Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 13
Socio-Demographic, Economic and Clinical Predictors for HAART Adherence Competence in HIV-Positive Adults at Felege Hiwot Teaching and Specialized Hospital, North West Ethiopia
Authors Tegegne AS
Received 14 May 2021
Accepted for publication 26 June 2021
Published 9 July 2021 Volume 2021:13 Pages 749—758
DOI https://doi.org/10.2147/HIV.S320170
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Bassel Sawaya
Awoke Seyoum Tegegne
Department of Statistics, Bahir Dar University, Bahir Dar, Ethiopia
Correspondence: Awoke Seyoum Tegegne
Department of Statistics, Bahir Dar University, Ethiopia
Tel +251 918779451
Email [email protected]
Background: Currently, around 36.7 million people in the world are living with HIV. Among these, 52% are living in sub-Saharan Africa. The main objective of this study was to identify socio-demographic economic and clinical factors associated with HAART adherence competence in successive visits among adult HIV patients after commencement of their treatment.
Methods: A retrospective cohort study design was conducted on a random sample of 792 treatment attendants. The samples were selected using stratified random samples technique considering their residence area as strata. Secondary data were used in this study. Structural equation modeling (SEM) was applied to identify predictors of HAART adherence competence over time.
Results: In this longitudinal study, factors affecting long-term HAART adherence competence in successive visits were identified. Among the predictors, marital status (mean = 3.97, variance = 0.6, p = 0.021), level of disclosure of the disease (mean = 6.24, variance = 0.29, p = 0.012), residence area (mean = 3.97, variance = 0.6, p = 0.021), level of education (mean = 2.04, variance= 0.81, p = 0.012), ownership of cell phone (mean = 2.99, variance = 0.68, p = 0.034), household income (mean = 6.37, variance = 0.53, p = 0.002), age of patients (mean = – 2.78, variance = 56.64, p = 0.023), sex of patients (mean = – 1.25, variance = 0.88, p = 0.036), weight (mean = – 2.89, 42.36, p = 0.001), initial CD4 cell count (mean = 2.57, variance = 158.48, p = 0.015) and WHO stages (mean = 2.37, variance = 0.78, p = 0.026) were directly associated with retention of medication care. On the other hand, medication care was significantly and independently associated with longitudinal adherence competence.
Conclusion: The outcome variable in successive visits increased with the number of follow-up visits, but the rate of increase was different for different groups, such as urban and rural, and for those patients disclosing and not disclosing the disease to family members. An integrated health-related education should be given for non-adherent patients like rural residents, patients living without partners, patients with no cell phone and aged patients.
Keywords: adherence, HAART, adults, socio-demographic, economic, clinical, individual characteristics
Background
Now in these days, around 36.7 million people in the world are living with HIV. Among these, 17 million are under ART and about 52% are living in sub-Saharan Africa.1 The rapid scale up of antiretroviral treatment (ART) in sub-Saharan Africa (SSA) has resulted in an increase of focus on patients’ adherence.2 With the introduction of antiretroviral therapies (ARTs), HIV is becoming a chronic and manageable disease.3 Ethiopia, one of the East African countries, is highly affected by the pandemic, with a prevalence rate of 1.5%.4
Non-adherence to medication prescribed by health staff leads to drug-resistance and achieves high numbers of viral suppression. A continuous spread of first-line ART-resistant HIV strains increases demand for second-line treatment often associated with poor patient health outcomes and increasing healthcare costs.
Recently conducted studies on outcome variables show that predictors and risk factors are different per region of the world. This further enables healthcare providers to offer effective services for non-adherent patients. Medication adherence improves health conditions and prolongs the lives of persons with HIV; it also decreases viral suppression and HIV/AIDS-related morbidity and mortality.6 For these reasons and others, understanding of factors associated with medication adherence competence is paramount. However, implementation of medication adherence competence has been affected by various factors and faces major challenges.7 Previous findings had certain controversies with regard to the initiation of medication adherence for HIV patients.8 Many of the HIV patients who started their treatment with a small number of baseline CD4 cells count did not recover to a normal CD4 cells count even after long-term adherence competence.9 Many studies had been conducted about factors affecting HAART adherence in sub- Saharan African countries like Ethiopia, Uganda, Zimbabwe and South Africa.10,11 However, some of these studies were cross-sectional and did not assess the HAART adherence competence level over time12 and some of the others did not assess the latent variables that cannot be measured directly but have significant effects for the variable of interest.13–15 These studies lack multivariate analysis, including the latent variables among socio-demographic, economic, clinical and individual factors. The retention of medication care, measured using follow-up visits, should also be assessed for its effect on the progress of HAART adherence competence.
