Back to Journals » Cancer Management and Research » Volume 11
Sorcin: a novel potential target in therapies of cancers
Received 14 March 2019
Accepted for publication 5 July 2019
Published 5 August 2019 Volume 2019:11 Pages 7327—7336
DOI https://doi.org/10.2147/CMAR.S208677
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Sanjeev K. Srivastava
Xinyi Zhou,1 Xue Wu,2 Baoan Chen2
1Department of Hematology and Oncology, Zhongda Hospital, Medical School, Southeast University, Nanjing, Jiangsu Province, People’s Republic of China; 2Department of Hematology and Oncology (Key Department of Jiangsu Medicine), Zhongda Hospital, Medical School, Southeast University, Nanjing, Jiangsu Province, People’s Republic of China
Abstract: Soluble resistance-related calcium-binding protein (sorcin) is a member of the penta-EF-hand protein family. Sorcin is widely distributed in normal human tissues, such as the brain, heart, lymphocytes, kidneys, breast and skin. Findings suggest that sorcin is associated with the regulation of calcium homeostasis, cell cycle and vesicle trafficking. It has been reported that many types of non-neoplastic diseases such as diabetes, viral infection, infertility, and nervous system diseases were affected by the expression of sorcin. One of the main issues is the role of sorcin in neoplastic diseases. Research proved that sorcin can be found to overexpress in cells of several cancers, particularly in the case of multidrug-resistant cancers. Additionally, the researchers proposed that the expression of sorcin was significantly associated with the foundation of multidrug resistance (MDR). All the findings mentioned above emphasized the importance of studying sorcin. This review mainly includes the following aspects: functions of sorcin, role in non-neoplastic and neoplastic diseases, and research related to drugs. To sum up, sorcin is a potential novel target to be studied to deal with MDR.
Keywords: soluble resistance-related calcium binding protein, sorcin, multidrug resistance, MDR, cancer, chemotherapy
Introduction
Nowadays, cancer has become one of the significant health problems that threaten human life. Great progress has been made in the field of cancer treatment methods, but still not enough. The treatment of cancer mainly relies on chemotherapy and molecular-targeted therapy. Whereas the non-specificity and toxicity of drugs greatly affect the therapeutic effect. Such as chemotherapy, which is one of the most common clinical treatments, is limited by the nonspecificity of drugs. The reason for the high level of drugs in normal tissues could be a clue. Due to side effects in normal tissues, there always exists a gap between the maximum dose and effective dose of the drugs.1–3 What is more, the difficulty of dealing with multidrug resistance (MDR) is compounded by the cost, risk, and complexity of reducing the concentration of drugs in normal tissues.4–6 Consequently, owing to the progress of MDR, the response of patient treatment is greatly affected, thus results in the prognosis.7
Moreover, the sensitivity of the cells to the drug has reduced the drug eliminated from the cells, and the intake of the drug is downregulated, which leads to a decrease in the concentration of the drug in patients’ cells. In the past years, most of the researchers usually focused on different kinds of cancer resistance proteins, such as P-glycoprotein (P-gp), MDR-associated protein 1 (MRP1), breast cancer resistance protein (BCRP/ABCG2), and lung resistance protein/major vault protein (LRP/MVP). The most to be studied is P-gp.8
P-gp was found in the Chinese hamster ovary cells which were resisted to colchicine for the first time in 1976 by Juliano and Ling.9–11 Its code gene can be divided into ABCB1 (MDR1) and ABCB4 (MDR2). The former is located on chromosome 7, q21.1 and has been proved to result in MDR. P-gp is significantly associated with inducing MDR by preventing the accumulation of intracellular chemotherapeutic drugs, thereby avoiding the functions of cytotoxicity or apoptosis. This event is achieved by the drug pumping action of the transmembrane transporter, and reduces the level of intracellular drug concentration.12,13
Some reports also revealed the biological processes of other types of cancer resistance proteins. In general, due to the upregulation of expression of these proteins, such as MRP1, ABCG2, and LRP, the chemotherapy drugs are excreted from the cells.14
However, in recent years, we find that MDR seems to be related to sorcin. Sorcin is a calcium-binding protein that is highly expressed and induces MDR in several cancer patients.15,16 Sorcin affects the functions of P-gp by the regulation of cellular calcium levels and the phosphorylation of P-gp. It suggests that sorcin can be a potential target for cancer diagnoses, treating strategies and predictions.17
Sorcin, namely soluble resistance-related calcium-binding protein, which is also called v19 or cp-22, has always been the hotspot of study since it was first introduced in the MDR Chinese hamster ovary cell line CHRC5 in 1986. It has typical “E-F hand” structures and is a 22-kd protein.18 As observed in Figure 1, the C-terminal is rather well conserved among the PEF proteins.19 The PEF protein family members are related to different periods of cell cycle. However, there still exist some similar features: 1) all these proteins have two domains, C-terminal domain and N-terminal domain. The former, which includes five EF-hand motifs, has binding sites that can bind calcium. Whereas the latter is a flexible and hydrophobic Gly/Pro-rich domain; 2) the PEF proteins are dimers, and monomer–monomer association appears through EF5 hand of the unpaired C-terminal; 3) it is a common case that translocation to membranes occurs when calcium binding. Due to the existence of the large subunit of calpains, though the N-terminal domain is usually short and flexible, the domain can also be significantly complicated. Human sorcin comprises 198 amino acids and it was reported to be highly similar to hamster sorcin. As a consequence, sorcin is presented to be highly conserved in evolution.20
