Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 12
Specific IgA against Pseudomonas aeruginosa in severe COPD
Authors Millares L, Martí S, Ardanuy C , Liñares J, Santos S, Dorca J, García-Nuñez M, Quero S, Monsó E
Received 12 May 2017
Accepted for publication 25 July 2017
Published 30 September 2017 Volume 2017:12 Pages 2807—2811
DOI https://doi.org/10.2147/COPD.S141701
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Laura Millares,1–3 Sara Martí,2,4 Carmen Ardanuy,2,4 Josefina Liñares,2,4 Salud Santos,2,5 Jordi Dorca,5 Marian García-Nuñez,1–3,6 Sara Quero,3,6 Eduard Monsó2,7,8
1Department of Respiratory Medicine, Fundació Parc Taulí, Sabadell, Spain; 2CIBER de Enfermedades Respiratorias, CIBERES, Bunyola, Spain; 3Universitat Autònoma de Barcelona, Esfera UAB, Barcelona, Spain; 4Department of Microbiology, Hospital Universitari de Bellvitge-Universitat de Barcelona-IDIBELL, L’Hospitalet de Llobregat, Spain; 5Department of Respiratory Medicine, Hospital Universitari de Bellvitge-Universitat de Barcelona-IDIBELL, L’Hospitalet de Llobregat, Spain; 6Infectious Diseases Unit, Fundació Insitut d’Investigació GermansTrias i Pujol, Badalona, Spain; 7Department of Respiratory Medicine, Hospital Universitari Parc Taulí, Sabadell, Spain; 8Department of Medicine, Universitat Autònoma de Barcelona (UAB), Barcelona, Spain
Background: The bronchial mucosa is protected by a specialized immune system focused on the prevention of colonization and infection by potentially pathogenic microorganisms (PPMs). Immunoglobulin A (IgA) is the principal antibody involved in this mechanism. A defective immune barrier may facilitate the recurrent presence of PPMs in COPD.
Purpose: The aim of this study was to determine IgA-mediated bronchial specific immune responses against Pseudomonas aeruginosa in stable patients with severe disease.
Methods: COPD patients with good-quality sputum samples obtained during stability were included and classified according to the presence or absence of chronic bronchial colonization by P. aeruginosa. Levels of specific IgA for P. aeruginosa in sputum were determined by ELISA and expressed as ratios, using the pooled level of 10 healthy subjects as reference (optical density450 patient/control).
Results: Thirty-six stable COPD patients were included, 15 of whom had chronic colonization by P. aeruginosa. Levels of specific IgA against P. aeruginosa in stable non-colonized patients were lower than those in healthy subjects (IgA ratio: median =0.15 [interquartile range {IQR} 0.05–0.36]). Colonized patients had higher levels, (1.56 [IQR 0.59–2.79]) (p<0.001, Mann–Whitney U test), with figures equivalent but not exceeding the reference value.
Conclusion: IgA-based immune response against P. aeruginosa was low in severe COPD patients. Levels of specific IgA against this microorganism were higher in colonized patients, but did not attain clear-cut levels above the reference. An impaired local response against P. aeruginosa may favor chronic colonization and recurrent infections in severe COPD.
Keywords: immunoglobulin A, sputum, COPD, colonization, ELISA
Introduction
The bronchial epithelium represents a first line of the respiratory mucosal host defense through the local production of antimicrobial peptides and proteins, the transport and secretion of immunoglobulins to the epithelial surface, and mucociliary clearance.1,2 Secretory immunoglobulin A (SIgA) is the main immunoglobulin in the bronchial mucosa.3 It is produced as dimeric IgA, which is able to bind to the polymeric immunoglobulin receptor (pIgR) in the epithelium, allowing the transcytosis of IgA across the epithelial cell.4 Once in the epithelial surface, proteolytic cleavage releases the dimeric IgA bound to the extracellular domain of the receptor to form SIgA.5
SIgA agglutinates airborne antigens and microorganisms through a process known as immune exclusion, preventing them from activating or directly injuring airway epithelial cells.6 In normal conditions, inhaled particles and microorganisms are trapped in the surface mucus, agglutinated by specific SIgA, and then removed via the mucociliary escalator.2 In chronic obstructive pulmonary disease (COPD), the airway epithelium is structurally and functionally abnormal and unable to maintain the normal dynamics of the mucosal barrier.2 In addition to an impairment in mucociliary clearance mechanisms, a decreased expression of pIgR also characterizes the bronchial epithelium in COPD, resulting in a deficiency of SIgA on the mucosal surfaces.7,8
Lower levels of SIgA in the bronchial tree may contribute to the impaired mucosal defense against pathogens that is characteristic of COPD, favoring bacterial colonization and recurrent bronchial infections by potentially pathogenic microorganisms (PPMs), which are common events in the natural history of COPD.
