Back to Journals » Cancer Management and Research » Volume 12
Spatial Variation in Lung Cancer Mortality and Related Men–Women Disparities in Iran from 2011 to 2014
Authors Ghasemi S , Mahaki B , Dreassi E , Aghamohammadi S
Received 24 January 2020
Accepted for publication 3 June 2020
Published 17 June 2020 Volume 2020:12 Pages 4615—4624
DOI https://doi.org/10.2147/CMAR.S247178
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eileen O'Reilly
Shadi Ghasemi,1 Behzad Mahaki,2 Emanuela Dreassi,3 Saeedeh Aghamohammadi4
1Student Research Committee, Department of Biostatistics and Epidemiology, School of Health, Isfahan University of Medical Sciences, Isfahan, Iran; 2Department of Biostatistics, Kermanshah University of Medical Sciences, Kermanshah, Iran; 3Department of Statistics, Computer Science, Applications (DiSIA), University of Florence, Florence, Italy; 4Iranian Ministry of Health and Medical Education, Tehran, Iran
Correspondence: Behzad Mahaki Tel +98 09128077960
Email [email protected]
Purpose: Lung cancer is considered as a common cause of cancer mortality. The disease represents the second and third causes of deaths from cancer among Iranian women and men, respectively. The present study aimed to evaluate the spatial variations in relative risk of lung cancer mortality in Iran and its relation to common risk factors between men and women and specific risk factors among women.
Methods: In this ecological study, the lung cancer mortality data were analyzed in Iran during 2011– 2014. Besag, York, and Mollie’s (BYM) model and shared component model (SCM) were used to compare the spatial variations of the relative risk of lung cancer mortality by applying OpenBUGS version 3.2.3 and R version 3.6.1.
Results: The median age for death due to lung cancer in Iran is 74 years. During 2011– 2014, the age-standardized lung cancer mortality rates among men and women were 12 and 5 per 100,000 individuals, respectively. In addition, almost similar spatial patterns were observed for both men and women. Further, risk factors, which are shared between men and women, were considered as the main cause of variation of lung cancer mortality relative risk in the regions under study for both men and women. The highest impact of the women-specific risk factors was estimated in northeastern and southwestern of the country while the lowest was related to Gilan province in northern part of Iran.
Conclusion: Based on the spatial pattern, lung cancer risk factors are at relatively high levels in most parts of Iran, especially in the northwest of the country. Regarding the women, the high-risk regions were considerably extended. Further, the highest concentration of the specific risk factors among women was observed in the eastern, central, and southwestern parts. The smoking effect, and the second-smoking effect and environmental pollutions could play more significant roles for men and women, respectively.
Keywords: ecological study, age-standardized mortality rate, BYM model, shared component model
Introduction
Lung cancer is regarded as a common cause of cancer mortality,1 and its rates of incidence and mortality are very close to one another by considering its very low survival rate.2 The lung cancer is the second reason for cancer mortality following the gastric cancer in Iran. According to the reports by Iranian Ministry of Health and Medical Education, the lung cancer represents the second and third cause of cancer mortality among Iranian men and women, respectively.3
The lung cancer incidence and mortality rates are different around the world, among different countries, and even within the same country.4 The main reason behind such variations is related to the difference in the prevalence of cigarette smoking.2,5,6 Smoking has been reported as the most significant risk factor of the lung cancer, being responsible for about 80% of the lung cancer incidence cases.4 In addition, genetic factors and environmental pollutions (eg air pollution, radon, and asbestos) have been referred to as other risk factors contributing to the lung cancer.2,5,6
In many regions around the world, the rate of lung cancer mortality among men is higher than that of women, mainly due to the wider extension of smoking among men rather than women.5,7 However, the lung cancer has been served as the second largest cause of cancer mortality among women in the world, with about half of the lung cancer incidence being caused by smoking.6
Although the number of male smokers is about six times as large as the number of female smokers in Iran,8 the age-adjusted rate of lung cancer mortality among Iranian men in 2012 has been only about 2 times as large as that of Iranian women,9 among whom only one out of each seven women fatalities from lung cancer is smoker.7 In general, the rate of lung cancer incidence among non-smoking women is higher than that of non-smoking men.5,10 In other words, women are engaged with special risk factors tending to increase lung cancer mortality.5
