Back to Journals » Therapeutics and Clinical Risk Management » Volume 19
Speckle-Tracking Echocardiography Predicts Adverse Left Ventricular Remodeling After Valve Replacement in Rheumatic Mitral Stenosis
Authors Zhang X , Zhang J, Cai Y , Li Y, Qin S, Li J , Zeng D, Huang T , Huang LL, Zhong Y, Wei L, Wu J
Received 28 April 2023
Accepted for publication 14 August 2023
Published 20 September 2023 Volume 2023:19 Pages 755—766
DOI https://doi.org/10.2147/TCRM.S419163
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Deyun Wang
Xiaofeng Zhang,1,* Jiaqi Zhang,1,* Yongzhi Cai,1 Yue Li,1 Shiyun Qin,1 Jingtao Li,1 Decai Zeng,1 Tongtong Huang,1 Liu Liu Huang,2 Yanfen Zhong,1 Lihui Wei,1 Ji Wu1
1Department of Ultrasonic Medicine, the First Affiliated Hospital of Guangxi Medical University, Nanning, People’s Republic of China; 2Department of Cardiothoracic Surgery, the First Affiliated Hospital of Guangxi Medical University, Nanning, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ji Wu, Email [email protected]
Background: Rheumatic mitral stenosis(RMS) may leads to left ventricular remodeling (LVR), which can persist even after valve surgery. Identifying markers for early structure and function in patients with rheumatic heart disease who are at risk for adverse LVR after surgery can help determine the optimal timing of intervention. This study aimed to investigate whether preoperative parameters of global left ventricular long-axis strain (LVGLS) and mechanical discretization (MD) could predict postoperative adverse LVR.
Methods: A total of 109 adult patients with RMS and 50 healthy controls were enrolled in this study. Baseline clinical features, conventional echocardiography results, LVGLS, and MD were compared between the two groups. Pre- and post-surgery echocardiography measurements were collected, and adverse LVR was defined as a> 15% increase in left ventricular end-diastolic volume or > 10% decrease in left ventricular ejection fraction. Binary regression analysis was used to determine independent predictors of poor left ventricular remodeling.
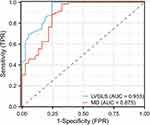
Results: The variables associated with adverse LVR in this study were LVGLS (P< 0.001, odds ratio: 1.996, 95% CI: 1.394– 2.856) and MD (P=0.011, odds ratio: 1.031, 95% CI: 1.007– 1.055). The poorly reconstructed group had lower absolute values of LVGLS and higher MD than the healthy control group and the non-poorly reconstructed group. A LVGLS cutoff of − 15.0% was the best predictor for patients with poorly reconstructed LVR (sensitivity: 75.7%; specificity: 100.0%; AUC: 0.93), and a MD cutoff of 63.8ms was the best predictor (sensitivity: 63.8%; specificity: 98.6%; AUC: 0.88).
Conclusion: Speckle tracking echocardiography has potential value for predicting the progression of adverse LVR and for identifying non-responders among patients with RMS undergoing surgery.
Keywords: rheumatic mitral stenosis, left ventricular remodeling, left ventricular global longitudinal strain, mechanical dispersion, speckle tracking echocardiography
Introduction
Rheumatic heart disease (RHD) is a severe and chronic condition resulting from acute rheumatic fever triggered by Group A streptococcus infection, leading to an autoimmune response.1 The main pathogenesis of rheumatic heart disease is valve involvement, and rheumatic mitral stenosis(RMS) is the most common manifestation of valve involvement; commissural fusion, thickening, and narrowing of the valve leaflets lead to obstruction of left ventricular (LV) filling.2 In RMS, reduced stroke volume usually relates to reduced LV preload, rather than ventricular contractile impairment. However, some patients might present true systolic dysfunction that is independent from LV preload.3
Left ventricular remodeling (LVR) is an unfavorable adaptation of the heart to various factors such as mechanical, neurohormonal, and inflammatory changes that regulate the size, morphology, and function of the ventricles.4 In the context of rheumatic valvular disease, left ventricular remodeling is characterized by pathological features such as increased myocardial interstitial space, collagen deposition, and muscle fiber loss, resulting in myocardial fibrosis, which is the hallmark of left ventricular remodeling. These alterations occur prior to the deterioration of left ventricular systolic function or the onset of symptoms in patients.5 Evidence suggests that myocardial fibrosis may remain irreversible even after valve surgery. Furthermore, myocardial fibrosis is associated with less improvement in clinical symptoms and recovery of left ventricular systolic function, ultimately leading to adverse LVR.6
Identifying additional markers of early structure and function in patients with rheumatic heart disease after surgery who develop adverse left ventricular remodeling will help redefine the optimal timing of intervention. Speckle tracking echocardiography (STE) is widely used for detecting of early subclinical ventricular dysfunction. It can independently predict subsequent cardiac adverse events, as these abnormal findings usually precede the reduction of LVEF.7–10 However, there are few studies on poor left ventricular remodeling predictors after valvular surgery for rheumatic heart disease. Global longitudinal strain (GLS) and mechanical dispersion (MD) have been applied to predict the poor prognosis of various heart diseases. Still, they have not been applied to the poor prognosis of valvular surgery for rheumatic heart disease. This study aimed to explore whether the assessment of global long-axis strain and mechanical dispersion of the left ventricle by STE can be used to identify poor LVR after surgery in patients with RMS.