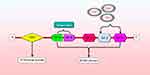 |
Figure 1 Structure of sorcin.21 Note: Reprinted from Translational Oncology, 11/6, Shabnam B, Padmavathi G, Banik K, Girisa S, Monisha J, Sethi G, Fan L, Wang L, Mao X, Kunnumakkara AB, Sorcin a Potential Molecular Target for Cancer Therapy, 1379-1389, Copyright (2018), with permission from Elsevier.Abbreviation: NTD, N-terminal domain. |
Sorcin is widely distributed in normal human tissues, such as lymphocytes, monocytes, kidneys, breast and skin, with high expression in the brain and heart. In addition, sorcin is also widely distributed in tumor-resistant cells and is overexpressed. Moreover, sorcin is also widely distributed even overexpressed in tumor-resistant cells, whose position in the cell is not fixed, but changes dynamically. First in the nucleus for instance, then cytoplasm, plasma membrane, endoplasmic reticulum, and endoplasmic reticulum secreted vesicles during the mitotic phase. When it comes to the end of the division, sorcin is concentrated in the cleavage furrow.22
Functions of sorcin
Regulation of calcium homeostasis
The structure foundation of calcium binding of proteins is the EF-hand, which is a commonplace helix-loop-helix structural motif. When it comes to how sorcin regulates calcium homeostasis, it is believed that sorcin can be involved in the regulation of calcium channels and exchangers which located at the plasma membrane and at the endo/sarcoplasmic reticulum (ER/SR) as a consequence of preventing ER stress. In the meantime, some researchers proposed that sorcin can also influence the unfolded protein response.19 Being associated with the regulation, calcium channels or exchangers are being studied, such as cardiac ryanodine receptors (RyR2), L-type Ca2+ channels, Na+/Ca2+ exchanger (NCX)23–25, and sarcoendoplasmic reticulum calcium transport ATPase (SERCA).26 Sorcin can inhibit RyR and activate SERCA therefore to regulate dimensions and calcium load of the ER vesicles.22 What is more, sorcin has another way to regulate calcium homeostasis. Sorcin can also bind with Ca2+.27 Among many researchers, EF3’s disruption is proved to have the largest functional impact as well.28 In order to confirm the impact of sorcin, a proving experiment was carried out, which is a sorcin knockout mouse model. Findings of the experiment also present more evidence that because of Ca2+ disturbances, cardiac ventricular arrhythmias may result from a lack of sorcin, particularly during the adrenergic response of the heart.29
Regulation of cell cycle
So far, there has been numerous information about calcium-dependent interactions with many proteins such as Polo-like kinase 1 (PLK1), Aurora A and Aurora B kinases, which has been proved from research of sorcin interactions. Sorcin is associated with the regulation of kinase activity by the interaction with PLK1. Through this reaction, sorcin undergoes phosphorylation and results in the autophosphorylation of PLK1.22
Regulation of vesicle trafficking
Figure 2 presents sorcin can inhibit RyRs and activate SERCA therefore to regulate dimensions and calcium load of the ER vesicles.22
 |
Figure 2 Function of sorcin.Abbreviations: NCX, Na+/Ca2+ exchanger; SERCA, sarcoendoplasmic reticulum calcium transport ATPase. |
Sorcin and non-neoplastic diseases
Role in diabetes
In order to reduce the global incidence of type 2 diabetes, it is critical to preserve β-cell function. Under high-fat diet, overexpressing sorcin in the β-cell may induce glucose intolerance, fasting blood decreased and cytosolic and ER Ca2+. Eventually, it may cause β-cell ER stress and apoptosis.30 Researchers designed a modified way which successfully identified 115 differentially expressed proteins (DEPs) from human islets by conducting the 2 and 7 days’ exposure of high-level palmitate. These DEPs including sorcin were closely related with insulin secretion increase stimulated by glucose, protein degradation changes, increased autophagy, altered redox condition, and hampered insulin processing caused by the impairment of islets’ functions. And these events can provide us with a novel target to intervene obesity-related type 2 diabetes mellitus.31
Role in viral infection
In terms of viral infection, sorcin is an important factor involved in the proliferation of viruses. Foot-and-mouth disease virus VP1 can interact with sorcin, leading to an interferon-suppressor to inhibit type I interferon response. This event also results in inhibition of vesicular stomatitis virus replication. Consequently, sorcin is associated with regulating cell response to viral infections.32 Furthermore, there are also some data that demonstrate that hepatitis C virus (HCV) modulates sorcin activity via nonstructural protein 5A (NS5A) protein for its own propagation. The transmission of HCV can be reduced by inhibiting the expression of sorcin.33
Role in infertility
The recent study indicated that sorcin may play an important role in the initiation and maintenance of the window of receptivity and subsequent embryo implantation. During early- and mid-secretory phases of endometrium of infertile women, the expression of sorcin was significantly decreased, and this event may be associated with changes and/or maturation of endometrium in infertile women that have not figured out the cause of disease.34 Furthermore, downregulation of sorcin expression may result in the failure of embryo implantation in both in vivo and in vitro events. Sorcin induces vascular endothelial growth factor (VEGF), an angiogenic factor, in the endometrium and activates its downstream PI3K/Akt signaling cascade. Akt activates nitric oxide synthase, which in turn enhances local nitric oxide levels, regulates cell proliferation, migration and invasion of endothelial cells, and leads to angiogenesis in the endometrium at the implanted site.35 Sorcin also regulates the calcium homeostasis in endometrium, which may cause the failure of recurrent implantation.