Generally speaking, spatial variations in lung cancer mortality should be considered as a necessary step toward attenuating the lung cancer incidence rate for both genders in order to identify high-risk areas and determine the priorities for research activities and the required policy settings.11 Besag, York, and Mollie’s (BYM)12 model is regarded as one of the most widely used models for mapping and identifying high-risk areas in which the correlation of the data among neighboring and geographical close areas is considered. On the other hand, based on the above-mentioned issues, Iranian men and women may suffer from different risk factors contributing to the lung cancer. Therefore, it seems necessary to identify those regions of Iran where men and women are engaged with common risk factors and identify the regions where women are exposed to excessive risk of the disease. Special disease mapping models can be used to identify the pattern of spatial variations in common and specific risk factors among men and women, among which Bayesian shared component modeling (SCM) can be emphasized.13,14 Several studies used this model to evaluate the common spatial variations of the multi-diseases15–17 or a single disease based on different variables such as gender13 or race.18
Common and specific risk factors among men and women in terms of latent variables are evaluated by using the SCM model in order to observe their spatial variations separately for different genders. In this model, the shared component represents the risk factors contributing to higher risk of mortality in both genders. On the other hand, the specific component represents the risk factors which just increase the risk of mortality in a specific gender.13
Although lung cancer is considered as one of the cancers with high mortality rates in Iran, no study, to the best of our knowledge, addressed the differences in spatial pattern of lung cancer mortality in both men and women in Iran. Thus, the present study aimed to evaluate the spatial variations in relative risk of lung cancer mortality in Iran and its relation to common risk factors between men and women and specific risk factors among women. To this aim, the BYM model was used to assess the spatial variations in the relative risk of lung cancer mortality. In addition, the Bayesian SCM was used to study the spatial pattern of common risk factors between men and women, as well as specific risk factors among women.
Materials and Methods
Data
In this ecological study, the lung cancer (topography code C33-C34) mortality data were analyzed among the Iranian men and women in Iran during 2011–2014. The data were collected by Network Management Center of Iranian Ministry of Health.19–21 The region under study included 30 provinces from the total 31 provinces of Iran excluding Tehran. The data included the count of deaths from lung cancer in different years and were classified based on province, gender, and age. This study was confirmed by the Ethical Committee of Isfahan University of Medical Sciences (IR.MUI.REC.1395.3.687).
First, the yearly age-standardized rates of lung cancer mortality were calculated for each gender. Then, the rates were adjusted based on the standard global population as per the World Health organization (WHO).22 Subsequently, the data corresponding to different years from 2011 to 2014 were pooled and gender-specific deaths were computed from lung cancer among the 20 or older individuals in each province.
Statistical Analysis
Let yik indicate the number of observed deaths from lung cancer among 20 or older residents of the i-th province (i = 1, 2, …, 30) and k-th gender (k = 1 for men, 2 for women) with a Poisson’s probability distribution function (PDF) with the parameter (Eik θik) where θik represents the relative risk in i-th province, and k-th gender and Eik indicate the expected cases.
(1)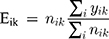
where ni1 and ni2 are the populations of men and women who are at risk in the i-th region, respectively.
BYM Model
In this stage, the model proposed by Besag, York and Mollie (BYM)12 was used to fit models for each gender. In this model, it is assumed that θik is a random variable. Thus, we have:
(2)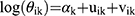
where αk is a gender-specific intercept. In both genders, ui indicates the random effect representing the correlation between the neighbouring provinces and vi shows the random effect included in the model to represent the unstructured heterogeneity in the relative risk of lung cancer mortality among the provinces.
It is assumed that the heterogeneity terms vik are independent and each vik follows normal distribution (0, λvk), while the terms uik follow some conditional autoregressive (CAR) normal distribution (W, λuk) to include the spatial correlation of the data, where W is the matrix representation of the spatial correlation and is obtained in relation to the adjacent provinces of the considered province. The adjacency is herein defined as having at least one common border. In addition, the parameters λvk and λuk are precision parameters indicating the levels of non-structured and structured spatial variations, respectively.