Method
Study Design and Population
A total of 109 adult inpatients who underwent cardiac surgery at the First Affiliated Hospital of Guangxi Medical University between September 2020 and November 2022 were included in this study. The study was approved by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University (No.2022-KY-E-132), which waived the requirement for informed consent as it involved only anonymous imaging data sets and did not collect individual patient data or human tissue samples.
Inclusion criteria of this study: ① Patients were clinically diagnosed with pure RMS or mixed lesions with predominant mitral stenosis were submitted to primary open mitral valve replacements, associated or not with tricuspid valve repairs and/or Cox-maze procedures; ② Routine echocardiography and left ventricular long-axis strain rate and mechanical dispersion evaluation were performed in all patients before operation. Exclusion criteria: ① Patients with heart diseases such as hypertension, coronary heart disease, cardiomyopathy and congenital heart disease that cause structural changes and function damage of the left ventricle; ② Patients with moderate or severe mitral regurgitation, aortic valve disease, previous heart surgery, and mitral valve repair; ③ Patients who failed to cooperate with echocardiography examination or whose low-quality images were not suitable for analysis.
Routine Transthoracic Echocardiography (TTE) and Follow-Up
All enrolled subjects were examined by transthoracic echocardiography pre-and post-operatively, with a 12-month follow-up of all patients participating in the study, with the first follow-up being six months post-operatively and the subsequent follow-up at 12 months with echocardiogram data recorded. The patients in this study were retrospectively recruited from the hospital’s picture archiving and communication system (PACS) and clinical medical record system. The LVEF, LVEDV, and left ventricular end-systolic volume(LVESV) before and after surgery were collected from the system. Conventional TTE was performed using Philips IE 33 or Philips EPIQ 7C systems (Philips Ultrasound System, the Netherlands) with an S5-1 ultrasound probe operating at a frequency range of 2.5–5.0MHz. The LVEF, LVEDV, and LVESV were measured using the biplane Simpson’s method in reference to the 2015 American Echocardiography Association (ASE) recommendations. Color Doppler was used to evaluate the degree of stenosis or regurgitation of each valve.11
Speckle Tracking Echocardiography
All enrolled patients were examined preoperatively using a Philip EPIQ 7C ultrasound diagnostic apparatus equipped with an S5-1 adult cardiac probe (frequency 2.5–5.0 MHz) and a Philips QLab quantitative analysis workstation.
The participants in the experiment chose the left lateral decubitus position and were told to keep calm and breathe steadily. The synchronous electrocardiograph(ECG) was connected and the sonograms of three consecutive cardiac cycles in the apical four-chamber view, three-chamber view, and two-chamber view were stored. When acquiring all the sectional images, it is required to minimize the fan angle area based on the complete and clear display of the left ventricular structure to acquire dynamic images with a frame rate greater than 30 frames/s and record each dynamic image for at least three cardiac cycles. The left ventricular endocardium and epicardium of 18 segments were delineated by STE to obtain the peak myocardial strain rates of each segment and the whole. LV mechanical dispersion (MD) was defined as the standard deviation of time from surface ECG to peak negative strain in 18 LV segments.12
Two echocardiologists with different experiences (X.Z., five years experience in cardiac imaging; J.W., 25 years of cardiac imaging experience) were involved in the preoperative LVGLS and MD measurement analyses, respectively. Twenty subjects were randomly selected. The same physician repeated the measurements for one month, and the parameters were measured by another physician (J. W.) who did not know the subject information. The repeatability was analyzed, and the consistency of the measured parameters was evaluated by calculating the interobserver correlation coefficient (ICC).