Role in nervous system diseases
In a recent study, in order to induce apoptotic neuronal injury, researchers used neurotoxin 1-methyl-4-phenyl-pyridinium ion (MPP+) to treat SH-SY5Y cells. The identification of the changing protein levels proved by two dimension difference gel electrophoresis (2D-DIGE) followed by Matrix-Assisted Laser Desorption/Ionization Time of Flight Mass Spectrometry (MALDI-TOF-MS). There are 22 proteins that have been found to significantly alter from proteomics analysis and sorcin was one of the proteins that decreased. This result indicates that sorcin might play an essential role in MMP+-induced DAergic neuron death due to its regulating calcium homeostasis and the apoptosis signaling pathway.36 One of the pathological features of Alzheimer’s disease (AD) is neurofibrillary tangles of microtubule-associated protein tau. Some recent studies strongly suggest that sequestration of sorcin by abnormal forms of tau impairs the function of sorcin, such as calcium homeostasis and cellular resistance by ER stress. These evidence may significantly support the researchabout AD.37
Functions in neoplastic diseases
According to previous research, sorcin plays an indispensable role in the invasion and migration of cancers, which is also a key to tumor proliferation. However, the mechanism remains unclear.38
Role in gastric cancer
Immunohistochemistry was adopted by some researchers to identify the difference of the expression of sorcin between cancer tissues and adjacent normal tissues. It is well acknowledged that sorcin is mainly concerned with migration and invasion of gastric (GC) cells from the findings of the wound healing and transwell. Many researchers used isobaric tags for relative and absolutely quantitation (iTRAQ) as a common means to confirm the function of sorcin in the field of GC metastasis. Consequently, they managed to identify the DEPs by checking cells with and without sorcin knockdown. Meanwhile, the ITRAQ date was confirmed by RT-PCR and Western blot, the results were assessed as well. In order to control the expression of sorcin, researchers can downregulate recombinant cathepsin Z, matrix metalloprotein 2, matrix metalloprotein 9, and p-STAT3 expression followed by suppression of tumor growth and metastasis.39 Given that the expression of sorcin was proved to be 5.4-fold upregulated in gastric cancer cells (SGC7901 cells) than the normal cells,sorcin can serve as a new molecular biomarker in the diagnosis, treatment, and prognosis of GC.40
Role in colorectal cancer
Recent researches suggest that overexpression of sorcin in colorectal (CRC) HCT116 cells facilitated the process of cell migration, invasion, and epithelial-mesenchymal transition (EMT). Sorcin regulates these cellular functions via activation of PI3K/Akt/mTOR pathway.41 What is more, sorcin can specifically interact with tumor necrosis factor receptor-associated protein 1 (TRAP1), which is related with MDR in CRC cells. Indeed, with depleted short hairpin RNA of TRAP1 in colorectal carcinoma cells, sorcin in mitochondria will be at a lower level. Consequently, the depletion of sorcin by small interfering RNA will also increase TRAP1 degradation.42
Role in breast cancer
Clearly, a small set of tumor-initiating cells (CSCs) has been proved to be associated with resistance to chemotherapy, cancer metastasis and recurrence, always accompanied with EMT activation.43–45 EMT activation is a crucial event that can be always activated during cancer invasion and metastasis.46 The depletion in MDA-MB-231 cells results in decrease of the populations of the CD44+/CD24- and acetaldehyde dehydrogenase 1 (ALDH1) high and leads to mammospheres and colonies formation in soft agar, though it cannot influence proliferation in vitro.
The findings shed light on the relationship between sorcin and CSCs.47 However, the issue of whether sorcin can directly or indirectly influence the event remains resolved. Also, sorcin can regulate VEGF, which is a kind of cytokine that plays a huge role in the tumor vasculature. It may lead to downregulation of VEGF if the level of sorcin decreases, including a large amount of changes such as cell proliferation, migration, invasion, and survival in many solid cancers.48,49 Therefore, the depletion of sorcin suppresses the growth and metastasis of breast cancer cells, impedes angiogenesis in vivo, reduces the CSC population, and sensitizes cancer cells to chemotherapeutics. All these serve as a novel way to develop the therapies in breast cancer.
Role in lung cancer
As previous reported, sorcin has been identified as a gemcitabine-resistance-related protein by proteomics, though immunohistochemistry within 62 non-small cell lung cancer (NSCLC) specimens. Sorcin staining has been found in 66.1% of NSCLC tumors and the overexpression of sorcin was linked to gemcitabine resistance and a poor prognosis in NSCLC patients from immunohistochemical results.50 Cisplatin is a kind of widely used chemotherapy drug for unresectable disease, especially for NSCLC. However, the effectiveness of this drug application is unsatisfactory. According to recent studies, the overexpression of sorcin protein is obvious in the human lung cancer cell line A549/DDP that was resistant to cisplatin. The role of the PI3K/Akt and MEK/ERK pathways are also indisputable in this aspect.51
Role in human nasopharyngeal carcinoma
It has been acknowledged that sorcin protein expression was associated with NPC. In order to establish the drug-resistance cell line called CNE2/DDP, researchers continue to expose CNE2 to cisplatin. The expression of sorcin was found to increase significantly in CNE2/DDP cells by Western blotting. Moreover, the cytotoxicity of cisplatin increased, intracellular rhodamine-123 accumulated and glutathione depleted by knockout of sorcin in human NPC CNE2/DDP cells and examined the function of sorcin in MDR reversal. Additionally, this operation led to a messenger RNA and protein expression of multidrug resistance gene (MDR1), multidrug resistance-associated protein (MRP1), excision repair cross-complementing gene 1 (ERCC1), glutathione S-transferase-p (GST-n), RhoE, Bcl-2, and surviving in sorcin silencing CNE2/DDP cells downregulated. It seems that the silence of sorcin protein induced the essential cellular signaling pathways from PTEN expression increasing and p-Akt and NF-jB expression decreasing.52
Role in hematological tumor
MDR is one of the major incentives of treatment failure in pediatric acute lymphoblastic leukemia. It has been proved that sorcin mRNA levels have a positive correlation with resistance to MTX by cell line data analyses. There exists no significant difference in the expression level of sorcin between the patients and the control group. Compared with patients with negative MRD, sorcin mRNA levels in patients with poor response to treatment were increased (up to 6.9 times).53 In addition, sorcin was remarkably upregulated over parental cells of doxorubicin-induced MDR leukemia cell line K562/A02. It was also proposed that the expression level of sorcin in leukemia patients was not only directly related to the expression of MDR1 gene, but opposite to the patients’ response to chemotherapy and overall prognosis.54 Besides, as another research presented, acute myeloid leukemia (AML) patients whose expression of sorcin was excessive had a higher rate to reach the complete remission (CR) than the patients with sorcin+(P<0.001). As noted above, the sorcin expression in AML patient was negatively correlated with MDR1 expression. Hence, the patients with sorcin-/MDR1- may have good response to induction chemotherapy.55 Meanwhile, some researchers demonstrated that sorcin were upregulated in doxorubicin-resistant myelogenous leukemia cell line.56 The conclusion can be drawn that the overexpression of sorcin is related to MDR of leukemia.
