Back to Archived Journals » International Journal of Clinical Transfusion Medicine » Volume 5
Spectra Optia apheresis system: experience with secondary plasma devices
Authors Rummler S, Volkholz S, Steinke T, Maier K, Jütte H, Hilge A , Ziehm P
Received 13 January 2017
Accepted for publication 23 April 2017
Published 24 May 2017 Volume 2017:5 Pages 61—67
DOI https://doi.org/10.2147/IJCTM.S117318
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Cees Th. Smit Sibinga
Silke Rummler,1 Sabine Volkholz,1 Thomas Steinke,2 Katrin Maier,1 Heike Jütte,1 Agnieszka Hilge,1 Patrice Ziehm1
1Institute of Transfusion Medicine, 2Department of Cardiothoracic Surgery, University Hospital Jena, Jena, Germany
Abstract: Performing clinical procedures and optimal management of Spectra Optia in combination with secondary plasma devices (SPDs) have not been described in the literature. To contribute to this topic, we examined 300 data log files from patients treated with the Spectra Optia from November 2015 until October 2016. Of these 300 procedures, 149 secondary plasma treatments were conducted in 13 patients (76 immunoadsorption [IA] and 73 lipoprotein apheresis [LA]). Nine patients had IA due to transplantation and autoimmune diseases. Four patients were treated with LA after heart transplantation. Of the procedures, 62% were performed using peripheral venous access, even in patients with a low inlet blood flow. A central catheter was required only in 15% cases, and an arteriovenous fistula in 23% of the procedures. Anticoagulation management differed in both procedures. The LA procedures required a higher amount of citrate as they were performed using only citrate. The IA procedures required less citrate because a mixture of citrate and heparin was used. We did not observe clotting or bleeding, regardless of heparin use. Due to strict calcium management, no patient showed signs of hypocalcemia. Despite our apheresis management, four procedures had to be canceled preliminarily due to patient complications. The targeted substances could be reduced significantly: lipoprotein (a) (Lpa) level by 68% (76%–45%), low-density lipoprotein-cholesterol by 57% (64%–37%), and IgG from 6.6 (1.09–21.0) to 2.61 (0.33–14.7) g/l. The Cobe could be replaced by the Optia system effectively. The Optia has a high plasma extraction efficiency, which allows SPD procedures with peripheral venous access and moderate inlet flow rates.
Keywords: adverse events, anticoagulation, peripher venous access, lipoprotein apheresis, immunoadsorption
Introduction
For more than 25 years, Cobe Spectra (Cobe; Terumo BCT, Lakewood, CO, USA) has been extensively used for cell separation. However, it was replaced with a new and refined apheresis system, Spectra Optia (Optia), which is based on the established Trima and Cobe technologies.
The Optia is used for the primary separation of blood components through continuous flow centrifugation as well as optical blood component detection technology. All input and process parameters are recorded and can be retrieved as data log files or are printed on a network printer.
The Optia is widely used in routine clinical practice as it enables the operators to perform a wide variety of procedures such as exchange of blood components, collection of blood components, or secondary processing of plasma. However, whether we can effectively and safely perform procedures using secondary plasma device (SPD) software on the Optia, as was done with the Cobe, still needs to be clarified.
Therapeutic plasma exchange (TPE) reduces the levels of pathological factors circulating in the patient’s plasma. It relieves symptoms or prevents further destruction of the involved organ or system.1 As an alternative to TPE, specific plasma purification methods such as lipoprotein apheresis (LA) or immunoadsorption (IA) have been developed for selective removal of the purported pathological substances from the separated plasma.1–3 As Schwartz et al reported, the availability of the selective removal systems and their superior efficacy in cholesterol removal make the use of TPE uncommon.4
In a recent PubMed search, we found only one report on LA, although LA is a commonly performed procedure in countries where Optia is used.5 When TPE fails, IA is the treatment of choice for acute life-threatening autoimmune diseases or during acute humoral transplant rejection episodes.
Our apheresis unit completely replaced the Cobe cell separation devices with Optia in 2015. To the best of our knowledge, there are no reports on IA performed with Optia, and only a few guidelines have been described for IA. Therefore, in this study, we report the routine use of Optia and the occurrence of adverse events (AEs).
Materials and methods
Since 2015, three Optia devices were regularly used for supporting clinical colleagues and their patients in the University Hospital Jena. All the procedures were documented using Optia, both in data log files and in manually recorded protocols. As each Optia can store up to 100 procedures, we examined the last 300 data log files from November 2015 until October 2016. We included all available procedures and did not apply any exclusion criteria.