Definition of Adverse Left Ventricular Remodeling
For LVR many previous researchers have conducted in-depth research and come to different definitions of LVR. Carrick et al13 reviewed 300 cases of ST-segment elevation myocardial infarction treated with reperfusion and defined poor left ventricular remodeling as a > 20% increase in the percentage of LVEDV. Bulluck et al14 used 12% of the LVEDV, LVESV, and LVEF, respectively, as the thresholds for poorly defined or reversed left ventricular remodeling. In a meta-study involving 4209 patients, Legallois et al15 proposed the follow-up composite left ventricular cutoff (12% to 15% increase in LVESV and 12% to 20% increase in LVEDV) as a common cutoff for determining adverse LVR. In this study, patients with a> 15% increase in LVEDV or > 10% decrease in LVEF were classified as the adverse remodeling group; Otherwise, the patients were assigned to the non-adverse remodeling group.
Statistical Analysis
Statistical analysis was performed using SPSS software v. 23.0 (SPSS Inc., Chicago, Illinois, USA). Continuous variables are expressed as mean standard deviation. Two independent samples t- test was used for comparison between groups, and χ²-test or Fisher exact probability method was used for comparison between enumeration data groups. The inter-group correlation coefficient (ICC) was used to evaluate the intra-group and inter-group consistency of the two groups, and ICC greater than 0.8 was considered good consistency. Binary logic regression was used to determine the predictor of the LVR. A subject-operator characteristic curve was analyzed, and the area under the curve (AUC) was plotted accordingly. The optimal cutoff value was selected by optimizing the sensitivity and specificity.
Results
Characteristic and Clinical Manifestations of Rheumatic Heart Disease
The patient selection process is shown in the flow chart (Figure 1). A total of 143 patients were diagnosed with rheumatic heart disease. Eleven patients were excluded from this study due to concurrent conditions of congenital heart disease (n=2, including one ventricular septal defect and one patent ductus arteriosus), infective endocarditis (n=3), and coronary heart disease (n=6). Four outpatients were also excluded. A total of 111 of 128 inpatient patients underwent valve surgery, and 17 did not receive surgery (11 patients underwent transcatheter balloon dilatation, 4 refused surgery, and 2 had no surgical indication). All patients who underwent surgery acquired of the cut planes required by conventional transthoracic echocardiography and spot-tracking techniques in our hospital pre-operatively. Four-chamber, two-chamber, and three-chamber views were collected preoperatively to analysis the Speckle tracking echocardiography, and two patients lost to follow-up postoperatively were excluded. A total of 109 patients were included in the study with an average follow-up time of 14.5±6.3 months.
 |
Figure 1 Flow chart of the study population. Abbreviation: RHD, rheumatic heart disease. |
A total of 50 healthy volunteers (mean age, 53.5±4.1 years; range, 46–60 years old; females, n=32; males, n=18) were recruited as HCg. Demographic data (including age, gender, height, weight, blood pressure, and heart rate) and their routine ultrasound and left ventricular long-axis strain rate imaging parameters were collected for all participants.
All patients simultaneously had rheumatic valvular disease involving the mitral valve or other valves. All 109 patients had clinical symptoms, and most complained of chest tightness or dyspnea (77/109, 70.6%). Some patients had concomitant palpitations (22/109, 20.2%), chest pain (5/109, 4.6%), and cough (17/109, 15.6%). Very few patients (6/109, 5.5%) had lower extremity edema. The above clinical symptoms may occur simultaneously. Most patients had concomitant atrial fibrillation (86/109, 78.9%). Functional status was evaluated by New York Heart Association (NYHA) classification.16 Thirteen patients (11.9%) were classified as NYHA Class I, 48 patients (44.0%) were classified as Class II, 38 patients (34.9%) were classified as NYHA Class III, and ten patients (9.2%) were classified as Class IV.
Comparison of Demographic Data in Patients with RHD and HCg
Demographic data and conventional echocardiography comparisons between the RHD and HCg groups (Table 1). No statistical significance was found in gender, age, body mass index, diastolic blood pressure, or heart rate between the two groups (P>0.05). Echocardiography showed that preoperative left ventricular ejection fraction of patients with rheumatic heart disease decreased, atrioventricular volume increased, and left ventricular weight increased (P<0.05).
 |
Table 1 Comparison of Patients with Rheumatic Heart Disease and Healthy Controls |
The LVGLS was statistically significantly higher in the RHD group than in the HCg (−17.6±4.3 vs −23.2±1.6, P<0.001). The parameter MD of the RHD group was significantly higher than that of the HCg group (46.6±38.1 ms vs.7.9±4.4 ms).