There is a scarcity of literature that evaluates latent variables/relationships between factors affecting adherence to HAART in successive visits over time; hence, this gap became an initiation for the current research to be conducted.
The objective of this study was to identify socio-demographic, economic, clinical and individual characteristics associated with HAART adherence competence among HIV-positive adults at Felege Hiwot Teaching and Specialized Hospital, North West Ethiopia. The result obtained in this study helps both health service providers and patients to make the initiation and continuation of the program and to run proper management and monitoring of healthcare interventions.
Methods and Materials
Study Area
The study was conducted at Felege Hiwot Teaching and Specialized Hospital, Amhara region, north-western part of Ethiopia. The hospital is also referral in which different patients in other district hospitals in Amhara region are referred to this hospital. In this hospital, about 6000 of patients are under ART.
Study Design and Setting
A retrospective longitudinal study design was conducted under this investigation. In the hospital, during the initial period of the treatment, patients were directed to visit the hospital weekly, for a month. Subsequently, patients visited the hospital monthly for five months in successive visits. Thereafter, patients were directed to visit the hospital quarterly for medical examination of HAART adherence competence. Patients were also directed to bring unused pills without awareness of why they were doing this. Patients in the hospital received regimens containing two nucleoside reverse transcriptase inhibitors and one non-nucleoside reverse transcriptase inhibitor. The hospital delivered different regimens for patients in the urban and rural areas. The reason for this was because they can be co-administered with anti-tuberculosis (TB) medication. Information related to HIV/AIDS at each visiting time of patients was recorded on data collection sheets prepared by the Ministry of Health. The quality of data was controlled by ART section in the hospital.
Variables Under Investigation
The variable of interest in this study was the HAART adherence competence in successive visits. The variable of interest was measured frequently using pill count and self-reported adherence performance. The patients were categorized as adherent or non-adherent. A patient is said to be adherent if he/she took at least 95% of the prescribed medication otherwise he/she was categorized as non-adherent
Predictor variables were as follows. Time invariant predictors under this investigation included sex (male, female), residence area (urban, rural), level of education (no education, primary, secondary and tertiary), ownership of cell phone (yes, no), marital status (living with partner, living without partner), level of income (low, middle and high), WHO stages (stage 1, stage 2, stage 3 and stage 4), disclosure of the disease (yes, no), age in years, and baseline CD4 cell count in cells/mm3. Visiting times were taken as count predictor variable consisting of value 1 for the first visit, 2 for the second visit, and 23 for the twenty-third follow-up visit. The other time variant covariate included under this investigation was weight in kilograms and CD4 cell count measured at every visit.
Sample size and sampling procedures were as follows. A random sample of 792 patients was selected using a stratified random sampling technique considering their residence area as strata. In calculating the sample size, 95% CI and 5% margin of error were taken into account. Assuming 10% of the respondents’ charts as being incomplete, the sample size was multiplied by 2 to consider the effect of the strata and to increase the power of precision. Moreover, attempts were made to compute the required sample size by employing two population proportion techniques. However, all were found to be below 792. Therefore, 792 samples were taken as the final sample size.
The data source for this study was secondary and collected from the charts of the selected patients by healthcare givers while patients visited the hospital for re-filling pills for the following months and to test their health status (progress of CD4 cell count and viral suppuration) by care givers.
