Sidak post-hoc test for multiple comparisons. All data are presented as mean ± SEM and for all analyses, p<0.05 were taken to be significant.
Results
Biochemical dysregulation of the ECM is observed after injury
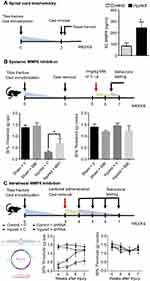
To assess the overall integrity of the SC ECM after peripheral injury, protein measurements of MMP8 were carried out. Compared to control mice, injured mice displayed decreased levels of ipsilateral SC MMP8 (F=3.7, p=0.02) 3 weeks after injury (Figure 1A).
Pharmacological inhibition of MMP ameliorates mechanical allodynia after injury
In order to link biochemical ECM stability to the phenotype of ongoing pain after injury, we systemically treated mice with a selective MMP8 inhibitor for 2 weeks. Behavioral measures of tactile allodynia at the 7-week timepoint demonstrate an amelioration of mechanical sensitivity in the group that was treated with the MMP8 inhibitor. Compared to vehicle-treated injured mice, M8I-treated injured mice showed an increase in mechanical threshold values on the ipsilateral hindpaw (mean difference=−0.38±0.18, Figure 1B).
shRNA downregulation of spinal MMP8 results in a progressive increase in mechanical thresholds after injury
In order to ascertain the role of MMP8 in spinal tissues, we complemented our systemic M8I administration studies with intrathecal injections of MMP8-downregulating shRNA using lentiviral vectors. Our data show that a single injection at the 3w timepoint was sufficient to elicit improvements in mechanical hypersensitivity. Compared to injured mice treated with control-shRNA, injured mice treated with MMP8-downregulating shRNA showed increased mechanical thresholds on the ipsilateral hindpaw at the 6- (mean difference=0.59±0.2) and 7- (mean difference=0.61±0.2) week timepoints (Figure 1C).
Discussion
In this report, we provide evidence for a novel spinal mechanism that may support the transition of acute to chronic pain. Our data show that spinal ECM dysregulation in the form of increased MMP8 is observed following peripheral trauma and that its normalization can reverse pain hypersensitivity in a mouse model of tibia fracture, thereby proposing a novel mechanism of pain-related SC plasticity.
Recent studies have outlined a role for MMP8 following frank injury to the SC. Acute MMP8 upregulation was recently demonstrated in a model of SC compression, and its inhibition was linked to neuroprotective effects in rats;10 similarly, clinical studies have shown the upregulation of serum MMP8 levels in patients with SC injury.11,12 However, to our knowledge, this is the first report of spinal MMP8 involvement in chronic pain due to peripheral trauma.
The CNS ECM is a dynamic entity that continues to change in adulthood. A wealth of studies support the hypothesis that CNS damage is accompanied by profound ECM alterations: traumatic spinal injury and multiple sclerosis are both paralleled by an upregulation in chondroitin sulfate proteoglycans, key components of the CNS ECM13 that are generally linked to restricted neuronal plasticity.14 Additionally, heparin sulfate proteoglycan involvement has been demonstrated in neurodegenerative disorders such as Alzheimer’s disease and Parkinson’s disease.15 While chronic pain is often the result of an injury to the periphery rather than CNS insult, significant SC and brain neuroplasticity is observed in various chronic pain conditions,16 suggesting the involvement of a plastic ECM. This direction is quickly gaining traction: for example, a recent manuscript describes the spinal upregulation of the astrocyte-secreted ECM protein thrombospondin-4 and its role in the development of aberrant excitatory synaptogenesis and peripheral neuropathy in rats.17 Finally, in our recent manuscript,3 we show structural and biochemical alterations in the hippocampal ECM that are linked to pain, cognitive dysfunction, and cellular plasticity in a mouse model of chronic pain.
How does a dysregulated ECM contribute to pain chronification? We propose the following possible mechanisms: first, the degradation of ECM structural components could result in a less rigid matrix that is no longer able to support the cytoarchitectural changes in neuronal dendrites,3 thus preventing adaptive neuronal plasticity. Second, a dysregulated ECM might be deficient in the storage of pronociceptive cytokines, thus making them more available to neurons and glia. In particular, proteoglycans have been shown to interact with cytokines and modify their binding to cell surface receptors as well as their storage within the ECM.18 Third, it is possible that the perineuronal net, a specialized ECM that predominantly surrounds inhibitory interneurons, is affected, thereby resulting in destabilized inhibitory interneurons. Diminished inhibition in the SC dorsal horn is a well-studied mechanism of chronic pain.19 Fourth, excessive proteolytic activity by MMPs could result in damage to tight junctions and the subsequent compromise of the blood–SC barrier.10 Increased blood-SC permeability has been reported in models of peripheral neuropathy.20 Future studies targeting each of these hypotheses will be undertaken.
The current report is the first statement of SC ECM involvement in pain regulation. While we show that MMP8 downregulation is sufficient in ameliorating the pain phenotype, further studies localizing these ECM changes, both to specific SC regions and to specific cell types, are needed.