Shared Component Model (SCM)
When the BYM was adopted for each of the genders, the shared component modeling (SCM) was utilized to evaluate the variations in the lung cancer mortality in both males and females. Originally developed by Knorr-Held and Best,14 the SCM was used to introduce a shared random effect at province level to consider the relationships between the mortality rate among men and women. Further, an independent random effect was introduced for women to evaluate the gender-specific variations. The women-specific component reflects the inter-provincial variations in lung cancer mortality in women, ie variations beyond the common variations in men and women.
Like the previous case, it is assumed that the observations follow the Poisson’s distribution function is as follows:
(3)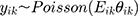
Regarding the second level, it is assumed that
(4) (5)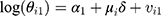
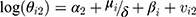
where μi represents the common risk factors among men and women, among which smoking is the most important one, and βi represents the women-specific risk factors which are the root cause of the difference between the corresponding spatial pattern and that of men. Both of these variables have spatial structures and it is assumed that those follow CAR Normal (W, λμ) and CAR Normal (W, λβ), respectively, where the λμ and λβ are considered as precision parameters. Furthermore, the parameters α1, α2, vi1, and vi2 are defined similarly to those in the BYM model.
Unfortunately, most of the dataset in the Iranian Ministry of Health and Medical Education like the one used in the present study is not available to researchers. In this case, using random variables as hidden variables in the model is considered as one of the ways to consider some of these variables which play the role of common or non-common risk factors between two or more diseases or between men and women such as smoking status, BMI, characteristics of the environment in which the individual lives and the like. In fact, the random variables μi and βi used in the model are successors for known or unknown risk factors. In this equation, δ is the scaling factor and its logarithm is assumed to follow normal distribution. In both models, the precision parameters are supposed to follow the Gamma (0.5, 0.0005) distribution function23 and both of α parameters are assumed to follow flat non-informative distribution.
Version 3.2.3 of OpenBUGS (The GNU General Public License) software and Markov chain Monte Carlo (MCMC) methods were used to obtain posterior probability distributions and approximation of the considered parameters. Based on this approach, the first 200,000 iterations were dropped, and a total of 2800 samples of the subsequent 224,000 iterations were stored at the spacing of 80 iterations. The convergence of the algorithm was verified and confirmed by Gelman-Robin test.24 In addition, the model fit was calculated by using deviance information criterion (DIC), which is appropriate for use with Bayesian hierarchical models. The lower DIC value indicates better model fit.25 The DIC value of the SCM was compared to the sum of the DIC values from the two individual BYM models. Finally, the version 3.6.1 of R software was used to plot the maps and diagrams.
Results
During the considered period, a total of 16,528 deaths from lung cancer were recorded across the considered regions, among whom 70% were men with 74 as the median age of death. Further, 12% were younger than 60, 53% ranged between 60 and 79, and 35% were older than 79. In 2014, the maximum age-specific rate of death from lung cancer among Iranian men and women was 136 and 69 cases per 100,000 individuals in the age range of 80–84, respectively (Figure 1). As shown in Figure 1, the slope of the diagram related to standardized lung cancer mortality rate in the population of Iran increases rapidly after the age of 50. Additionally, lung cancer mortality rate in the age range of 70–74 and 80–84 is 5 and 11 times more compared to that of 50–54, respectively. In all of the age groups beyond 40, the rate of lung cancer mortality was considerably higher among men rather than women and the average mortality rate for age-standardized lung cancer among men and women was 12 and 5 per 100,000 individuals, respectively (Table 1).