Inclusion and exclusion criteria were as follows. The number of follow-ups and age were the criteria used for including or excluding patients in this study. Adult patients whose follow-ups were between September 2012 and August 2017, with a minimum of two follow-ups, were included in this investigation. However, patients with less than two follow-ups and children under age 15 were excluded.
Data analysis and models used in current investigation were as follows. Data were entered and analyzed using AMOS and SAS (version 9.2) software. Dummy variables were created in the case of categorical variables. Structural equation modeling (PROC CALIS) was used for assessing factors affecting HAART adherence competence in successive visits over time with the inclusion of correlated data obtained in repeated measures. To test for the goodness of fit of the given model, socio-demographic, economic, individual and clinical characteristics were considered as latent variables on the first level. Similarly, retention on HAART medication care was considered as a latent variable on the second level. Data analysis was carried out to obtain descriptive statistics like means and standard deviations (SD) for each of the independent variables. The estimated values of both observed and latent variables were computed. In this regard, an estimated negative value indicates that the covariate and dependent variables were negatively correlated whereas a positive value indicates that the predictor and dependent variables were positively correlated. The conceptual framework which shows relations between different predictors with variables of interest are indicated in Figure 1.
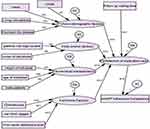 |
Figure 1 Conceptual framework for relationship between observed and latent variables with the variable of interest (HAART adherence competence). |
In Figure 1, W1, W2, … … W18 stands for weight or magnitude of estimated predictors (mean value of estimated predictors at 5% CI). D1, D2, … D5 stands for disturbance or residual terms of unobserved/latent exogenous variable. e1 stands for error terms of the dependent variable (HAART adherence competence)
In Figure 1, socio-demographic/latent variables are expressed using level of education, marital status, level of disclosure of the disease and residence area. Similarly, economic factors are expressed in terms of ownership of cell phone and level of income. Individual characteristics are expressed in terms of age, sex and weight of patients and clinical variables are expressed in terms of initial CD4 cell count, WHO stages and first month adherence. Hence, socio-demographic, economic, individual and clinical variables were latent variables and all the variables indicated in Table 1 were observed variables.
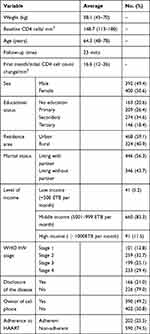 |
Table 1 Baseline Socio-Demographic and Clinical Variables of 792 Patients in the Study Area |
Figure 1 indicates a network of relationships among exogenous and endogenous variables. The parameter estimation for relationships between observed and latent variables was constructed using AMOS software version 22. All Ws are estimated and the estimated magnitude of each covariate with its variance and sig values is indicated in Figure 2. The goodness of fit index (GFI), the adjusted goodness of fit index (AGFI), the comparative fit index (CFI), and the root mean square error of approximation (RMSEA) were assessed using PROC CALIS in SAS software.
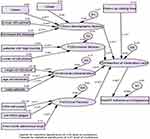 |
Figure 2 Path analysis of predictor variables for estimating mean values (Wi), variances, disturbance terms (Di) and errors (ei) on the dependent variable (HAART adherence competence). |
Results
The baseline characteristics of participants are indicated in Table 1.
As shown in Table 1, out of the sample of 792 patients, 40.9% were rural residents, 50.6% were females, 56.3% were living with their partner, 21% had disclosed their disease to family members, and 49.2% were owners of cell phones. Lastly, only 25.5% of the patients were adherent and the rest were non-adherent.
The estimates were conducted using maximum likelihood estimation technique. The mean and variance of exogenous variables are indicated in Table 2.
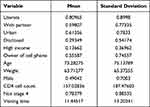 |
Table 2 Mean and Variance of Exogenous Variables |
As it is indicated in Table 2, critical region | C.R| = |Estimates/S.E| was greater than 1.96 for 0.05 level of confidence for all covariates and this further indicates that parameter estimates by all covariates under investigation were statistically significant.30,31
The magnitude of mean and standard deviation for each exogenous variable indicates that standard deviation was greater than mean for all variables and this is an indication that distribution was over-dispersed. The estimated values of covariates for CR, variance and their significance value (p-values) are indicated in Figure 2.