Echocardiographic Findings in Patients with Rheumatic Heart Disease Before and After Surgery
Patients with > 15% increase in LVEDV or > 10% decrease in LVEF in this study were classified as the adverse remodeling group (n=37) and vice versa (n=72). The values of LVEDV (non-adverse remodeling vs adverse remodeling: 122.5±39.0mL vs.104.0±31.1mL; P=0.014), LVESV non-adverse remodeling and adverse remodeling group (47.6±22.8 vs.36.6±16.3mL; P=0.01), LVEF non-adverse remodeling group and adverse remodeling group (61.90%±8.41% vs.57.68%±6.79%, P=0.009) (Table 2).
 |
Table 2 Preoperative and Postoperative Echocardiography in Patients with RHD |
Predictors of Postoperative Poor Left Ventricular Remodeling
To compare the clinical features, conventional echocardiography, LVGLS, and MD parameters of patients with left ventricular non-poor remodeling and poor remodeling. Surgical information, including Wilkins’ score, mitral valve regurgitation, prosthetic valve size, the number of cases of atrial fibrillation radiofrequency ablation and metal prosthetic valve, and duration of cardiopulmonary bypass and mechanical ventilation, showed no significant difference between the non-adverse left ventricular remodeling group and the adverse left ventricular remodeling group (P>0.05) (Table 3). Preoperative parameters including TAPSE, LVMI, GLS, and MD (P≤0.10) were collected and analyzed by binary logistic regression (Table 4). Variables associated with adverse LVR were left ventricular GLS (p<0.001, odds rate:1.996, 95% CI:1.394–2.856) and MD(P=0.011, odds rate:1.031, 95% CI:1.007–1.055). As shown in Figure 2, the adverse LVR group had lower absolute values of GLS and higher values of MD than the HCg and non-poorly reconstructed groups.
 |
Table 3 Comparison of Characteristics Between Patients with Non-Adverse Reconstructions and Adverse Reconstructions |
 |
Table 4 Binary Logistic Regression Analysis for Independent Prediction of Left Ventricular Adverse Remodeling |
ROC Curve Analysis
The ROC curve indicated that −15.0% was the best LVGLS cutoff for poorly identified LVR patients (sensitivity: 75.7%; Specificity: 100.0%; AUC: 0.93), and the best cutoff for MD was 63.8ms (sensitivity: 63.8%; Specificity: 98.6%; AUC: 0.88) (Figure 3).
Consistency Check
The intraclass correlation coefficient (ICC) for GLS, assessed from 20 consecutive patients, showed excellent interrater reliability (ICC: 0.969, 95% CI: 0.924–0.988) and inter‐rater reliability (ICC: 0.959, 95% CI: 0.900–0.984). For the parameter MD, the ICC also showed excellent inter-group reliability (ICC: 0.876, 95% CI: 0.713–0.949) and inter-group reliability (ICC, 0.792, 95% CI: 0.546–0.912). The obtained data are shown in Table 5.
 |
Table 5 Inter-and Intra-Observer Repeatability Analysis of Each Parameter |
Discussion
This study employed two-dimensional speckle tracking imaging to evaluate global left ventricular long-axis strain and mechanical discrete parameters in patients with rheumatic mitral stenosis before surgery, and investigated their significance in predicting adverse left ventricular remodeling after surgery. The findings indicated that LVGLS absolute value ≤15.0% and MD≥63.8ms could serve as predictors of adverse LVR after RHD valve surgery, with a sensitivity of 75.7% for LVGLS and 63.8% for MD, and a specificity of 100.0% for LVGLS and 98.6% for MD. This noninvasive ultrasound-based technique is simple, precise, repeatable, and exhibits good predictive value.