Patients
All patients provided written informed consent. This study was approved by the ethical committee of the University Hospital Jena, Germany (approval no. 4943-10/16).
Of the 300 recorded procedures, 149 secondary plasma treatments were conducted in 13 patients.
For Optia evaluation, it was important to analyze the following patient and procedural data: gender, age, body weight, venous access, hematocrit, processed inlet volume, plasma volume (PV) treated, duration of the treatment, quantity of anticoagulant used, and AEs.
Optia uses details of gender, height, and weight to determine the total blood volume of the patient.6 The maximum infusion rate of anticoagulant citrate dextrose solution formula A (ACD-A) is limited. If a maximum ACD-A infusion rate of 1.2 ml/min/l/total blood volume is reached, the device switches into a warning mode. Here, it is possible to proactively adapt the infusion rate via the inlet flow.
The estimation of treatment results involves PV, which is calculated using the following formula: PV = [0.065 × body weight in kg] × [1 − hematocrit].7
SPD – online plasma processing and plasma regeneration
An adsorption matrix, Immunosorba® (Fresenius Medical Care Deutschland GmbH, Bad Homburg, Germany), was used for IA, and Monet® (Fresenius Medical Care Deutschland GmbH,) or Evaflux™ (Kawasumi Laboratories, Tokyo, Japan) was used for LA. The IA columns need an additional device for secondary plasma processing. Adasorb® devices (Medicap GmbH, Ulrichstein, Germany) allow adsorption and desorption (regeneration) of the columns. In one session, the device treats up to 10,000 ml of plasma. The setting of the SPD is done according to the PV calculated by Optia. IA can almost completely clear all circulating Ig. For example, on treating 2.5 times the PV on 5 consecutive days, the IgG antibodies are lowered by 95%.8
The plasma flow rate through the columns is set depending on the number of IA performed or the current IgG level. A high amount of IgG allows a maximum plasma flow of 35 ml/min; a low IgG level at the end of IA cycles requires a plasma flow of 10–20 ml/min.9 According to our standard operating procedures, we fixed the target PV for autoantibodies to 2.2 times and alloantibodies to 2.5 times the PV.
The LA filter is based on the pore size and allows the removal of high-molecular weight substances (eg, low-density lipoprotein-cholesterol [LDL-cholesterol], lipoprotein [a] [Lpa], or IgM). The LA of 3 l PV reduces the level of LDL-cholesterol by 55%.10
The target PV for LA was set as 1.1-fold PV following our standard operating procedures, and the plasma rate was adjusted not to exceed a filter pressure limit of 200 mmHg.
To connect the filter to the Optia and rinse the filter, a filter connection and rinsing set (Cell-Max GmbH, Munich, Germany) were utilized.
All procedures were strictly performed following the SPD instructions of Optia.
The anticoagulation in LA was realized with ACD-A alone, with a ratio of 12:1 (11 parts whole blood [WB] to 1 part ACD-A) as recommended by the manufacturer.
In IA, we continuously infused heparin (10,000 U in 50 ml saline, 5 ml/h), in addition to ACD-A with a ratio of 15:1. A bolus of heparin prior to IA was not administered.
Results
Our dataset of 149 plasma treatment procedures (PTPs; 76 IA and 73 LA) was taken from 300 runs, which were stored in our three Optia systems. The other procedures were mononuclear cell collection, TPE, and white blood cell depletion.
Seventy-three LA procedures were performed in four patients with severe drug-refractory transplant vasculopathy (Stanford IIIa–IV) several years after cardiac transplantation (Table 1). All patients had an elevated level of Lpa or LDL-cholesterol and a history of vascular events.
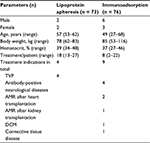  | Table 1 Patient characteristics: total, mean, and parameters in range Abbreviations: TVP, transplant vasculopathy; AMR, acute antibody-mediated rejection; DCM, dilated cardiomyopathy. |
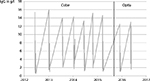
At least 3 years earlier, before LA treatment was initiated, three of the four patients with dyslipoproteinemia had high initial isolated Lpa, ranging from 596 to 1802 mg/l with a mean of 1072 mg/l (N < 300 mg/l). Several treatments later, the Lpa successively dropped down to 668 (262–1236) mg/l. Looking at the trend line, a decrease of Lpa can be seen over the years (Figure 1). With one session of LA, the Lpa level could be reduced significantly by 68% (76%–45%). When treating the patients with LA, no cardiac events, rejection episodes, or stenting requirements were observed.