As it is indicated in Figure 2, there were three levels of relationship between observed and latent variables. On the first level, the association between observed predictor variables and latent variables (socio-demographic, economic, individual and clinical factors) was investigated. On the second level, the association between socio-demographic, economic, individual and clinical factors and retention of medication care was assessed. On the third level, the association between retention medication care and HAART adherence competence was investigated.
On the first level, the magnitude of critical region (CR) and variance and its p-value for different variables were: literate (mean = 2.04, variance = 0.81, p = 0.012); patients living in urban area (mean = 3.97, variance = 0.6, p = 0.021); patients living with partners (mean = 8.16, variance = 0.62, p = 0.024); patients had disclosed the disease to family members (mean = 6.24, variance = 0.29, p = 0.012); high income (mean = 6.37, variance = 0.53, p = 0.002); ownership of cell phone (mean = 2.99, variance = 0.68, p = 0.034); weight (mean = –2.89, 42.36, p- value = 0.001); age of patients (mean = –2.78, variance = 56.64, p = 0.023); male patients (mean = –2.75, variance = 0.88, p = 0.036); CD4 cell count (mean = 2.57, variance = 158.48, p = 0.015), not WHO stage 4 (mean = 2.37, variance = 0.78, p = 0.026); and first month adherence level (mean = 3.07, variance = 0.72, p = 0.034). Hence, all | Wi’s|= | CR| were >1.96 at 5% CI. This indicates that covariates were highly correlated with/affected by socio-demographic, economic, individual and clinical factors.
Similarly, on the second level of the model, socio-demographic factors, economic factors, individual characteristics and clinical factors statistically and independently affected the retention of medication adherence. Follow-up visits of patients were also significantly associated with retention of medication care. Finally, on the third level of the model, retention medication care was significantly and statistically associated with the variable of interest (HAART adherence medication) (Figure 2).
The results obtained from the given data indicated that the model was an acceptable fit on CFI (0.965). Unacceptable fit was found with chi-square (39.67; p-value <0.0001) and RMSEA (0.218). However, the covariate model was found acceptable for CFI (0.994), RMSEA (0.001) and unacceptable for chi-square (40.23, p-value <0.0001).
In the estimation of the covariance structure, the following important (key) indicators of goodness of fit were provided: chi-square = 983.45, with a p-value for chi-square of <0.01. This indicates that the chi-square statistic is not closer to zero and the corresponding p-value is very small (significant), which is an indicator of weak fit. This indicates that the model is inadequate. However, RMSEA was estimated to be 0.01, CFI = 0.97, non-normed fit index (NNFI) = 0.96 and NFI = 0.95. Hence, RMSEA, CFI and NFI assured for the model to be a good fit.
Discussion
Socio-Demographic Factors
Education had a significant effect on the variable of interest in this study. More educated patients may easily understand the use of proper adherence.40 On the other hand, illiterate HIV-positive adults are less likely to know what AIDS is and to have had less follow-ups on their prescribed medication (HAART adherence competence), which is further associated with lower CD4 cell count and high viral suppression.18,19
Marital status of patients indicates that the risk of HIV prevalence remained significantly high among unmarried compared with married people.20 Patients living with partners are medication adherent and competent as compared to those living without partners. This might be because partners living together support each other in using the prescribed medication or remind each other the time to take pills and visit the hospital. This result contradicts a previous study32 and is supported by another study.39
Consistent with results from recent longitudinal studies,28 the findings in this study showed that the HAART adherence competence improved through time. The HAART adherence competence at starting period of the treatment was similar for urban and rural patients; however, as visiting time increased, the variable of interest was higher for urban patients as compared to that of rural residents. The reason for this disparity might be the reason that urban residents reside near health institution (in towns) as compared to rural patients.33,35 The later diagnosis of rural patients for their health status might be another reason for the variation in progression rates of the disease for the two groups (urban and rural). Hence, rural patients may not have regular checkups or HIV diagnosis; and most of them come to hospital after severity of illness. This finding agrees with another previous research.32
Level of disclosure of the disease to family members has a significant effect. Hence, patients disclosing the disease are medication adherent and this further leads to HAART adherent competency as compared to those patients not disclosing the disease.