Furthermore, the high level of expression of sorcin is significantly associated with MDR of myeloma.57 The silencing of sorcin increases the chemosensitivity of myeloma cell lines KM3/DDP and U266/ADM, which may lead to cell cycle arrest and apoptosis by reducing cell proliferation.58
Diffuse large B cell lymphoma (DLBCL) is well known to be sensitive to induction therapy with R-CHOP and it has a high cure rate.59 In approximately 40% of DLBCL patients, the emergence of MDR to CHOP chemotherapy regimens (cyclophosphamide, doxorubicin, vincristine, prednisone) remains the main cause of treatment failure and death. Upon verification, sorcin protein played roles in calcium binding and the expression of sorcin was upregulated in CHOP-resistant DLBCL cells.60
Last yet important, sorcin was also upregulated in vincristine-resistant HOB1 lymphoma cells, but the mechanism is yet uncertain.17,61
Role in cervical cancer
The researchers established the MDR1/P-gp-overexpressing multidrug-resistant subline called Hvr100-6 from the human cervical carcinoma cell line HeLa-Ohio (HeLa). It suggests that if the expression of sorcin was suppressed, the expression level for MDR1 mRNA would increase by three times. In addition, the activity and expression of caspase-3 in HeLa cells (sorcin knockdown) were higher than those (scrambled siRNA) cells. From the findings above, we can conclude that the expression of sorcin may be associated with suppression of the expression MDR1 partially. Furthermore, sorcin may induce suppression of apoptosis by dysfunction of caspase-3.62,63
Other approaches related to MDR
The overexpression of ATP-binding cassette transporters including ABCB1, also known as MDR1/P-gp, is outstandingly related to the mechanism of MDR in cancer cells.64–66 Recently, some researchers illustrated that sorcin induces markedly the expression of MDR1/P-gp by activating the cAMP response element in mdr1/p-gp promoter called CREB. Instead, sorcin increased the expression of P-gp by upregulating the combination of sequence binding of p-CREB1 and CREB.67 The sorcin gene is located on chromosome 7 and is homogeneously stained regions in the same homologous region as the MDR1 gene encoding P-gp.68
Most of drug-resistant cells have efflux transporters. In addition to P-gp, there are also different expressions of MRP1 and MRP2. These transporters can excrete drugs with different structures and different mechanisms of action, resulting in decreased drug concentration in tumor cells, affecting the toxic effects of chemotherapy drugs on tumor cells.69–71 The expression of sorcin and transporters in tumor-resistant cells is summarized as shown in Tables 1 and 2.
 |
Table 1 The expression of sorcin and transporters in hematological drug-resistance tumor cells |
 |
Table 2 The expression of sorcin and transporters in solid drug-resistance tumor cells |
Regulation of drugs
Researchers set different concentrations of tetrandrine (Tet), respectively, are 0, 0.5, 1.0, and 2.0 mg/L, to determine the role of sorcin expression in the reversal of MDR in the leukemia cell line of K562/A02 cells. The expression of sorcin was lowest when the concentration was 1.0 mg/L. The results suggested that Tet reversed MDR of K562/A02 cells with concentration dependence and Tet finished this event through regulation of expression of sorcin gene and protein but not fully correlated to the reverse effect.78
Haishengsu (HSS) is a shell protein that has been found to exhibit anticancer activity in recent in vitro studies. Some researchers brought 228 AL patients together for a double-blind, placebo-controlled study. The result showed that HSS can improve the CR rates and quality of life in patients with AL, when HSS was added to conventional chemotherapy. Moreover, in vitro studies, the findings indicated that downregulation of P-gp and sorcin genes in leukemia cells may be associated with the beneficial effects of HSS. In addition, HSS is involved in inducing the apoptosis of drug-resistant K562/ADM tumors in a dose-dependent way.79,80
According to recent studies, PH II-7, being a new kind of anti-tumor drug, could either have a significant effect on inhibiting the proliferation of different human tumor cells, or on MDR tumor cells such as HL60,HL60/ADR, and K562/A02, yet the mechanism remains unclear. PH II-7 may increase the drug concentration in cells through suppressing the MDR-related genes mdr1 and sorcin and result in cell apoptosis.81
There are also some researchers show great interest in the effect of cyclosporine A (CSA) on sorcin gene expression and clinical effect in multiple myeloma patients with MDR. They conducted a comprehensive analysis of all randomized controlled trials. The results showed that CSA could reverse MDR and enhance clinical effect while the mechanism had no correlation with sorcin gene expression.82
In another previous study, it has been found that sorcin could bind directly and with high affinity to doxorubicin, vincristine, paclitaxel, and cisplatin and give these drugs MDR. It is shown that sorcin cell localization changed after doxorubicin treatment, which indicated that the protein responded to doxorubicin and may also bind the drug in the cell soon after the drug entered. They also believed that sorcin can limit the cytotoxic effects of chemotherapies. In addition, after doxorubicin treatment, sorcin silencing increased cell death and doxorubicin accumulating in the nucleus, whereas decreased MDR1 expression and doxorubicin efflux through MDR1.16