RHD represents a significant factor in cardiovascular mortality and morbidity in developing countries.17 In RMS, reduced stroke volume usually relates to reduced LV preload, rather than ventricular contractile impairment. However, some patients might present true systolic dysfunction that is independent from LV preload.18 More recently, with the advent of techniques for visualization of blood flow within the heart, it has been shown that LV systolic function depends not only on diastolic filling volume but also on filling patterns, which may be disturbed by mitral stenosis.19 Prolonged LV hypofilling, endomyocardial fibrosis, and subvalvular tissue stiffness lead to a diastolic dysfunction characterized by altered ventricular compliance and elevated end-diastolic pressure, although EF remains normal at this time.20 If left untreated or not treated effectively, it often leads to chronic heart failure.21 The exact mechanism remains unclear, but it is probably multifactorial, but it is mainly characterized by diffuse fibrosis resulting from valve orifice stenosis or insufficiency in patients. Diffuse myocardial fibrosis is caused by fibroblast proliferation, collagen metabolic disorders, and cardiomyocyte hypertrophy, which can lead to abnormal myocardial stiffness and decreased contractility, ultimately leading to left ventricular decompensation.22 Arantxa González et al23 have reported that myocardial fibrosis is associated with poor prognoses, such as left ventricular dysfunction and heart failure, and monitoring the degree of myocardial stiffness caused by myocardial fibrosis is important. Previous studies have shown a linear relationship between mitral valve area(MVA) in severe MS patients and systolic and diastolic myocardial velocities determined by Doppler tissue imaging. Moreover, a positive correlation was found between MVA and impaired longitudinal strain rates.24,25 Nevertheless, there is limited research on predictors of adverse left ventricular remodeling after surgery for rheumatic valvular disease.Active monitoring based on echocardiography screening is a routine test method for early efficacy.26 LVGLS can reflect the contraction of myocardial fibers under the intima and effectively reflect the abnormal contraction movement of myocardial function.27 Karamitsos et al28 proposed a negative correlation between strain and systolic and diastolic dysfunction, and dysfunction during fibrosis was identified earlier than in conventional echocardiography.
Endomyocardial biopsy is a gold standard for evaluating the degree of myocardial fibrosis. Still, it has been largely limited in clinical practice due to its invasive nature, serious operational difficulty, and high examination risk and complication probability.29,30 Cardiac magnetic resonance imaging has a promising application prospect in the assessment of myocardial damage and fibrosis by combining natural and advanced gadolinium enhancement (LGE) with hematocrit (ECV), reflecting the expanded extracellular matrix.31 However, since CMR is expensive and has a long imaging time, it is not convenient for repeated examinations. It is unsuitable for cardiac examinations after renal dysfunction and metal valve replacement. Echocardiography, as a non-invasive and affordable imaging technology, remains a first-line choice for cardiac evaluations, owing to its portability and high accessibility. Furthermore, recent studies have demonstrated a relationship between myocardial strain in the two ventricles and the left atrium and the degree of myocardial fibrosis, indicating that echocardiography can also be used to assess myocardial fibrosis non-invasively.32
Recent studies have shown that GLS can be used to evaluate heart failure and valvular heart disease.27 A multicenter study coordinated by the National University of Singapore Heart Center (NUHCS) showed that in asymptomatic/mild symptomatic patients with moderate and severe AS, the GLS of patients with alternative myocardial fibrosis was impaired even if the LVEF was preserved. The left ventricular GLS had a moderately strong correlation with the indexed left ventricular mass with a consistent independent association with adverse outcomes.33 Although studies have used GLS as a predictor of hypertrophic cardiomyopathy, aortic stenosis, dilated cardiomyopathy, and other diseases, STE has not been reported as a marker for predicting cardiac decompensation and prognosis in patients with rheumatic heart disease.34–36
Previous ultrasound studies of rheumatic valvular heart disease have focused on valvular, hemodynamic, and cardiac function. LVEF reflects its systolic function by evaluating the pump function of the left ventricle and cannot directly reflect the changes in the systolic characteristics of the left ventricle myocardium, which is a late marker of cardiac decompensation.37 RHD-induced mitral stenosis results in a sudden decrease in left ventricular volume load, resulting in a significant decrease in myofibril density and a progressive decrease in systolic function. However, the change is slow because the pressure load is also reduced. At the same time, the decreased blood filling volume of the left ventricle leads to a decrease in cardiac output, causing insufficient coronary artery perfusion, which in turn leads to the poor myocardial blood supply. The longitudinal myocardium located under the endocardium is more sensitive to myocardial ischemia, and myocardial fibrosis occurs earlier.38
In our preliminary study, we utilized echocardiography to evaluate preoperative and postoperative changes in parameters of patients with rheumatic heart disease and to identify preoperative predictors of postoperative left ventricular poor remodeling, which is crucial for clinicians to prevent further postoperative cardiac damage promptly and to guide future treatment strategies. The study results indicated that the degree of preoperative absolute value reduction of LVGLS was negatively correlated with postoperative adverse left ventricular remodeling. The more the LVGLS was reduced, the greater the possibility of adverse left ventricular remodeling. Additionally, MD demonstrated a positive correlation with postoperative adverse left ventricular remodeling. As preoperative MD increases, the likelihood of adverse left ventricular remodeling occurring also increases.