 |
Table 1 Age-Standardized Mortality Rate of Lung Cancer by Gender in Iran During 2011–2014 |
 |
Figure 1 Age-specific mortality rate based on gender in Iran during 2014. |
The results of the BYM model indicated almost similar spatial patterns for men and women, especially in northwestern and southeastern regions of the country. Accordingly, the relative risk of death from lung cancer in men varied from 0.34 in Sistan and Baluchestan (southeastern Iran) to 1.79 in West Azerbaijan (northwestern Iran). Furthermore, regarding women, the lowest rate related to the risk of lung cancer mortality (0.41) belonged to Sistan and Baluchestan, while the women in East Azerbaijan (northwestern Iran) were at the highest relative risk (1.68) of lung cancer mortality. Figure 2 and Table 1 indicate the results obtained from the BYM. As shown, there is correlation and spatial patterns among neighboring and geographical close areas in both males and females. In addition, the maps plotted for men and women (Figure 2) indicate a common spatial pattern suggesting high mortality rates in northwestern areas of Iran and very low mortality rates in southeastern part of the country. Also, in Table 2 identified high and low risk Iranian provinces for both men and women.
 |
Table 2 Relative Risk of Lung Cancer Mortality in Iranian Provinces |
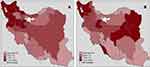 |
Figure 2 Map of the estimated relative risk in the BYM Model for lung cancer mortality in men (A) and women (B) in Iran during 2011–2014. Abbreviations: BYM Model, Besag, York, and Mollie’s model. |
Finally, Figure 3 displays the results of the SCM. Based on this mothed, 90.4% of the spatial variations in lung cancer mortality among men can be explained by the shared components, while the corresponding percentage to the women is 78.5%. The remaining portion of variations in women is related to the gender-specific component, among which 13.6% and 7.9% are related to specific unstructured and structured spatial variations, respectively. The largest portion of spatial variations in both males and females is explained by the shared component, the effect of which is the largest in the northwestern part of the country. In other words, in both genders, common risk factors of men and women are the main cause of variation in lung cancer mortality relative risk in the regions under study, while a small portion is related to uncommon risk factors. The posterior mean of the scaling parameter δ was estimated at 1.02 (95% confidence interval: 0.83, 1.16). Therefore, the effect of common risk factors on lung cancer mortality in men is slightly greater than that of women. Given the pattern of spatial variations in the women-specific risk factors, the highest intensity of this factor was observed in South Khorasan (northwestern Iran) and Bushehr (southern Iran) Provinces, while the lowest was estimated in Gilan Province (northern Iran). In addition, the gender-specific factor could be used to classify the country into low-risk and high-risk regions. The sum of the DIC values from the two BYM models and the DIC value from the SCM are 527 and 487.1, respectively, indicating the superiority of the SCM over their modeling individually.
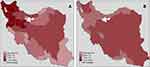 |
Figure 3 Shared risk in lung cancer mortality among men and women (A) and specific risk for women (B) from SCM in Iran during 2011–2014. Abbreviation: SCM, shared component model. |
Discussion
The highest percentage of lung cancer mortality was observed among the 60–79-aged individuals. In addition, an increasing trend of age-specific lung cancer mortality rate was observed among both men and women during 2011–2014, while the rate of this increasing trend was slightly higher among men rather than women. In 2014, age-standardized lung cancer mortality rates among men and women were 12.2 and 5.3 per 100,000 individuals, which are 1.6 times and 2.5 times higher than in 2002,26 respectively. Based on the obtained information, the gender ratio of the lung cancer mortality in Iran is lower than some special Asian countries such as Turkey and Kuwait, while it is still higher than many other Asian states such as China, Japan, Thailand, and India.2 The ratio (2.4) was slightly less than the global estimated value (2.72).27
Regarding the age-standardized rates of the 20 or older Iranian men and women, a significant percentage of deaths from lung cancer occurred in the age group of 20 years and above, and they were considered as the main at-risk populations.
The present study assessed the relative risk of lung cancer mortality among the 20 or older men and women residing in different Iranian provinces. Relatively strong spatial patterns were observed in both genders. Regarding men, the highest relative risk of the disease was related to the northwestern region of the country, with extensions toward the north and west. Further, high levels of risk were observed in the eastern area of the country toward the central Iran. Regarding women, the high-risk regions such as northwestern, western, central, and parts of eastern areas of Iran (RR>1.2) were extended more. Furthermore, high levels of relative risk were observed in Bushehr province (southwestern Iran). Given the plotted maps, a common spatial pattern was evident, while high mortality rates were anticipated in northwest of Iran and very low rates were observed in southeastern part of the country (Figure 2).