We have known that inhibition of P-gp and sorcin played a significant role in the reversal of MDR. And dihydromyricetin (DMY) is one of the drugs which can make this happen. Some researchers proposed a new approach to reverse MDR more effectively, which is applying DMY and OND at the same time to avoid the emergence of MDR when patients are doing chemotherapy. Meanwhile, they revealed the relationship between P-gp and sorcin. The combination of DMY and OND strongly enhanced the antiproliferative efficiency of doxorubicin (ADR) due to increased accumulation of ADR in K562/ADR resistant cell lines. DMY can downregulate the expression of SORCIN and P-gp via the ERK/Akt pathway, while OND does not. Finally, the combination of DMY, OND, and ADR results in G2/M cell cycle arrest and apoptosis by restoring P53 function and inhibiting expression of related proteins. These basic findings provide a promising approach to further treatment of MDR.83
Conclusion
Currently, MDR remains one of the biggest difficulties which faced in clinical treatment. In recent years, numerous research dealing with clinical treatment show that sorcin is closely related to MDR. Basic research suggest that sorcin not only plays a disputable role in the formation of MDR in cancer cells, but also is significantly associated with tumor malignancy and poor prognosis. As a further matter, inhibition of sorcin can reverse MDR. Nevertheless, the mechanism of sorcin on MDR still has not been settled, it is believed that sorcin can eventually become a new target for MDR diagnosis and treatment along with the deepened study gradually.
Acknowledgments
This work was supported by the Key Medical Projects of Jiangsu Province (BL2014078), Key Medical of Jiangsu Province (ZDXKB2016020), Southeast University Project (2018yy-jccx003), Jiangsu Social Development Project (BE2018711), and The National Natural Science Foundation of China (81800197).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Alfarouk KO, Stock C-M, Taylor S, et al. Resistance to cancer chemotherapy: failure in drug response from ADME to P-gp. Cancer Cell Int. 2015;15:71. doi:10.1186/s12935-015-0221-1
2. Luqmani YA. Mechanisms of drug resistance in cancer chemotherapy. Med Princ Pract. 2005;14(Suppl 1):35–48. doi:10.1159/000086183
3. Gao Z, Zhang L, Sun Y, et al. Nanotechnology applied to overcome tumor drug resistance. J Control Release. 2012;162(1):45–55. doi:10.1016/j.jconrel.2012.07.021
4. Dong X, Mumper RJ, van der Lelie D, Gang O. Nanomedicinal strategies to treat multidrug-resistant tumors: current progress. Nanomedicine (Lond). 2010;5(4):597–615. doi:10.2217/nnm.10.2
5. Kedar U, Phutane P, Shidhaye S, et al. Advances in polymeric micelles for drug delivery and tumor targeting. Nanomedicine. 2010;6(6):714–729. doi:10.1016/j.nano.2010.05.005
6. Gothwal A, Khan I, Gupta U. Polymeric micelles: recent advancements in the delivery of anticancer drugs. Pharm Res. 2016;33(1):18–39. doi:10.1007/s11095-015-1784-1
7. Kesharwani SS, Kaur S, Tummala H, et al. Multifunctional approaches utilizing polymeric micelles to circumvent multidrug resistant tumors. Colloids Surf B. 2019;173:581–590. doi:10.1016/j.colsurfb.2018.10.022
8. Yousefi B, Azimi A, Majidinia M, et al. Balaglitazone reverses P-glycoprotein-mediated multidrug resistance via upregulation of PTEN in a PPARgamma-dependent manner in leukemia cells. Tumour Biol. 2017;39(10):1010428317716501. doi:10.1177/1010428317716501
9. Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta. 1976;455(1):152–162. doi:10.1016/0005-2736(76)90160-7
10. Cordon-Cardo C, O’Brien JP, Casals D, et al. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc Natl Acad Sci U S A. 1989;86(2):695–698. doi:10.1073/pnas.86.2.695
11. Kartner N, Evernden-Porelle D, Bradley G, et al. Detection of P-glycoprotein in multidrug-resistant cell lines by monoclonal antibodies. Nature. 1985;316(6031):820–823. doi:10.1038/316820a0
12. Wang Z, Chen Y, Liang H, et al. P-glycoprotein substrate models using support vector machines based on a comprehensive data set. J Chem Inf Model. 2011;51(6):1447–1456. doi:10.1021/ci2001583
13. Chen L, Li Y, Yu H, et al. Computational models for predicting substrates or inhibitors of P-glycoprotein. Drug Discov Today. 2012;17(7–8):343–351. doi:10.1016/j.drudis.2011.11.003
14. de Moraes AC, Maranho CK, Rauber GS, et al. Importance of detecting multidrug resistance proteins in acute leukemia prognosis and therapy. J Clin Lab Anal. 2013;27(1):62–71. doi:10.1002/jcla.2013.27.issue-1
15. Gong Z, Chaaban SA, Meouchy J, et al. Overexpression of sorcin in multidrug-resistant human breast cancer. Oncol Lett. 2014;8(6):2393–2398. doi:10.3892/ol.2014.2426
16. Genovese I, Nikolic A, Kentish SJ, et al. Binding of doxorubicin to Sorcin impairs cell death and increases drug resistance in cancer cells. Cell Death Dis. 2017;8(7):e2950. doi:10.1038/cddis.2017.518
17. Yu X, Mao J, Mahmoud S, et al. Soluble resistance-related calcium-binding protein in cancers. Clin Chim Acta. 2018;486:369–373. doi:10.1016/j.cca.2018.08.034
18. Van der Bliek AM, Meyers MB, Biedler JL, et al. A 22-kd protein (sorcin/V19) encoded by an amplified gene in multidrug-resistant cells, is homologous to the calcium-binding light chain of calpain. Embo J. 1986;5(12):3201–3208. doi:10.1002/embj.1986.5.issue-12
19. Colotti G, Cao Z-H, Wang B, et al. Sorcin, a calcium binding protein involved in the multidrug resistance mechanisms in cancer cells. Molecules. 2014;19(9):13976–13989. doi:10.3390/molecules190811211
20. Wang SL, Tam M-F, Ho Y-S, et al. Isolation and molecular cloning of human sorcin a calcium-binding protein in vincristine-resistant HOB1 lymphoma cells. Biochim Biophys Acta. 1995;1260(3):285–293. doi:10.1016/0167-4781(94)00206-I
21. Shabnam B, Padmavathi G, Banik K, et al. Sorcin a potential molecular target for cancer therapy. Transl Oncol. 2018; 11(6):1379–1389. doi:10.1016/j.tranon.2017.10.004 Reprinted from Transl Oncol, Volume 11, Issue 6, Shabnam, B., et al., Sorcin a Potential Molecular Target for Cancer Therapy, Pages 1379-1389, 2018, with permission from Elsevier. No modifying material in this review. The link of formal publication: https://doi.org/10.1016/j.tranon.2018.08.015. Under a Creative Commons user license: https://creativecommons.org/licenses/by-nc-nd/4.0/.