One of these four patients presented an increased LDL-cholesterol – an average of 6.79 (5.13–8.14) mmol/l (N < 2.59 mmol/l) – and a high LDL/HDL ratio of 2.5 (1.8–3.5). The LDL-cholesterol could be reduced by 57% (64%–37%) as well as the LDL/HDL ratio to 1.15 (0.7–1.9). Unfortunately, this patient was nonadherent, and hence, could not be treated regularly, and died after a severe cardiac infarction.
Seventy-six IA procedures were performed in 9 nine patients. Three patients suffered from alloantibodies after transplantation, and autoimmune diseases occurred in six patients (Table 1).
The circulating amount of IgG was considerably reduced from a mean initial value of 6.6 (1.09–21.0) to 2.61 (0.33–14.7) g/l. One patient with connective tissue disease received a high dose of intravenous Ig before IA. Here, IA eliminated IgG to only half of the baseline. Another patient with Rasmussen encephalitis was repeatedly treated with the Cobe and afterward with the Optia. Since the beginning of IA treatments, he has been free of partial epileptic episodes (Figure 2).
We were able to complete 71 out of 73 LA and 74 out of 76 IA (Table 2). One LA had to be stopped due to breast pain, and another due to venous access problems. Two IA treatments were stopped because of severe hypotonia. With regard to the Optia alarms, a total of 28 alarms in LA and IA occurred due to problems with peripheral venous access that were easily resolved. No alarms such as filter-pressure alarms, valve malfunction, or those due to fractionated plasma occurred during SPD.
  | Table 2 Total, mean, and parameters in range of whole-blood separation and secondary plasma treatment Abbreviations: AV, arteriovenous; ACD-A, anticoagulant citrate dextrose solution formula A. |
The utilization of citrate per milliliter of treated plasma for LA procedures was significantly higher than for IA (Table 2). For the IA treatment, we utilized a mixed anticoagulation regimen of citrate and heparin. In both regimens, we did not observe clotting or bleeding.
All patients received 10% calcium gluconate as an infusion during SPD treatment (Table 2). No patient showed signs of hypocalcemia.
Discussion
Our findings showed that the Optia works precisely as intended. The same procedures could be performed with Cobe, but not with such a low amount of resources.
One of the biggest advantages of the Optia is its high plasma removal efficiency. Ideal plasma flow rates for filter or IA columns could be reached faster. To comply with our Institute’s standards for IA or LA, the operator adjusted the plasma flow. This measure can extend the time required for plasma processing.
A limitation of our report is that each Optia can only store up to 100 procedures. Therefore, our dataset starts at the end of 2015. Further, the collected data do not cover the patients’ clinical findings or outcome, thus favoring either the Spectra or the Optia.
Furthermore, one of the main uses of LA is the treatment of patients with homozygous familial hypercholesterolemia. In this report, only patients with dyslipoproteinemia due to immunosuppression after transplantation have been treated. As this is indeed a different entity, it limits our results concerning the efficacy of the system for LA. One patient presented an Lpa below 300 mg/ml before treatment a few times. Here, an exception from our 300-mg/ml limit was made due to his transplant vasculopathy and his proven biweekly treatment (Figure 1).
The reduction of the total amount of IgG and circulating human leukocyte antibodies prevented further acute antibody-mediated humoral rejection episodes. As experience has shown, autoimmune patients, especially those with encephalitis, do not necessarily benefit from Ig reduction. It should be noted that one patient with Rasmussen encephalitis maintained a stable clinical condition since the beginning of IA treatment. His clinical course is demonstrated in Figure 2.
In centrifugal apheresis procedures, citrate has become the anticoagulant of choice. The World Apheresis Registry data show that 98.3% of centrifugal apheresis procedures are performed with ACD-A alone.11 Citrate is easy to use and neutralized within 36 min in patients without compromised liver function.12 The recommended citrate ratio during PTPs with Optia is 12:1 (WB:ACD-A). Nevertheless, the volume of anticoagulant utilized depends on the PTP. The mean ACD-A utilization volume to process 1 ml plasma in TPE is 0.16 ml for Optia.13 We observed a utilization volume of 0.17 ml ACD-A for LA and 0.14 ml for IA to process 1 ml of plasma.
To prevent bleeding and filter plugging in LA, we refrained from using heparin as an additional anticoagulant. We found that pore size-based filters deplete coagulation factors such as factor XIII in the same amount as lipoproteins. When heparinized plasma is cooled to below 35°C, the formation of cryoprecipitates blinds the filter and leads to a high filter pressure.14 This pressure has to be regulated immediately, or the procedure will need to be stopped.