Bronchial colonization by PPMs such as Haemophilus influenzae has been previously related to lower levels of specific IgA in moderate COPD.9 The objective of the present study was to determine the levels of specific IgA against Pseudomonas aeruginosa in sputum samples from severe COPD patients in their stable periods, either non-colonized or colonized by this PPM, in order to assess the relationships between the bronchial immunoglobulin defense barrier and chronic colonization by P. aeruginosa in these patients.
Methods
Ethics approval and consent to participate
The study was reviewed and approved by the Comité Etic d’Investigació Clínica de l’Hospital Universitari de Bellvitge (HUB). Sputum samples and bacterial strains were recorded in an anonymized database. Written informed consents were collected from patients and controls according to the HUB ethics committee requirements.
Design, patients, and definitions
A cross-sectional analysis of the bronchial IgA-mediated immune response against P. aeruginosa in severe COPD patients was performed. Participants were selected from a previously described hospital-based prospective cohort regularly attending an outpatient respiratory clinic for scheduled and exacerbation visits.10 Patients with good-quality sputum samples during stability were included and classified according to the presence of chronic bronchial colonization by P. aeruginosa, which was diagnosed when this PPM was recovered from the sputum sample studied and in two or more additional samples in the interval of a year.
Clinical and functional variables
Demographic and clinical data were recorded at baseline. Patients answered an epidemiologic questionnaire that covered smoking habits, respiratory symptoms, and previous exacerbations. Functional characterization included forced spirometry and reversibility testing.11 Forced vital capacity and forced expiratory volume in 1 second (FEV1) were measured with the same dry rolling seal spirometer (Sibelmed, Sibelgroup, Barcelona, Spain) and expressed as absolute values (mL) and percentages of the reference values obtained from age- and height-adjusted selected volunteers from the Barcelona area.12 COPD was diagnosed in accordance with the criteria of the Global Initiative for Chronic Obstructive Lung Disease (GOLD).15
Sputum collection and microbiology processing
Spontaneous sputum samples were collected from each patient in stability. Only good-quality sputum samples were considered for the study.13 Quantitative cultures and PCR detection of atypical bacteria were performed, as previously described.10 For the measurement of specific immunity against P. aeruginosa, the sputum supernatant was separated after dilution of the sputum with 1/10 dilution of dithiothreitol (Sputasol, Oxoid; Thermo Fisher Scientific, Waltham, MA, USA) and neutralization with phosphate-buffered saline (PBS).
Specific immunity against P. aeruginosa in sputum
ELISA was used to determine the IgA antibody level against P. aeruginosa in the supernatant of the sputum samples recovered. The capture antigen was prepared as follows: 10 clinical isolates (5 mucoid and 5 non-mucoid) were grown overnight on Tryptone Soy Agar (TSA) plates (Oxoid) at 37°C and 5% CO2. These P. aeruginosa strains were colonizing strains isolated from respiratory patients attending a chest outpatient clinic. Several colonies were inoculated to brain heart infusion (BHI) broth (Oxoid) and grown until logarithmic (log) phase. Bacterial concentration was adjusted to optical density (OD) 0.5–0.6 at a wavelength of 600 nm (~1×106 cfu/mL), and the ten isolates were mixed to obtain a suspension of 1×106 cfu/mL. Fifty microliters of this P. aeruginosa suspension was used to coat each well on a microtiter plate (Corning, NY, USA) and were incubated overnight at 37°C.
Between each of the following steps, the wells were washed four times with PBS (400 μL/well). After washing, the wells were blocked by the addition of 200 μL of PBS-1% bovine serum albumin (BSA) (Sigma-Aldrich Co., St Louis, MO, USA) and incubated at 37°C for 2 hours. The wells were then washed, and sputum supernatants diluted in PBS were added and incubated for 1 hour at 37°C. After washing, horseradish peroxidase-conjugated mouse-anti human IgA antibody (Abcam, Cambridge, UK) diluted 1:1000 in PBS-1% BSA was added and incubated for 1 hour at 37°C. Following another washing, 50 μL of 3,3′,5,5′-tetramethylbenzidine substrate (Sigma-Aldrich Co.) was added to the wells, and after 30 minutes, the reaction was stopped with 50 μL of H2SO4 1M and the OD was read at 450 nm. All samples were run in triplicate, and the final result was the average of the scores.
Specific IgA determinations were performed in the supernatant of sputum of all patients studied and in a sputum supernatant pool of 10 healthy controls from the general population who were used as the reference. Results were expressed as the OD ratio between patients and the healthy control reference (OD patients/OD pool of healthy controls).