22. Lalioti VS, Ilari A, O’Connell DJ, et al. Sorcin links calcium signaling to vesicle trafficking, regulates Polo-like kinase 1 and is necessary for mitosis. PLoS One. 2014;9(1):e85438.
23. Suarez J, Belke DD, Gloss B, et al. In vivo adenoviral transfer of sorcin reverses cardiac contractile abnormalities of diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2004;286(1):H68–75. doi:10.1152/ajpheart.00245.2003
24. Seidler T, Miller SLW, Loughrey CM, et al. Effects of adenovirus-mediated sorcin overexpression on excitation-contraction coupling in isolated rabbit cardiomyocytes. Circ Res. 2003;93(2):132–139. doi:10.1161/01.RES.0000081596.90205.E2
25. Meyers MB, Fischer A, Sun Y-J, et al. Sorcin regulates excitation-contraction coupling in the heart. J Biol Chem. 2003;278(31):28865–28871. doi:10.1074/jbc.M302009200
26. Matsumoto T, Hisamatsu Y, Ohkusa T, et al. Sorcin interacts with sarcoplasmic reticulum Ca(2+)-ATPase and modulates excitation-contraction coupling in the heart. Basic Res Cardiol. 2005;100(3):250–262. doi:10.1007/s00395-005-0518-7
27. Ilari A, Johnson KA, Nastopoulos V, et al. The crystal structure of the sorcin calcium binding domain provides a model of Ca2+-dependent processes in the full-length protein. J Mol Biol. 2002;317(3):
28. Mella M, Colotti G, Zamparelli C, et al. Information transfer in the penta-EF-hand protein sorcin does not operate via the canonical structural/functional pairing. A study with site-specific mutants. J Biol Chem. 2003;278(27):24921–24928. doi:10.1074/jbc.M213276200
29. Chen X, Weber C, Farrell ET, et al. Sorcin ablation plus β-adrenergic stimulation generate an arrhythmogenic substrate in mouse ventricular myocytes. J Mol Cell Cardiol. 2018;114:199–210. doi:10.1016/j.yjmcc.2017.11.017
30. Marmugi A, Richardson CC, Ravishankar A, et al. Sorcin links pancreatic beta-cell lipotoxicity to ER Ca2+ stores. Diabetes. 2016;65(4):1009–1021. doi:10.2337/db15-1058
31. Groebe K, Cen J, Schvartz D, et al. Palmitate-induced insulin hypersecretion and later secretory decline associated with changes in protein expression patterns in human pancreatic islets. J Proteome Res. 2018;17(11):3824–3836. doi:10.1021/acs.jproteome.8b00239
32. Li X, Yildirim-Aksoy M, Klesius PH, et al. Engagement of soluble resistance-related calcium binding protein (sorcin) with foot-and-mouth disease virus (FMDV) VP1 inhibits type I interferon response in cells. Vet Microbiol. 2013;166(1–2):35–46. doi:10.1016/j.vetmic.2013.07.025
33. Tran GVQ, Luong TTD, Park E-M, et al. Nonstructural 5A protein of hepatitis C virus regulates soluble resistance-related calcium-binding protein activity for viral propagation. J Virol. 2016;90(6):2794–2805. doi:10.1128/JVI.02493-15
34. Lim HJ, Kocarnik JM, Bush WS, et al. Alteration in endometrial proteins during early- and mid-secretory phases of the cycle in women with unexplained infertility. PLoS One. 2014;9(11). doi:10.1371/journal.pone.0089791
35. Gupta K, Sirohi VK, Kumari S, et al. Sorcin is involved during embryo implantation via activating VEGF/PI3K/Akt pathway in mice. J Mol Endocrinol. 2018;60(2):119–132. doi:10.1530/JME-17-0153
36. Xie H, Chang M, Hu X, et al. Proteomics analysis of MPP+-induced apoptosis in SH-SY5Y cells. Neurol Sci. 2011;32(2):221–228. doi:10.1007/s10072-010-0340-3
37. Kim SI, Li H-M, Lee YZ, et al. Sequestration of sorcin by aberrant forms of tau results in the defective calcium homeostasis. Korean J Physiol Pharmacol. 2016;20(4):387–397. doi:10.4196/kjpp.2016.20.2.177
38. Krakhmal NV, Zavyalova MV, Denisov EV, et al. Cancer invasion: patterns and mechanisms. Acta Naturae. 2015;7(2):17–28.