Economic Factors
Level of income can be defined in terms of individual income or household income. Previous researches indicate that economically poor individuals tend to be exposed to greater dangers in the course of their everyday life than the relatively wealthy.21 The adherence levels of patients are highly affected by level of income such that patients with a high level of income had good competence level of adherence.22 Thus, it is said that a person is HAART adherence competent when he/she uses those resources to do what is expected, as would be the case in consuming medication as prescribed.23 Hence, patients with high income may use different alternatives to get pills and he/she also uses proper food adherence schedules for the treatment to be effective and this encourages the patient to attend all visits to the health institution.42
Cell phone ownership of patients can play a significant role in taking pills on time and to remind the date that the patient should visit the hospital. Cell phones helped patients to be HAART adherent because of their alarm (memory aid) for reminding the time pills are taken.43 This finding is consistent with findings from another study44 and suggests the need for making cell phones available to the needy HAART attendants. This finding is also consistent with another previously conducted research.14
Individual Characteristics
Age of patients is highly correlated with retention to medication adherence. Hence, age is negatively correlated with retention medication care but retention medication care is positively correlated with the dependent variable (HAART adherence competence). In previous research, it is indicated that patients aged 40 years and above presented lower rates of CD4 increase over the period of the cohort and this is associated with low HAART adherence competence.22,23 Recent studies also indicate that age has a significant association with HAART adherence competence.32 Hence, as age of a patient increased, HAART adherence competence decreased. This result confirms the literature that states immune function decreases with an increase of age.
Clinical Variables
CD4 cell count of patients at each follow-up visit indicates that patients with rapid progression of HIV had small CD4 cell counts.23 Different researches conducted recently declared that HAART adherence competence is strongly associated with optimal CD4 cell count change compared to non-adherence.24 Patients with a high CD4 cell count encouraged the patient to be HAART adherent as compared to patients with a lower CD4 cell count.38,44–46 This result indicates that this clinical factor, CD4 cell count, is positively associated with retention of medication care.
The importance of early diagnosis to initiate HAART was indicated in this study. Patients who started HAART at WHO stage 1 increased the absolute difference in CD4 cell count as compared to those patients initiated at other stages (stage 2, stage 3, stage 4). This encourages patients to be HAART adherent. This result agrees with findings obtained from other similar studies,36,37 and contradicts other findings.38 Hence, the result needs further investigation.
As visiting time/follow-up visits increased, the absolute difference in CD4 cell count also increased, and this result further leads patients to be HAART adherent.41 Retention in HIV medication care is a crucial activity, positively associated with HAART adherence competence. This result is supported by one of the previous research.25 The HIV care guideline also recognizes the importance of retention in HIV care as an originator to adherence.26 Successful strategies to improve retention in HIV care and HAART adherence competence are the requirement and understanding of retention and adherence behavior.27
Conclusion
The current investigation indicates that socio-demographic variables, economic variables, individual characteristics and clinical variables significantly affected HAART adherence competence. Socio-demographic variables (level of education, residence area, marital status and level of disclosure of the disease to family members living together) had direct and significant effects on retention medication care. Similarly, close follow-ups, economic variables (level of income, ownership of cell phone), individual characteristics (age and weight of patients) and clinical variables (initial CD4 cell count, baseline adherence and WHO stages) had direct and significant effects on retention adherence.
On the other hand, retention adherence had a direct and statistically significant effect on the response variable (HAART adherence competence). The result in current investigation indicates that rural residents, patients not owning a cell phone, patients living without partners and aged patients were at a relatively maximum risk of treatment response.
Poorly adherent patients had low results on the variable of interests, which indicates that adherence to HAART and the absolute difference in CD4 cell count are positively correlated with each other. The current study indicated that the HAART adherence competence increased over time. However, its progress was different for different groups.