Statistical analysis
Data were analyzed using the SPSS statistical software package version 19 (IBM Corporation, Armonk, NY, USA). Results for categorical variables are expressed as absolute and relative frequencies and results for continuous variables as means and standard deviations (SD) or as medians and interquartile ranges (IQR) when the distribution was not normal.
Patients included in the study were categorized as non-colonized and chronically colonized by P. aeruginosa. The OD of specific IgA against P. aeruginosa (PA-IgA) in patients in non-colonized and colonized patients, expressed as a ratio of the OD in healthy subjects, was calculated and used for the comparison of non-colonized and colonized severe COPD.
All analyses were performed using chi-square, Fisher exact, or Mann–Whitney U tests as required. Statistical tests were two-sided, and a p-value ≤0.05 was reported as statistically significant.
Results
Patient characteristics
The original cohort consisted of 111 severe COPD patients who were followed for a minimum of 1 year after enrolment and reported one or more exacerbations during this period.10 Good-quality sputum samples obtained from a stability period were available from 36 participants in the cohort, who were the target for this study. This population sample had a mean age of 70.5 (SD 7) years and severe COPD (mean FEV1 36 [SD 13] % of the predicted value, range 25–45.0) (Table 1). Sputum cultures obtained during stability were negative for P. aeruginosa in 21 patients and positive for this PPM in the other 15.
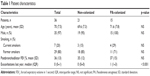  | Table 1 Patient characteristics |
Specific IgA against P. aeruginosa in sputum
The levels of PA-IgA in non-colonized patients, expressed as a ratio of the value in healthy controls, were below 1 (median =0.15 [0.05–0.36]) (Figure 1) and confirm the low level of specific IgA against this microorganism in severe COPD patients. Specific PA-IgA levels were significantly higher in colonized than in non-colonized patients (1.56 [IQR 0.59–2.78] vs 0.15 [0.05–0.36], p<0.001, Mann–Whitney U test) and attained the levels found in healthy subjects, without reaching higher figures.
Discussion
In this study, specific PA-IgA levels in severe COPD were below the reference values and increased to the levels found in healthy subjects only when P. aeruginosa was recovered from bronchial secretions. Bronchial colonization by P. aeruginosa was not able to stimulate a higher specific response in these patients. These results suggest that the production of specific IgA against P. aeruginosa is similarly impaired in non-colonized and colonized COPD patients with advanced disease, given that they do not attain levels higher than the non-colonized healthy population when they chronically harbor P. aeruginosa in the bronchial tree.
The blunted IgA response of the bronchial mucosa in severe COPD patients shown in our study may favor chronic colonization and recurrent infections. Dimeric IgA is produced in subepithelial plasma cells, bound to pIgR, and transported to the apical surface of epithelial cells, where it is cleaved to form SIgA. Several studies have found a decreased expression of pIgR in COPD, which leads to a SIgA deficiency in the airway mucosa5,7,8 which is only partially compensated by the direct secretion of SIgA by submucosal glands.2 Furthermore, the SIgA in the bronchial lumen is often trapped within mucus plugs in COPD, which disrupt its normal distribution along the mucosal surface and prevent it from reaching its expected protective functionality.2 The low levels of specific IgA against P. aeruginosa found in our study confirm the defective production and/or transport of specific IgA against common respiratory pathogens in patients with severe COPD, whose levels of specific IgA only approach those of healthy subjects when colonized by this PPM, and even then are unable to surpass these levels.
Severe COPD patients colonized by P. aeruginosa in our study showed higher levels of specific PA-IgA than non-colonized patients. Nonetheless, in spite of the bronchial colonization, they did not attain figures above healthy controls. Therefore, our results confirm that the presence of P. aeruginosa in the bronchial tree is able to increase the production of specific PA-IgA in severe COPD patients, but only to levels equivalent to those in non-colonized healthy subjects, probably not enough to efficiently remove the PPM from the airways in chronically colonized patients. A previous study focusing on specific IgA against H. influenzae (HI-IgA) in moderate COPD patients obtained different results, with higher levels of this immunoglobulin in bronchial secretions of non-colonized COPD patients than in healthy controls.9 Another recent study14 also found higher levels of HI-IgA in COPD patients with early disease than in healthy controls. These differences in the specific IgA response may point to a specific blunted response against P. aeruginosa in COPD which favors chronic colonization by this microorganism but may also suggest that abnormal IgA responses are restricted to severe disease, since the studies that reported high IgA levels against H. influenzae had not included patients with advanced disease. In mild and moderate COPD, SIgA secreted by both epithelial cells and submucosal glands maintains the level of total luminal SIgA; in severe COPD, however, in which there is a profound surface SIgA deficiency, the total luminal content of SIgA decreases, as has been shown in bronchoalveolar lavage samples.2
The present study has some limitations that should be taken into account. The main limitation is the small sample size. We obtained good-quality sputum samples from only 36 COPD patients, and 15 of them were colonized by P. aeruginosa. The other limitation is that we only included severe or very severe COPD patients so we were not able to analyze the relationship between FEV1 and IgA levels. Therefore, future studies with larger COPD cohorts including patients with moderate disease are needed to confirm our results and its validity in patients with early stage COPD.