In patients with severe myocardial fibrosis, we hypothesize that left ventricular myocardial elasticity may become decompensated when STE measures LVGLS and MD values reach cutoff points. Even after correcting for hemodynamic abnormalities through valve surgery, the LVEF and EDV may not be recovered, leading to poor left ventricular remodeling. Our study confirms that two-dimensional strain prediction of postoperative left ventricular poor remodeling is independent of traditional parameters and is associated with cardiac events in surgically treated patients with RHD. Objective echocardiogram parameters can aid in decision-making and monitor postoperative adverse cardiac events more effectively than identifying subtle clinical symptoms. By identifying impaired systolic function through preoperative GLS reductions and standardizing GLS and MD to left ventricular end-diastolic volume, clinicians can control load conditions and administer therapy before postoperative adverse LVR occurs, potentially slowing or stopping the disease’s progression. Future studies should investigate whether GLS-based assessment of sub-endocardial myocardial contractile movements can aid in detecting myocardial changes early.
Study Limitations
The present study has several limitations that require further consideration. Firstly, this was a single-center study with a small sample size. Therefore, the results of this study need to be validated in larger sample sizes from different centers. Secondly, the accuracy of STE analysis depends on image quality, and we excluded patients with poor image quality, which may limit the generalizability of the findings. Thirdly, although we have speculated in the discussion that left ventricular myocardial fibrosis is the cause of poor left ventricular remodeling in patients with rheumatic mitral stenosis, at the same time, mitral stenosis leads to reduced left ventricular blood filling and reduced cardiac output, The resulting hypoperfusion of the coronary arteries may lead to pathological changes in cardiomyocytes, but clinically, the changes in coronary hemodynamics cannot be effectively verified. Lastly, long-term follow-up data of the included patients could not be obtained due to patient loss of follow-up.
Conclusions
The present study has confirmed that measurements of LVGLS and MD are characterized by simplicity, reliability, and good repeatability, owing to their high accuracy. As a result, STE may be able to predict the progression of adverse LVR and identify non-responders among patients with RMS undergoing surgery. Studies with a larger sample size are needed in the future.
Data Sharing Statement
Data applied in the course of this study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The present study was a retrospective case–control study. It met the requirements laid out in the Declaration of Helsinki. Approval for this study was provided by the Ethics Committee of The First Affiliated Hospital of Guangxi Medical University (No.2022-KY-E-132), waiving the right to sign the informed consent form; It relates only to anonymous imaging data set; No individual patient data or human tissue samples were collected.
Acknowledgments
X.Z. and J.Z are co-first authors.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported in part by Natural Science Foundation of Guangxi Zhuang Autonomous Region (Grant No. 2017GXNSFAA198246); Key project of Guangxi Science and Technology Program (No. 2023GXNSFDA026010); Key R&D projects in Hubei Province (Grant No.2022BCE004).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Dooley LM, Ahmad TB, Pandey M., et al. Rheumatic heart disease: a review of the current status of global research activity. Autoimmun Rev. 2021;20(2):102740. doi:10.1016/j.autrev.2020.102740
2. Unger P, Pibarot P, Tribouilloy C, et al. Multiple and mixed valvular heart diseases. Circ Cardiovasc Imaging. 2018;11(8):e007862. doi:10.1161/CIRCIMAGING.118.007862