39. Huan Tuo FS, She S, Yang M, et al. Sorcin induces gastric cancer cell migration and invasion contributing to STAT3 activation. Oncotarget 2017;8(61):104258–104271.
40. Deng L, Li M, Zhang G, et al. Upregulation of soluble resistance-related calcium-binding protein (sorcin) in gastric cancer. Med Oncol. 2010;27(4):1102–1108. doi:10.1007/s12032-009-9208-x
41. Tong W, Sun D, Wang Q, et al. Sorcin enhances metastasis and promotes epithelial-to-mesenchymal transition of colorectal cancer. Cell Biochem Biophys. 2015;72(2):453–459. doi:10.1007/s12013-014-0486-3
42. Landriscina M, Laudiero G, Maddalena F, et al. Mitochondrial chaperone Trap1 and the calcium binding protein Sorcin interact and protect cells against apoptosis induced by antiblastic agents. Cancer Res. 2010;70(16):6577–6586. doi:10.1158/0008-5472.CAN-10-1256
43. Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2(1):48–58. doi:10.1038/nrc706
44. Kakarala M, Wicha MS. Cancer stem cells: implications for cancer treatment and prevention. Cancer J. 2007;13(5):271–275. doi:10.1097/PPO.0b013e318156da4e
45. Bastid J. EMT in carcinoma progression and dissemination: facts, unanswered questions, and clinical considerations. Cancer Metastasis Rev. 2012;31(1–2):277–283. doi:10.1007/s10555-011-9344-6
46. Singh A, Settleman J, Navarro M, et al. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29(34):4741–4751. doi:10.1038/onc.2010.378
47. Hu Y, Li S, Yang M, et al. Sorcin silencing inhibits epithelial-to-mesenchymal transition and suppresses breast cancer metastasis in vivo. Breast Cancer Res Treat. 2014;143(2):287–299. doi:10.1007/s10549-013-2809-2
48. Patton AM, Kassis J, Doong H, et al. Calcium as a molecular target in angiogenesis. Curr Pharm Des. 2003;9(7):543–551. doi:10.2174/1381612033391559
49. Lodola F, Laforenza U, Bonetti E, et al. Store-operated Ca2+ entry is remodelled and controls in vitro angiogenesis in endothelial progenitor cells isolated from tumoral patients. PLoS One. 2012;7(9):e42541.
50. Qu Y, Li M, Zhang G, et al. Comparative proteomic profiling identified sorcin being associated with gemcitabine resistance in non-small cell lung cancer. Med Oncol. 2010;27(4):
51. Gao Y, LI W, LIU X, et al. Reversing effect and mechanism of soluble resistance-related calcium-binding protein on multidrug resistance in human lung cancer A549/DDP cells. Mol Med Rep. 2015;11(3):2118–2124. doi:10.3892/mmr.2014.2936
52. Liu X, Chen L, Feng B, et al. Reversing effect of sorcin in the drug resistance of human nasopharyngeal carcinoma. Anat Rec (Hoboken). 2014;297(2):215–221. doi:10.1002/ar.v297.2
53. Dabaghi M, Rahgozar S, Moshtaghian J, et al. Overexpression of SORCIN is a Prognostic Biomarker for Multidrug-Resistant Pediatric Acute Lymphoblastic Leukemia and Correlates with Upregulated MDR1/P-gp. Genet Test Mol Biomarkers. 2016;20(9):516–521. doi:10.1089/gtmb.2016.0031
54. Zhou Y, Xu Y, Tan Y, et al. Sorcin, an important gene associated with multidrug-resistance in human leukemia cells. Leuk Res. 2006;30(4):469–476. doi:10.1016/j.leukres.2005.08.024
55. Tan Y, Li G, Zhao C, et al. Expression of sorcin predicts poor outcome in acute myeloid leukemia. Leuk Res. 2003;27(2):125–131. doi:10.1016/S0145-2126(02)00083-8
56. Qinghong S, Shen G, Lina S, et al. Comparative proteomics analysis of differential proteins in respond to doxorubicin resistance in myelogenous leukemia cell lines. Proteome Sci. 2015;13(1):1. doi:10.1186/s12953-014-0057-y
57. Li GY, Wang GX, Yang XM, et al. Relationship of sorcin gene expression and multidrug resistance in multiple myeloma patients. Shandong Med J. 2002;(06):1–4.
58. Xu P, Jiang YF, Wang JH. shRNA-mediated silencing of sorcin increases drug chemosensitivity in myeloma KM3/DDP and U266/ADM cell lines. Int J Clin Exp Pathol. 2015;8(3):2300–2310.
59. Till BG. Maintenance therapy in diffuse large B cell lymphoma and mantle cell lymphoma. Curr Treat Options Oncol. 2018;19(9):45. doi:10.1007/s11864-018-0561-x
60. Maxwell SA, Cherry EM, Bayless KJ, et al. Akt, 14-3-3zeta, and vimentin mediate a drug-resistant invasive phenotype in diffuse large B-cell lymphoma. Leuk Lymphoma. 2011;52(5):849–864. doi:10.3109/10428194.2010.535182
61. Lee WP. Purification, cDNA cloning, and expression of human sorcin in vincristine-resistant HOB1 lymphoma cell lines. Arch Biochem Biophys. 1996;325(2):217–226. doi:10.1006/abbi.1996.0027
62. Kawakami M, Nakamura T, Okamura N, et al. Knock-down of sorcin induces up-regulation of MDR1 in HeLa cells. Biol Pharm Bull. 2007;30(6):1065–1073. doi:10.1248/bpb.30.1065
63. Demidova NS, Ilyinskaya GV, Shiryaeva OA, et al. Decreased sensitivity of multidrug-resistant tumor cells to cisplatin is correlated with sorcin gene co-amplification. Neoplasma. 1995;42(4):195–201.