In the next step, the relationship between the mortality rates in men and women was evaluated by identifying the spatial pattern of common risk factors, among which cigarette smoking is considered as the most significant one. Further, given the findings of the previous studies confirming the presence of women-specific risk factors and the larger contribution of environmental pollution into lung cancer mortality among women rather than men, a specific component was used to recognize the spatial pattern of women-specific risk factors. Subsequently, a major portion of spatial variations in the relative risk of lung cancer among men and women could be explained by the shared component by fitting the model into the data. The impact of the shared component of lung cancer mortality among men and women was maximized in the northwest of Iran, with the extensions toward west, and central and eastern parts of the country at lower intensities. However, the highest concentration of the women-specific risk factors was observed in the eastern, central, and southwestern parts, with the extensions toward southeast. Additionally, as shown in Figure 3, the effect of this risk factor is relatively high in other parts of Iran.
It is worth noting that the prevalence of cigarette smoking among men is six times more than that of women in Iran,28 and the lung cancer mortality ratio among men and women is expected to be very large since the smoking is considered among the major factors contributing to the lung cancer. However, the ratio is just above 2. On the other hand, a large portion of variations in lung cancer mortality among women can be explained by the shared component, which can be related to one of the following reasons:
- Iranian women tend to conceal their cigarette smoking habit due to cultural constraints in the Iranian society, upon which the cigarette smoking is an extremely unfavorable act for women, which may lead to the underestimation of the prevalence of cigarette smoking among women.8
- The number of women smoking water pipe is almost 7 times as large as the number of those smoking cigarettes.29 On the other hand, in some parts of the country, smoking water pipe by a woman is considered as a culturally accepted act.29 For instance, Bushehr Province, located in southwest of Iran, suffers from the higher risk of lung cancer mortality among women, compared to the adjacent provinces, possibly due to the spread of smoking water pipe among the women in this province, compared to other provinces in Iran.30
- In those areas where more prevalence of cigarette smoking is common among men, the rate of lung cancer mortality among women is higher due to the so-called second-smoking effect.
- The areas of higher shared risk are likely to suffer from higher levels of environmental pollution, which may boost the rate of lung cancer mortality in these areas in two ways. First, tobacco smoking and environmental pollution have interaction effects on lung cancer mortality,5,31 which means that the presence of one of them can increase the negative impact of the other one. Second, the environmental pollutions contribute to the rate of lung cancer mortality among non-smoking individuals, especially women.7,32
There are some differences between the population in Iran and that of the developed countries regarding some issues such as the epidemiology of molecular alterations in the genes involved in cancer immunosurveillance, xenobiotic metabolism, and DNA repair.33 These differences are related to unique environmental exposures and population genetics of the Iranian populace.34 Also, the unique environmental exposures in the different areas of Iran and genetic diversity and difference in different Iranian ethnic groups can be considered as one of the important reasons for the expansion and variations observed in the RR of lung cancer mortality in these areas. Studying these issues can abundantly help to prevent lung cancer, develop targeted therapeutic approaches, and improve patients’ survival.35 However, a few studies were conducted in this area in Iran.33
Based on the results, the highest rates of lung cancer mortality among men and women were observed in the age groups of 80–84 (Figure 1). In addition, the smoking age among Iranian men was considerably younger than that their Iranian counterparts. Further, the maximum prevalence of cigarette smoking among the men occurred in the age group of 35–44, while it was 55–65 for the same range among the women.8 The incidence of lung cancer because of cigarette smoking usually occurs after several decades of smoking, which can be served as a preferential factor contributing to the increased rate of lung cancer mortality among older men.4 However, the second-smoking effect and environmental pollutions may play more significant roles in Iranian women. Additionally, the reduction in the rate of lung cancer mortality in older ages can be related to competitive risks in both males and females.4
During the last few decades, the mortality of lung cancer, especially non-small cell lung cancer, which is the most common type of this cancer,36 has increased among the elderly,37 while few studies were conducted among this group of individuals.38 Although there are potential therapies and approaches of decreasing the rate of lung cancer progression for elderly patients,39 to the best of our knowledge, there is no study in this area in Iran.