3. Chandrashekhar Y, Westaby S, Narula J. Mitral stenosis. Lancet. 2009;374(9697):1271–1283. doi:10.1016/S0140-6736(09)60994-6
4. Frantz S, Hundertmark MJ, Schulz-Menger J, et al. Left ventricular remodelling post-myocardial infarction: pathophysiology, imaging, and novel therapies. Eur Heart J. 2022;43(27):2549–2561. doi:10.1093/eurheartj/ehac223
5. Malahfji M, Shah DJ. Cardiac magnetic resonance in valvular heart disease: assessment of severity and myocardial remodeling. Methodist Debakey Cardiovasc J. 2020;16(2):106–113. doi:10.14797/mdcvj.964
6. Podlesnikar T, Delgado V, Bax JJ. Cardiovascular magnetic resonance imaging to assess myocardial fibrosis in valvular heart disease. Int J Cardiovasc Imaging. 2018;34(1):97–112. doi:10.1007/s10554-017-1195-y
7. Lotti R, DE Marzo V, Della Bona R, et al. Speckle-tracking echocardiography: state of art and its applications. Minerva Med. 2021;114(4):500–515. doi:10.23736/S0026-4806.21.07317-1
8. McGregor PC, Moura FA, Banchs J, et al. Role of myocardial strain imaging in surveillance and management of cancer therapeutics-related cardiac dysfunction: a systematic review. Echocardiography. 2021;38(2):314–328. doi:10.1111/echo.14944
9. Arciniegas Calle MC, Sandhu NP, Xia H, et al. Two-dimensional speckle tracking echocardiography predicts early subclinical cardiotoxicity associated with anthracycline-trastuzumab chemotherapy in patients with breast cancer. BMC Cancer. 2018;18(1):1037. doi:10.1186/s12885-018-4935-z
10. Oikonomou EK, Kokkinidis DG, Kampaktsis PN, et al. Assessment of Prognostic value of left ventricular global longitudinal strain for early prediction of chemotherapy-induced cardiotoxicity: a systematic review and meta-analysis. JAMA Cardiol. 2019;4(10):1007–1018. doi:10.1001/jamacardio.2019.2952
11. Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1–39.e14. doi:10.1016/j.echo.2014.10.003
12. Voigt JU, Pedrizzetti G, Lysyansky P, et al. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. J Am Soc Echocardiogr. 2015;28(2):183–193. doi:10.1016/j.echo.2014.11.003
13. Carrick D, Haig C, Rauhalammi S, et al. Prognostic significance of infarct core pathology revealed by quantitative non-contrast in comparison with contrast cardiac magnetic resonance imaging in reperfused ST-elevation myocardial infarction survivors. Eur Heart J. 2016;37(13):1044–1059. doi:10.1093/eurheartj/ehv372
14. Bulluck H, Go YY, Crimi G, et al. Defining left ventricular remodeling following acute ST-segment elevation myocardial infarction using cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2017;19(1):26. doi:10.1186/s12968-017-0343-9
15. Legallois D, Hodzic A, Alexandre J, et al. Definition of left ventricular remodelling following ST-elevation myocardial infarction: a systematic review of cardiac magnetic resonance studies in the past decade. Heart Fail Rev. 2022;27(1):37–48. doi:10.1007/s10741-020-09975-3
16. Hurst JW. The value of using the entire New York Heart Association’s classification of heart and vascular disease. Clin Cardiol. 2006;29(9):415–417. doi:10.1002/clc.4960290909
17. Watkins DA, Johnson CO, Colquhoun SM, et al. Global, regional, and national burden of rheumatic heart disease, 1990-2015. N Engl J Med. 2017;377(8):713–722. doi:10.1056/NEJMoa1603693
18. Al‐Taweel A, Almahmoud MF, Khairandish Y, et al. Degenerative mitral valve stenosis: diagnosis and management. Echocardiography. 2019;36(10):1901–1909. doi:10.1111/echo.14495
19. Samaan AA, Said K, Aroussy WE, et al. Left Ventricular remodeling following balloon mitral valvuloplasty in rheumatic mitral stenosis: magnetic resonance imaging study. Front Cardiovasc Med. 2021;8:674435. doi:10.3389/fcvm.2021.674435
20. Eleid MF, Nishimura RA, Lennon RJ, et al. Left ventricular diastolic dysfunction in patients with mitral stenosis undergoing percutaneous mitral balloon valvotomy. Mayo Clin Proc. 2013;88(4):337–344. doi:10.1016/j.mayocp.2012.11.018