The way forward: due attention should be given to address the specific needs of each group of patients. Non-adherent patients in this long-term treatment program were at risk and should receive interventional treatment. Health-related education should be given to patients to initiate their treatment before reaching its cut-off-point (<200 cells/mm3). This helps for easy recovery to normal situation (>500 cells/mm3).32,34 A single intervention strategy cannot improve risks of non-adherent patients. The current study corroborates that successful attempts to improve patients’ HAART adherence competence depend upon a set of key factors. These include realistic assessment of patients’ knowledge/level of education and understanding of the regimen, residence area of patients and age of patients.
This study had certain limitations. One of the limitations is that the data were taken in one treatment site. Considering two or more sites in the investigation may have provided additional information; further study on the variable of interest in successive visits and its determinants with additional sites is recommended. Measuring HAART adherence competence using pill count may not be reliable since some patients may discard the pills not used and this leads to inflation of adherence competence.
Abbreviations
CI, confidence interval; HAART, highly active retroviral therapy; RMSEA, residual mean square error approximation; CFI, comparative fit index; NNFI, non-normed fit index; NFI, normal fit index; GFI, goodness of fit; Statistic, AGFI, adjusted goodness of fit statistic; RMR, root mean square residual; SAS, statistical analysis system.
Data Sharing Statement
The author confirmed that the data used for this research is available from him.
Ethical Approval and Consent to Participate
The data used in the current investigation was collected previously by the health staff for treatment/diagnosis of HIV/AIDS and to start ART. To use this previously collected data, an ethical approval certificate had been obtained from Bahir Dar University Ethical Approval Committee, Bahir Dar University, Ethiopia, reference number: RCS/1412/2012. Hence, this study was conducted in accordance with the Declaration of Helsinki.
Consent for Publication
This manuscript has not been published elsewhere and is not under consideration by any other journal. Hence, the author approved the final manuscript and agreed with its submission.
Acknowledgment
The author acknowledged the Amhara Region Health Research & Laboratory Center at Felege Hiwot Teaching and Specialized Hospital, Ethiopia, for the data they supplied for the success of this research.
Funding
There is no funding to report.
Disclosure
There is no financial and non-financial competing interest between the author and institutions.
References
1. Mahayana M. Self-Regulation of Pain Management by Hospice Patients. University of Illinois at Chicago, Health Sciences Center; 2009.
2. Smith AD, Tapsoba P, Peshu N, et al. Men who have sex with men and HIV/AIDS in sub-Saharan Africa. Lancet. 2009;374(9687):416–422. doi:10.1016/S0140-6736(09)61118-1
3. Pettifor AE, Rees HV, Kleinschmidt I, et al. Young people’s sexual health in South Africa: HIV prevalence and sexual behaviors from a nationally representative household survey. Aids. 2005;19(14):1525–1534. doi:10.1097/01.aids.0000183129.16830.06
4. Demographic E. Health Survey 2011 Central Statistical Agency Addis Ababa. USA: Ethiopia ICF International Calverton; 2012.