Mahaki et al40 used lung cancer data in 2007, irrespective of gender factor. Like the data used in the present study, they focused on north western regions as the areas with the highest risk while the south eastern regions indicated the lowest risk. A significant difference was observed in the pattern of the present model, which could be attributed to various factors, among which the change in risk factors during 2007–2014 was highlighted. It is worth noting that the extent of high-risk areas in the present study is higher than that of Mahaki et al’s study.
In another study, Khazaei et al41 evaluated the geographic distribution for age-standardized incidence rate (ASR) of lung cancer in Iran, the results of which are inconsistent with those in the present study. The reasons for this difference can be summarized as follows. First, they used data from the incidence of lung cancer in Iran in 2008 and presented their results without using a model and considering the spatial correlation between the provinces. Second, all ages were included in their study, while only the data related to the age group above 20 years, who were at higher risk, were considered for analysis in this study.
The main advantage of the present study can be found in a separate use of common component model for men and women. Separate analysis can determine the spatial pattern of risk factors better. Further, the application of more recent data and consideration of adults above 20 years old, as the main people exposed to risk factors, can present more accurate estimations.
Lack of access to the required information in Tehran Province is considered as the main limitation in the present study. Thus, the data were analyzed for other Iranian provinces without considering the adjacency of Tehran to the other provinces.
Conclusion
In general, the rate of lung cancer mortality has almost doubled in n Iran during 2002–2014.26 The increase can be represented as an alarm indicating boosted impact of the risk factors, especially among women. The smoking effect among men and the second-smoking effect and environmental pollutions among women can play significant roles in this regard. Failure to identify and control such risk factors may end up with increasing trends in not only the rate of lung cancer mortality, but also the rate deaths from other cancers, which have common risk factors with lung cancer.
Based on the spatial pattern obtained in this study, the lung cancer risk factors are at relatively high levels in most parts of Iran, particularly in northwestern areas. In the present study, those areas in which lung cancer mortality risk factors have a high prevalence were considered. Identifying these areas can be helpful in setting health policies, as well as focusing on high-risk areas or in examining the underlying causes of a lower risk of lung cancer death in one region or among male or female.
Acknowledgments
The authors are grateful to express their sincere thanks to the department of information and statistics network of Education Medical and Health, Iran. This article was supported by a grant [395687] from Isfahan University of Medical Sciences, Isfahan, Iran.
Disclosure
The authors of this article declare that they have no conflict of interests.
References
1. Fitzmaurice C, Dicker D, Pain A, et al. The global burden of cancer 2013. JAMA oncol. 2015;1(4):505–527. doi:10.1001/jamaoncol.2015.0735
2. Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends: an update. Cancer Epidemiol Biomarkers Prev. 2016;25(1):16–27. doi:10.1158/1055-9965.EPI-15-0578
3. Khosravi A, Aghamohammadi S, Kazemi E. Mortality Profile in the Islamic Republic of Iran 2015 (20 Leading Cause of Death by Sex and Age Group), Vol. 3. Education MoHaM, ed. Tehran, Iran: 2015.
4. Torre LA, Siegel RL, Jemal A. Lung cancer statistics. In: Ahmad A, Gadgeel SM, editors. Lung Cancer and Personalized Medicine: Current Knowledge and Therapies. New York: Springer; 2016:1–19.