For patients with compromised liver or heart function and muscle weakness, it is important to reduce citrate infusions. One method of reduction is to utilize a heparin and ACD-A mix for IA procedures. In addition, it is possible to minimize the inlet blood volume per minute. We applied a low inlet flow rate for LA and IA, compared to other published data. In the study by Kes et al,15 the inlet flow was maintained twice as high as that in our study. Here, a further reduction of the inlet flow was not presented as an option presented.15
The amount of citrate that the patient receives depends on the efficiency of plasma separation. Calculating the quotient of WB processed/PV removed, from the data of Cid et al, Optia had the best efficiency (1.55), followed by Amicus (1.68) and Cobe (1.86).13 Moreover, Tormey et al have demonstrated higher plasma removal efficiency for Optia in comparison to the Cobe.16 At first, we did not consider increased plasma efficiency when the devices were switched for SPD procedures. Rather than focusing on inlet flow, the Optia SPD software can be conveniently programmed to cap the plasma pump flow rate automatically, if necessary. The Optia is also designed to achieve high plasma extraction rates even at low inlet blood flows and peripheral venous access.17 To prevent clotting, especially in the case of IA with low inlet blood flow, mixed anticoagulation with heparin is helpful. In 1999, Braun and Risler described anticoagulation for IA. Anticoagulation was achieved in addition to ACD-A at a ratio of 12:1 (WB:ACD-A) with a 2,500 IU sodium heparin bolus and a continuous infusion of 1,000 IU sodium heparin/h.9 We performed the IA with a ratio of 15:1 (WB:ACD-A) and added 1,000 U heparin/h. Handschel et al observed clumping or granularity in the buffy coats at a ratio of 20:1/25:25 (WB:ACD-A). The clumping was cleared without operator intervention with the application of 16:1/18:1 (WB:ACD-A). All patients in this study received a bolus of heparin (2,500 U). Furthermore, they added 10,000 U heparin to a 600-ml ACD-A bag.5 In contrast, we refrained from a bolus delivery of heparin because alteration of shear stress in blood vessels, which can activate coagulation, does not occur until the start of the apheresis.18 We detected no clotting, despite the altered procedure. Bramlage et al suggested that every unit of heparin administered increases the risk of complications by 0.3%.19 Whether a heparin bolus delivery is actually required remains to be discussed. Similarly, Kes et al15 observed no clotting for the Optia. This AE occurred more often (five out of 27 procedures) with membrane technology (Diapact).15 Two procedures even required premature termination.
Mortzell et al20 evaluated data from the World Apheresis Registry and revealed that filtration technology for primary separation had more AEs than centrifugation. The severity of AEs and the volume processed were weakly correlated. However, a central access was related to severe AEs.20
In relation to the central access, the Optia enables the physician to prefer a peripheral venous access, as the device is designed to perform well at low blood flow rates. One of the greatest advantages of peripheral venous access is its extremely low infection rate. Furthermore, peripheral venous access reduces the risk of bacteremia associated with central venous catheters.2,21 In 62% of all examined treatments, a peripheral venous access was feasible. A central catheter or an arteriovenous (AV) fistula was used in 38% of cases because it was readily available. We estimated that more than 62% of severely ill patients could be treated with peripheral venous access with the Optia system. Registry data from other cell-separating systems indicated that peripheral venous access was used in 71% of apheresis procedures, central catheter in 21.2%, and AV fistula in 4%.11
Besides vascular access, the anticoagulant type, replacement fluid, underlying diseases, as well as the type of PTP have a great impact on the appearance of AEs and complications.2 As citrate exclusively anticoagulates the extracorporeal circuit, citrate-induced AEs occur only in 0.08%–1.2% of the procedures.1 The administration of ionized Ca to each PTP is helpful to prevent citrate reactions.21,22 Cid et al recommend the administration of 1 mmol ionized calcium and 0.5 mmol magnesium to 10 mmol citrate.13 With our citrate regimen and administration of ionized calcium, averaging 0.029 mmol/min, we observed no signs of hypocalcemia, similar to Handschel et al’s study.5 However, the results reported by Mortzell et al20 were in contrast with our results. If one of their patients received intravenous calcium as prophylaxis, he/she experienced more AEs than those who did not receive calcium, due to citrate load and metabolism or a secondary change of electrolytes.20
However, further studies should be conducted to discuss the different anticoagulation regimens and clarify how the patient can benefit the most from LA and IA treatments.