Conclusion
The specific immune response of the bronchial mucosa against P. aeruginosa is impaired in severe COPD patients, as shown by their abnormally low levels of specific IgA. Specific PA-IgA levels increase in colonized patients without attaining levels above those of healthy subjects. These findings confirm a blunted response to P. aeruginosa in severe COPD, which may favor chronic colonization and recurrent infections by this PPMs.
Acknowledgments
We thank Michael Maudsley for providing an outline for this manuscript and support in editing and journal styling.
This work has been funded by Fundació Catalana de Neumología (FUCAP), FIS 15/00157, and Centro de Investigación Biomédica en Red de Enfermedades Respiratorias (CIBERES), CB06/06/0037 and CB06/06/1089. CIBERES is an initiative of the Instituto de Salud Carlos III. Financial support was also provided by the European Regional Development Fund (ERDF) and CERCA Programme/Generalitat de Catalunya.
Author contributions
LM and EM conceived and designed the experiments and wrote the paper. LM performed the experiments and analyzed the data. SM, CA, JL, SS, JD, MGN, and SQ provided samples and contributed reagents/materials/analysis tools. All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Pilette C, Durham SR, Vaerman J-P, Sibille Y. Mucosal immunity in asthma and chronic obstructive pulmonary disease: a role for immunoglobulin A? Proc Am Thorac Soc. 2004;1(2):125–135. | ||
Du R-H, Richmond BW, Blackwell TS, et al. Secretory IgA from submucosal glands does not compensate for its airway surface deficiency in chronic obstructive pulmonary disease. Virchows Arch Int J Pathol. 2015;467(6):657–665. | ||
Corthésy B. Multi-faceted functions of secretory IgA at mucosal surfaces. Front Immunol. 2013;4:185. | ||
Ladjemi MZ, Lecocq M, Weynand B, et al. Increased IgA production by B-cells in COPD via lung epithelial interleukin-6 and TACI pathways. Eur Respir J. 2015;45(4):980–993. | ||
Gohy ST, Detry BR, Lecocq M, et al. Polymeric immunoglobulin receptor down-regulation in chronic obstructive pulmonary disease. Persistence in the cultured epithelium and role of transforming growth factor-β. Am J Respir Crit Care Med. 2014;190(5):509–521. | ||
Richmond BW, Brucker RM, Han W, et al. Airway bacteria drive a progressive COPD-like phenotype in mice with polymeric immunoglobulin receptor deficiency. Nat Commun. 2016;7:11240. | ||
Pilette C, Godding V, Kiss R, et al. Reduced epithelial expression of secretory component in small airways correlates with airflow obstruction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163(1):185–194. | ||
Polosukhin VV, Cates JM, Lawson WE, et al. Bronchial secretory immunoglobulin a deficiency correlates with airway inflammation and progression of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;184(3):317–327. | ||
Millares L, Marin A, Garcia-Aymerich J, et al. Specific IgA and metalloproteinase activity in bronchial secretions from stable chronic obstructive pulmonary disease patients colonized by Haemophilus influenzae. Respir Res. 2012;13:113. | ||
Domenech A, Puig C, Martí S, et al. Infectious etiology of acute exacerbations in severe COPD patients. J Infect. 2013;67(6):516–523. | ||
Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152(3):1107–1136. | ||
Roca J, Sanchis J, Agusti-Vidal A, et al. Spirometric reference values from a Mediterranean population. Bull Eur Physiopathol Respir. 1986;22(3):217–224. | ||
Murray PR, Washington JA. Microscopic and baceriologic analysis of expectorated sputum. Mayo Clin Proc Mayo Clin. 1975;50(6):339–344. | ||
Staples KJ, Taylor S, Thomas S, et al. Relationships between mucosal antibodies, non-typeable Haemophilus influenzae (NTHi) infection and airway inflammation in COPD. PLoS One. 2016;11(11):e0167250. | ||
Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for Diagnosis, Management, and Prevention of COPD – 2016. Available from: http://goldcopd.org/global-strategy-diagnosis-management-prevention-copd-2016/. Accessed July 3, 2017. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