Understanding ECM mechanisms of CNS plasticity can significantly improve our understanding of chronic pain. Additionally, it can provide valuable tools in research methodology: for instance, a recent study described an approach to magnetomechanical neuromodulation of dorsal root ganglion neurons via a 3D magnetic hyaluronic acid hydrogel.21 Finally, this direction can offer novel therapeutic venues where relevant ECM components, rather than cell types, can be targeted. Such interventions, administered after injury, have the potential to prevent the chronification of pain, thereby greatly enhancing recovery.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10(9):895–926. doi:10.1016/j.jpain.2009.06.012
2. Tsuda M, Koga K, Chen T, Zhuo M. Neuronal and microglial mechanisms for neuropathic pain in the spinal dorsal horn and anterior cingulate cortex. J Neurochem. 2017;141(4):486–498. doi:10.1111/jnc.14001
3. Tajerian M, Hung V, Nguyen H, et al. The hippocampal extracellular matrix regulates pain and memory after injury. Mol Psychiatry. 2018;23(12):2302–2313. doi:10.1038/s41380-018-0209-z
4. Tajerian M, Sahbaie P, Sun Y, et al. Sex differences in a murine model of complex regional pain syndrome. Neurobiol Learn Mem. 2015;123:100–109. doi:10.1016/j.nlm.2015.06.004
5. Tajerian M, Leu D, Yang P, Huang TT, Kingery WS, Clark JD. Differential efficacy of ketamine in the acute versus chronic stages of complex regional pain syndrome in mice. Anesthesiology. 2015;123(6):1435–1447. doi:10.1097/ALN.0000000000000889
6. Li WW, Guo TZ, Shi X, et al. Substance P spinal signaling induces glial activation and nociceptive sensitization after fracture. Neuroscience. 2015;310:73–90. doi:10.1016/j.neuroscience.2015.09.036
7. Gallagher JJ, Tajerian M, Guo T, et al. Acute and chronic phases of complex regional pain syndrome in mice are accompanied by distinct transcriptional changes in the spinal cord. Mol Pain. 2013;9:40. doi:10.1186/1744-8069-9-40
8. Tajerian M, Clark JD. The role of the extracellular matrix in chronic pain following injury. Pain. 2015;156(3):366–370. doi:10.1097/01.j.pain.0000460323.80020.9d
9. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63.
10. Kumar H, Jo MJ, Choi H, et al. Matrix metalloproteinase-8 inhibition prevents disruption of blood-spinal cord barrier and attenuates inflammation in rat model of spinal cord injury. Mol Neurobiol. 2018;55(3):2577–2590. doi:10.1007/s12035-017-0509-3
11. Moghaddam A, Heller R, Daniel V, et al. Exploratory study to suggest the possibility of MMP-8 and MMP-9 serum levels as early markers for remission after traumatic spinal cord injury. Spinal Cord. 2017;55(1):8–15. doi:10.1038/sc.2016.104
12. Light M, Minor KH, DeWitt P, Jasper KH, Davies SJ. Multiplex array proteomics detects increased MMP-8 in CSF after spinal cord injury. J Neuroinflammation. 2012;9:122. doi:10.1186/1742-2094-9-122
13. Stephenson EL, Yong VW. Pro-inflammatory roles of chondroitin sulfate proteoglycans in disorders of the central nervous system. Matrix Biol. 2018;71–72:432–442. doi:10.1016/j.matbio.2018.04.010
14. Bradbury EJ, Moon LD, Popat RJ, et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416(6881):636–640. doi:10.1038/416636a
15. Heindryckx F, Li JP. Role of proteoglycans in neuro-inflammation and central nervous system fibrosis. Matrix Biol. 2018;68–69:589–601. doi:10.1016/j.matbio.2018.01.015
16. Ji RR, Nackley A, Huh Y, Terrando N, Maixner W. Neuroinflammation and central sensitization in chronic and widespread pain. Anesthesiology. 2018;129(2):343–366. doi:10.1097/ALN.0000000000002130
17. Park JF, Yu YP, Gong N, Trinh VN, Luo ZD. The EGF-LIKE domain of thrombospondin-4 is a key determinant in the development of pain states due to increased excitatory synaptogenesis. J Biol Chem. 2018;293:16453–16463. doi:10.1074/jbc.RA118.003591
18. Schonherr E, Hausser HJ. Extracellular matrix and cytokines: a functional unit. Dev Immunol. 2000;7(2–4):89–101.
19. Takazawa T, Choudhury P, Tong CK, et al. Inhibition mediated by glycinergic and GABAergic receptors on excitatory neurons in mouse superficial dorsal horn is location-specific but modified by inflammation. J Neurosci. 2017;37(9):2336–2348. doi:10.1523/JNEUROSCI.2354-16.2017
20. Cahill LS, Laliberte CL, Liu XJ, et al. Quantifying blood-spinal cord barrier permeability after peripheral nerve injury in the living mouse. Mol Pain. 2014;10:60. doi:10.1186/1744-8069-10-60
21. Tay A, Sohrabi A, Poole K, Seidlits S, Di Carlo D. A 3D magnetic hyaluronic acid hydrogel for magnetomechanical neuromodulation of primary dorsal root ganglion neurons. Adv Mater. 2018;30:e1800927. doi:10.1002/adma.201800927
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.