Conclusion
The Cobe could be replaced by the Optia system effectively. The Optia has a high plasma extraction efficiency that allows SPD procedures with peripheral venous access and moderate inlet flow rates. The use of peripheral venous access as well as the reduction of ACD-A utilization increases patient’s safety.
Acknowledgment
The authors would like to express their gratitude to Tom Westhäuser for his advice and assistance.
Disclosure
Silke Rummler works as an “In Country Reviewer” for Terumo BCT. The other authors declare no conflicts of interest in this work.
References
Linenberger ML, Price TH. Use of cellular and plasma apheresis in the critically ill patient: part 1: technical and physiological considerations. J Intensive Care Med. 2005;20(1):18–27. | ||
Ward DM. Conventional apheresis therapies: a review. J Clin Apher. 2011;26(5):230–238. | ||
Madore F. Plasmapheresis. Technical aspects and indications. Crit Care Clin. 2002;18(2):375–392. | ||
Schwartz J, Padmanabhan A, Aqui N, et al. Guidelines on the use of therapeutic apheresis in clinical practice-evidence-based approach from the Writing Committee of the American Society for Apheresis: The Seventh Special Issue. J Clin Apher. 2016;31(3):149–162. | ||
Handschel D, Etienne Janssens M, Gericke M, De Reys S, Borberg H. Comparative evaluation of a heparin-citrate anticoagulation for LDL-apheresis in two primary apheresis systems. J Clin Apher. Epub 2016 Sep 27. | ||
Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51(2):224–232. | ||
Kaplan AA. A simple and accurate method for prescribing plasma exchange. ASAIO Trans. 1990;36(3):M597–M599. | ||
Gjorstrup P, Watt RM. Therapeutic protein A immunoadsorption. A review. Trans Sci. 1990;11(3–4):281–302. | ||
Braun N, Risler T. Immunoadsorption as a tool for the immunomodulation of the humoral and cellular immune system in autoimmune disease. Ther Apher. 1999;3(3):240–245. | ||
Bambauer R, Bambauer C, Lehmann B, Latza R, Schiel R. LDL-apheresis: technical and clinical aspects. ScientificWorldJournal. 2012;2012:314283. | ||
Stegmayr B, Ptak J, Wikstrom B, et al. World apheresis registry 2003-2007 data. Transfus Apher Sci. 2008;39(3):247–254. | ||
Kramer L, Bauer E, Joukhadar C, et al. Citrate pharmacokinetics and metabolism in cirrhotic and noncirrhotic critically ill patients. Crit Care Med. 2003;31(10):2450–2455. | ||
Cid J, Molina JM, Mustieles MJ, Perianez M, Lozano M. Comparison of plasma exchange procedures using three apheresis systems. Transfusion. 2015;55(5):1001–1007. | ||
Siami GA, Siami FS. Membrane plasmapheresis in the United States: a review over the last 20 years. Ther Apher. 2001;5(4):315–320. | ||
Kes P, Janssens ME, Basic-Jukic N, Kljak M. A randomized crossover study comparing membrane and centrifugal therapeutic plasma exchange procedures. Transfusion. 2016;56(12):3065–3072. | ||
Tormey CA, Peddinghaus ME, Erickson M, et al. Improved plasma removal efficiency for therapeutic plasma exchange using a new apheresis platform. Transfusion. 2010;50(2):471–477. | ||
Lambert C, Gericke M, Smith R, Hermans C. Plasma extraction rate and collection efficiency during therapeutic plasma exchange with Spectra Optia in comparison with Haemonetics MCS+. J Clin Apher. 2011;26(1):17–22. | ||
Kroll MH, Hellums JD, McIntire LV, Schafer AI, Moake JL. Platelets and shear stress. Blood. 1996;88(5):1525–1541. | ||
Bramlage CP, Schroder K, Bramlage P, et al. Predictors of complications in therapeutic plasma exchange. J Clin Apher. 2009;24(6):225–231. | ||
Mortzell Henriksson M, Newman E, Witt V, et al. Adverse events in apheresis: an update of the WAA registry data. Transfus Apher Sci. 2016;54(1):2–15. | ||
Okafor C, Ward DM, Mokrzycki MH, Weinstein R, Clark P, Balogun RA. Introduction and overview of therapeutic apheresis. J Clin Apher. 2010;25(5):240–249. | ||
Messmore H, Jeske W, Wehrmacher W, Walenga J. Benefit-risk assessment of treatments for heparin-induced thrombocytopenia. Drug Saf. 2003;26(9):625–641. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.