21. Liu M, Lu L, Sun R, et al. Rheumatic Heart Disease: causes, Symptoms, and Treatments. Cell Biochem Biophys. 2015;72(3):861–863. doi:10.1007/s12013-015-0552-5
22. Díez J, González A, Kovacic JC. Myocardial Interstitial Fibrosis in Nonischemic Heart Disease, Part 3/4: JACC Focus Seminar. J Am Coll Cardiol. 2020;75(17):2204–2218. doi:10.1016/j.jacc.2020.03.019
23. González A, Schelbert EB, Díez J, et al. Myocardial Interstitial Fibrosis in Heart Failure: biological and Translational Perspectives. J Am Coll Cardiol. 2018;71(15):1696–1706. doi:10.1016/j.jacc.2018.02.021
24. Erturk M, Aksu HU, Celik O, et al. Evaluation of the effect of mitral stenosis severity on the left ventricular systolic function using isovolumic myocardial acceleration. Cardiol J. 2014;21(4):442–448. doi:10.5603/CJ.a2013.0114
25. Ferreira MVS, Cunha CRD, Oliveira GS, et al. Left Ventricular Remodeling Shortly after Open Mitral Valve Replacement for Rheumatic Mitral Stenosis. Braz J Cardiovasc Surg. 2021;36(4):468–475. doi:10.21470/1678-9741-2020-0641
26. Zühlke L, Mayosi BM. Echocardiographic screening for subclinical rheumatic heart disease remains a research tool pending studies of impact on prognosis. Curr Cardiol Rep. 2013;15(3):343. doi:10.1007/s11886-012-0343-1
27. Potter E, Marwick TH. Assessment of left ventricular function by echocardiography: the case for routinely adding global longitudinal strain to ejection fraction. JACC Cardiovasc Imaging. 2018;11(2 Pt 1):260–274. doi:10.1016/j.jcmg.2017.11.017
28. Karamitsos TD, Arvanitaki A, Karvounis H, et al. Myocardial Tissue Characterization and Fibrosis by Imaging. JACC Cardiovasc Imaging. 2020;13(5):1221–1234. doi:10.1016/j.jcmg.2019.06.030
29. Khan T, Selvakumar D, Trivedi S, et al. The value of endomyocardial biopsy in diagnosis and guiding therapy. Pathology. 2017;49(7):750–756. doi:10.1016/j.pathol.2017.08.004
30. Martinez-Naharro A, Hawkins PN, Fontana M. Cardiac amyloidosis. Clin Med. 2018;18(Suppl 2):s30–s35. doi:10.7861/clinmedicine.18-2-s30
31. Li S, Wang S, Yu J, et al. Myocardial extracellular volume assessed by cardiovascular magnetic resonance may predict adverse left ventricular remodeling in rheumatic heart disease after valvular surgery. Quant Imaging Med Surg. 2022;12(4):2487–2497. doi:10.21037/qims-21-678
32. Lisi M, Cameli M, Mandoli GE, et al. Detection of myocardial fibrosis by speckle-tracking echocardiography: from prediction to clinical applications. Heart Fail Rev. 2022;27(5):1857–1867. doi:10.1007/s10741-022-10214-0
33. Le TT, Huang W, Singh GK, et al. Echocardiographic global longitudinal strain is associated with myocardial fibrosis and predicts outcomes in aortic stenosis. Front Cardiovasc Med. 2021;8:750016. doi:10.3389/fcvm.2021.750016
34. Liu H, Pozios I, Haileselassie B, et al. Role of global longitudinal strain in predicting outcomes in hypertrophic cardiomyopathy. Am J Cardiol. 2017;120(4):670–675. doi:10.1016/j.amjcard.2017.05.039
35. Ng ACT, Prihadi EA, Antoni ML, et al. Left ventricular global longitudinal strain is predictive of all-cause mortality independent of aortic stenosis severity and ejection fraction. Eur Heart J Cardiovasc Imaging. 2018;19(8):859–867. doi:10.1093/ehjci/jex189
36. Kažukauskienė I, Balčiūnaitė G, Baltrūnienė V, et al. Left ventricular global longitudinal strain predicts elevated cardiac pressures and poor clinical outcomes in patients with non-ischemic dilated cardiomyopathy. Cardiovasc Ultrasound. 2021;19(1):21. doi:10.1186/s12947-021-00254-1
37. Pandian NG, Kim JK, Arias-Godinez JA, et al. Recommendations for the use of echocardiography in the evaluation of rheumatic heart disease: a report from the American society of echocardiography. J Am Soc Echocardiogr. 2022;36:3–28. doi:10.1016/j.echo.2022.10.009
38. Wunderlich NC, Dalvi B, Ho SY, et al. Rheumatic mitral valve stenosis: diagnosis and treatment options. Curr Cardiol Rep. 2019;21(3):14. doi:10.1007/s11886-019-1099-7
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.