64. Wu CP, Calcagno AM, Ambudkar SV. Reversal of ABC drug transporter-mediated multidrug resistance in cancer cells: evaluation of current strategies. Curr Mol Pharmacol. 2008;1(2):93–105. doi:10.2174/1874467210801020093
65. Robert J, Larsen AK. Drug resistance to topoisomerase II inhibitors. Biochimie. 1998;80(3):247–254. doi:10.1016/S0300-9084(98)80007-2
66. Ambudkar SV, Dey S, Hrycyna CA, et al. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol Toxicol. 1999;39:361–398. doi:10.1146/annurev.pharmtox.39.1.361
67. Yamagishi N, Nakao R, Kondo R, et al. Increased expression of sorcin is associated with multidrug resistance in leukemia cells via up-regulation of MDR1 expression through cAMP response element-binding protein. Biochem Biophys Res Commun. 2014;448(4):430–436. doi:10.1016/j.bbrc.2014.04.125
68. Van der Bliek AM, Baas F, Van der Velde-Koerts T, et al. Genes amplified and overexpressed in human multidrug-resistant cell lines. Cancer Res. 1988;48(21):5927–5932.
69. Xue C, Wang C, Sun Y, et al. Targeting P-glycoprotein function, p53 and energy metabolism: combination of metformin and 2-deoxyglucose reverses the multidrug resistance of MCF-7/Dox cells to doxorubicin. Oncotarget. 2017;8(5):8622–8632.
70. Xue C, Wang C, Liu Q, et al. Targeting P-glycoprotein expression and cancer cell energy metabolism: combination of metformin and 2-deoxyglucose reverses the multidrug resistance of K562/Dox cells to doxorubicin. Tumour Biol. 2016;37(7):8587–8597. doi:10.1007/s13277-015-4478-8
71. Chen T, Jane EP, Pollack IF, et al. Dasatinib reverses the multidrug resistance of breast cancer MCF-7 cells to doxorubicin by downregulating P-gp expression via inhibiting the activation of ERK signaling pathway. Cancer Biol Ther. 2015;16(1):106–114. doi:10.4161/15384047.2014.987548
72. Hu Y, Cheng X, Li S, et al. Inhibition of sorcin reverses multidrug resistance of K562/A02 cells and MCF-7/A02 cells via regulating apoptosis-related proteins. Cancer Chemother Pharmacol. 2013;72(4):789–798. doi:10.1007/s00280-013-2254-2
73. Beyer-Sehlmeyer G, Hiddemann W, Wörmann B, et al. Suppressive subtractive hybridisation reveals differential expression of serglycin, sorcin, bone marrow proteoglycan and prostate-tumour-inducing gene I (PTI-1) in drug-resistant and sensitive tumour cell lines of haematopoetic origin. Eur J Cancer. 1999;35(12):1735–1742. doi:10.1016/S0959-8049(99)00202-6
74. Padar S, Kuroda Y, Sawa S, et al. Differential regulation of calcium homeostasis in adenocarcinoma cell line A549 and its Taxol-resistant subclone. Br J Pharmacol. 2004;142(2):305–316. doi:10.1038/sj.bjp.0705575
75. Wang T, Pei FY, Wang Q, et al. Relationship of sorcin over-expression to multidrug resistance of human lung cancer cell line NCI-H446. J Dalian Med Uni. 2013;35(05):
76. Chuthapisith S, Layfield R, Kerr ID, et al. Proteomic profiling of MCF-7 breast cancer cells with chemoresistance to different types of anti-cancer drugs. Int J Oncol. 2007;30(6):1545–1551.
77. He Q, Tang B, Liu Y, et al. Overexpression of sorcin results in multidrug resistance in gastric cancer cells with up-regulation of P-gp. Oncol Rep. 2011;25(1):237–243. doi:10.3892/or.2011.1175
78. Li J, Kang Z-J, Huang S-Y, et al. [Influence of tetrandrine on SORCIN gene expression in K562/A02 cell line]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2008;16(1):65–69.
79. Li GY, Zhang L, Liu J-Z, et al. Marine drug Haishengsu increases chemosensitivity to conventional chemotherapy and improves quality of life in patients with acute leukemia. Biomed Pharmacother. 2016;81:160–165. doi:10.1016/j.biopha.2016.04.005
80. Li GY, Liu JZ, Zhang B, et al. Tegillarca granosa extract Haishengsu (HSS) suppresses expression of mdr1, BCR/ABL and sorcin in drug-resistant K562/ADM tumors in mice. Adv Med Sci. 2013;58(1):112–117. doi:10.2478/v10039-012-0069-8
81. Tan Y, Qi J, Liu WJ, et al. Preliminary studies on the mechanisms of a new anti-tumor agent PH Ⅱ-7 with special preference to multidrug resistant tumor cells. Acta Academiae Medicinae Sinicae. 2002;(02):134–139.
82. Xing CL, Li GY, Wang J, et al. Effect of cyclosporin A on sorcin gene expression and clinical effect in multiple myeloma patients. Shandong Med J. 2007;(11):10–11.
83. Sun Y, Liu W, Wang C, et al. Combination of dihydromyricetin and ondansetron strengthens antiproliferative efficiency of adriamycin in K562/ADR through downregulation of SORCIN: a new strategy of inhibiting P-glycoprotein. J Cell Physiol. 2018.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