5. Aynalem Tesfay F, Dejenie Habtewold T. Assessment of prevalence and determinants of occupational exposure to HIV infection among healthcare workers in selected health institutions in Debre Berhan town, North Shoa Zone, Amhara Region, Ethiopia, 2014. AIDS Res Treat. 2014;2014:1–11. doi:10.1155/2014/731848
6. Gelaw B, Mengitsu Y. The prevalence of HBV, HCV and malaria parasites among blood donor in Amhara and Tigray regional states. Ethiop J Health Dev. 2008;22(1):3–7. doi:10.4314/ejhd.v22i1.10056
7. Abebe Y, Schaap A, Mamo G, et al. HIV prevalence in 72 000 urban and rural male army recruits, Ethiopia. Aids. 2003;17(12):1835–1840. doi:10.1097/00002030-200308150-00013
8. Beach MC, Keruly J, Moore RD. Is the quality of the patient-provider relationship associated with better adherence and health outcomes for patients with HIV? J Gen Intern Med. 2006;21(6):661. doi:10.1111/j.1525-1497.2006.00399.x
9. Lawn SD, Myer L, Bekker L-G, et al. CD4 cell count recovery among HIV-infected patients with very advanced immunodeficiency commencing antiretroviral treatment in sub-Saharan Africa. BMC Infect Dis. 2006;6(1):1. doi:10.1186/1471-2334-6-59
10. Kurth AE, Celum C, Baeten JM, et al. Combination HIV prevention: significance, challenges, and opportunities. Curr HIV/AIDS Rep. 2011;8(1):62–72. doi:10.1007/s11904-010-0063-3
11. Kharsany AB, Karim QA. HIV infection and AIDS in Sub-Saharan Africa: current status, challenges and opportunities. Open AIDS J. 2016;10(1):34. doi:10.2174/1874613601610010034
12. Seyoum A, Ndlovu P, Zewotir T. Quasi-Poisson versus negative binomial regression models in identifying factors affecting initial CD4 cell count change due to antiretroviral therapy administered to HIV-positive adults in North–West Ethiopia (Amhara region). AIDS Res Ther. 2016;13(1):36. doi:10.1186/s12981-016-0119-6
13. Gezie LD. Predictors of CD4 count over time among HIV patients initiated ART in Felege Hiwot Referral Hospital, northwest Ethiopia: multilevel analysis. BMC Res Notes. 2016;9(1):377. doi:10.1186/s13104-016-2182-4
14. Amberbir A, Woldemichael K, Getachew S, et al. Predictors of adherence to antiretroviral therapy among HIV-infected persons: a prospective study in Southwest Ethiopia. BMC Public Health. 2008;8(1):1.
15. Muyingo SK, Walker AS, Reid A, et al. Patterns of individual and population-level adherence to antiretroviral therapy and risk factors for poor adherence in the first year of the DART trial in Uganda and Zimbabwe. J Acquir Immune Defic Syndr. 2008;48(4):468–475. doi:10.1097/QAI.0b013e31817dc3fd
16. Edwards LJ. Modern statistical techniques for the analysis of longitudinal data in biomedical research. Pediatr Pulmonol. 2000;30(4):330–344. doi:10.1002/1099-0496(200010)30:4<330::AID-PPUL10>3.0.CO;2-D
17. Byrne BM. Structural Equation Modeling with Mplus: Basic Concepts, Applications, and Programming. Routledge; 2013.
18. Langebeek N, Gisolf EH, Reiss P, et al. Predictors and correlates of adherence to combination antiretroviral therapy (ART) for chronic HIV infection: a meta-analysis. BMC Med. 2014;12(1):142.
19. Do NT, Phiri K, Bussmann H, et al. Psychosocial factors affecting medication adherence among HIV–1 infected adults receiving combination antiretroviral therapy (cART) in Botswana. AIDS Res Hum Retroviruses. 2010;26(6):685–691. doi:10.1089/aid.2009.0222
20. Nosyk B, Lourenço L, Min JE, et al. Characterizing retention in HAART as a recurrent event process: insights into ‘cascade churn’. AIDS. 2015;29(13):1681. doi:10.1097/QAD.0000000000000746
21. Howard AA, Arnsten JH, Lo Y, et al. A prospective study of adherence and viral load in a large multi-center cohort of HIV-infected women. Aids. 2002;16(16):2175–2182. doi:10.1097/00002030-200211080-00010
22. Belle D. Poverty and women’s mental health. Am Psychol. 1990;45(3):385. doi:10.1037/0003-066X.45.3.385
23. Alok R, Das SK, Agarwal GG, et al. Problem-focused coping and self-efficacy as correlates of quality of life and severity of fibromyalgia in primary fibromyalgia patients. J Clin Rheumatol. 2014;20(6):314–316.