5. de Groot PM, Wu CC, Carter BW, Munden RF. The epidemiology of lung cancer. Transl Lung Cancer Res. 2018;7(3):220–233. doi:10.21037/tlcr.2018.05.06
6. Torre LA, Islami F, Siegel RL, Ward EM, Jemal A. Global cancer in women: burden and trends. Cancer Epidemiol Biomarkers Prev. 2017;26(4):444–457. doi:10.1158/1055-9965.EPI-16-0858
7. Hosseini M, Naghan PA, Karimi S, et al. Environmental risk factors for lung cancer in Iran: a case–control study. Int J Epidemiol. 2009;38(4):989–996. doi:10.1093/ije/dyp218
8. Moosazadeh M, Ziaaddini H, Mirzazadeh A, Ashrafi Asgarabad A, Haghdoost AA. Meta-analysis of smoking prevalence in Iran. Addiction Health. 2013;5(3–4):140–153.
9. Mohebbi E, Nahvijou A, Hadji M, et al. Iran cancer statistics in 2012 and projection of cancer incidence by 2035. Basic Clin Cancer Res. 2017;9(3):3–22.
10. Hosseini M, Alinaghi SAS, Naghan PA, et al. A clinicopathologic study of lung cancer cases in Iran. Tanaffos. 2009;8(3):36.
11. Shibuya K, Mathers CD, Boschi-Pinto C, Lopez AD, Murray CJ. Global and regional estimates of cancer mortality and incidence by site: II. Results for the global burden of disease 2000. BMC Cancer. 2002;2(1):37. doi:10.1186/1471-2407-2-37
12. Besag J, York J, Mollié A. Bayesian image restoration with two applications in spatial statistics. Ann Inst Stat Math. 1991;43(1):1–59. doi:10.1007/BF00116466
13. Ibáñez-Beroiz B, Librero-López J, Peiró-Moreno S, Bernal-Delgado E. Shared component modelling as an alternative to assess geographical variations in medical practice: gender inequalities in hospital admissions for chronic diseases. BMC Med Res Methodol. 2011;11(1):172. doi:10.1186/1471-2288-11-172
14. Knorr-Held L, Best NG. A shared component model for detecting joint and selective clustering of two diseases. J R Stat Soc. 2001;164(1):73–85. doi:10.1111/1467-985X.00187
15. Best N, Hansell AL. Geographic variations in risk: adjusting for unmeasured confounders through joint modeling of multiple diseases. Epidemiology. 2009;20(3):400–410. doi:10.1097/EDE.0b013e31819d90f9
16. Mahaki B, Mehrabi Y, Kavousi A, Schmid VJ. Joint spatio-temporal shared component model with an application in Iran cancer data. Asian Pac J Cancer Prev. 2018;19(6):1553.
17. Raei M, Schmid VJ, Mahaki B. Bivariate spatiotemporal disease mapping of cancer of the breast and cervix uteri among Iranian women. Geospat Health. 2018;13:1. doi:10.4081/gh.2018.645
18. Rossen LM, Khan D, Schoendorf KC. Mapping geographic variation in infant mortality and related Black–White disparities in the US. Epidemiology. 2016;27(5):690–696. doi:10.1097/EDE.0000000000000509
19. Aghamohamadi S, Khosravi A, Kazemi E, Shariati M. Mortality Profile in the Islamic Republic of Iran from 2012 to 2015. Tehran: Ministry of Health and Medical Education; 2018.
20. Khosravi A, Aghamohamadi S, Kazemi E. Mortality Profile in Iran (30 Provinces) in 2011. Tehran: Ministry of Health and Medical Education; 2015.
21. Khosravi A, Aghamohamadi S, Kazemi E, Azizollah A, Vasegh H. Mortality Profile in Iran (30 Provinces) in 2012. Tehran: Ministry of Health and Medical Education; 2016.
22. Ahmad OB, Boschi-Pinto C, Lopez AD, Murray CJL, Lozano R, Inoue M Age standardization of rates: a new who standard. GPE Discussion Paper Series: No. 31. Geneva: World Health Organization; 2002.
23. Kelsall J, Wakefield J. Discussion of ‘Bayesian models for spatially correlated disease and exposure data’, by Best et al. Bayesian Stat. 1999;6:151.
24. Gelman A, Rubin DB. Inference from iterative simulation using multiple sequences. Stat Sci. 1992;7(4):457–472. doi:10.1214/ss/1177011136
25. Spiegelhalter DJ, Best NG, Carlin BP, Van Der Linde A. Bayesian measures of model complexity and fit. J Royal Stat Soc. 2002;64(4):583–639. doi:10.1111/1467-9868.00353
26. Youlden DR, Cramb SM, Baade PD. The International Epidemiology of Lung Cancer: geographical distribution and secular trends. J Thorac Oncol. 2008;3(8):819–831. doi:10.1097/JTO.0b013e31818020eb
27. Wong MC, Lao XQ, Ho KF, Goggins WB, Shelly L. Incidence and mortality of lung cancer: global trends and association with socioeconomic status. Sci Rep. 2017;7(1):14300. doi:10.1038/s41598-017-14513-7
28. Islami F, Stoklosa M, Drope J, Jemal A. Global and regional patterns of tobacco smoking and tobacco control policies. Eur Urol Focus. 2015;1(1):3–16. doi:10.1016/j.euf.2014.10.001
29. Mirahmadizadeh A, Nakhaee N. Prevalence of waterpipe smoking among rural pregnant women in Southern Iran. Med Princ Pract. 2008;17(6):435–439. doi:10.1159/000151563
30. Yousefi F, Darabi H, Nabipour I, et al. Prevalence of tobacco smoking in bushehr province: comparison of two phases of the Persian gulf healthy heart study. ISMJ. 2014;17(3):487–495.
31. Krewski D, Lubin JH, Zielinski JM, et al. Residential radon and risk of lung cancer: a combined analysis of 7 North American case-control studies. Epidemiology. 2005;16:137–145. doi:10.1097/01.ede.0000152522.80261.e3
32. Kligerman S, White C. Epidemiology of lung cancer in women: risk factors, survival, and screening. AJR Am J. 2011;196:287–295. doi:10.2214/AJR.10.5412
33. Fathi Z, Syn NL, Zhou J-G, Roudi R. Molecular epidemiology of lung cancer in Iran: implications for drug development and cancer prevention. J Hum Genet. 2018;63(7):783–794. doi:10.1038/s10038-018-0450-y
34. Syn NL, Yong WP, Lee SC, Goh BC. Genetic factors affecting drug disposition in Asian cancer patients. Expert Opin Drug Metab Toxicol. 2015;11(12):1879–1892. doi:10.1517/17425255.2015.1108964
35. Fathi Z, Mousavi SAJ, Roudi R, Ghazi F. Distribution of KRAS, DDR2, and TP53 gene mutations in lung cancer: an analysis of Iranian patients. PLoS One. 2018;13:7. doi:10.1371/journal.pone.0200633
36. Zhu J, Li R, Tiselius E, et al. Immunotherapy (excluding checkpoint inhibitors) for stage I to III non‐small cell lung cancer treated with surgery or radiotherapy with curative intent. Cochrane Database Syst Rev. 2017;12.
37. Wingo PA, Cardinez CJ, Landis SH, Greenlee RT, Ries LAG. Long-term trends in cancer mortality in the United States, 1930–1998. Cancer. 2003;97(S12):3133–3275. doi:10.1002/cncr.11380
38. Rowe J, Patel S, Mazo-Canola M, et al. An evaluation of elderly patients (C70 years old) enrolled in Phase I clinical trials at University of Texas Health Science Center at San AntonioCancer Therapy Research Center from 2009 to 2011. J Geriatr Oncol. 2014;5:65–70. doi:10.1016/j.jgo.2013.08.005
39. Roviello G, Zanotti L, Cappelletti MR, et al. Are EGFR tyrosine kinase inhibitors effective in elderly patients with EGFR-mutated non-small cell lung cancer? Clin Exp Med. 2018;18(1):15–20. doi:10.1007/s10238-017-0460-7
40. Mahaki B, Mehrabi Y, Kavousi A, et al. Multivariate disease mapping of seven prevalent cancers in Iran using a shared component model. Asian Pac J Cancer Prev. 2011;12:2353–2358.
41. Khazaei S, Mansori K, Soheylizad M, et al. Epidemiology of lung cancer in Iran: sex difference and geographical distribution. Mid-East J Cancer. 2017;8(4):223–228.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