24. Okonji JA, Zeh C, Weidle PJ, et al. CD4, viral load response, and adherence among antiretroviral-naive breast- feeding women receiving triple antiretroviral prophylaxis for prevention of mother-to-child transmission of HIV in Kisumu, Kenya. J Acquir Immune Defic Syndr. 2012;61(2):249–257. doi:10.1097/QAI.0b013e318262514f
25. Schwimmer JB, Burwinkle TM, Varni JW. Health-related quality of life of severely obese children and adolescents. JAMA. 2003;289(14):1813–1819. doi:10.1001/jama.289.14.1813
26. Sagarduy JLY, López JAP, Ramírez MTG, et al. Psychological model of ART adherence behaviors in persons living with HIV/AIDS in Mexico: a structural equation analysis. Rev Saude Publica. 2017;51:81. doi:10.11606/s1518-8787.2017051006926
27. Rao D, Feldman BJ, Fredericksen RJ, et al. A structural equation model of HIV-related stigma, depressive symptoms, and medication adherence. AIDS Behav. 2012;16(3):711–716. doi:10.1007/s10461-011-9915-0
28. Shmueli A. Socio-economic and demographic variation in health and in its measures: the issue of reporting heterogeneity. Soc Sci Med. 2003;57(1):125–134. doi:10.1016/S0277-9536(02)00333-7
29. Guerrero M, Rialp J, Urbano D. The impact of desirability and feasibility on entrepreneurial intentions: a structural equation model. Int Entrepreneurship Manag J. 2008;4(1):35–50. doi:10.1007/s11365-006-0032-x
30. Marsh HW, Hocevar D. Application of confirmatory factor analysis to the study of self- concept: first-and higher order factor models and their invariance across groups. Psychol Bull. 1985;97(3):562. doi:10.1037/0033-2909.97.3.562
31. MacCallum RC, Austin JT. Applications of structural equation modeling in psychological research. Ann Rev Psychol. 2000;51(1):201–226. doi:10.1146/annurev.psych.51.1.201
32. Adams M, Luguterah A. Longitudinal analysis of change in CD4+ cell counts of HIV–1 patients on antiretroviral therapy (ART) in the Builsa district hospital. Eur Sci J. 2013;9(33).
33. Berg KM, Demas PA, Howard AA, et al. Gender differences in factors associated with adherence to antiretroviral therapy. J Gen Intern Med. 2004;19(11):1111–1117. doi:10.1111/j.1525-1497.2004.30445.x
34. Asfaw A, Ali D, Eticha T, et al. CD4 cell count trends after commencement of antiretroviral therapy among HIV-infected patients in Tigray, Northern Ethiopia: a Retrospective Cross-Sectional Study. PLoS One. 2015;10(3):e0122583. doi:10.1371/journal.pone.0122583
35. Skhosana NL, Struthers H, Gray GE, et al. HIV disclosure and other factors that impact on adherence to antiretroviral therapy: the case of Soweto, South Africa. Afr J AIDS Res. 2006;5(1):17–26. doi:10.2989/16085900609490363
36. Lima VD, Fink V, Yip B, et al. Association between HIV–1 RNA level and CD4 cell count among untreated HIV- infected individuals. Am J Public Health. 2009;99(S1):S193–S196. doi:10.2105/AJPH.2008.137901
37. Kulkarni H, Okulicz JF, Grandits G, et al. Early postsero conversion CD4 cell counts independently predict CD4 cell count recovery in HIV–1–positive subjects receiving antiretroviral therapy. J Acquir Immune Defic Syndr. 2011;57(5):387. doi:10.1097/QAI.0b013e3182219113
38. Montarroyos UR, Miranda-Filho DB, César CC, et al. Factors related to changes in CD4+ T-cell counts over time in patients living with HIV/AIDS: a multilevel analysis. PLoS One. 2014;9(2):e84276. doi:10.1371/journal.pone.0084276
39. Mair C, Hawes SE, Agne HD, et al. Factors associated with CD4 lymphocyte counts in HIV‐negative Senegalese individuals. Clin Exp Immunol. 2008;151(3):432–440. doi:10.1111/j.1365-2249.2007.03573.x
40. Maqutu D, Zewotir T. Optimal HAART adherence over time and time interval between successive visits: their association and determinants. AIDS Care. 2011;23(11):1417–1424. doi:10.1080/09540121.2011.565028
41. Kaufmann GR, Perrin L, Pantaleo G, et al. CD4 T-lymphocyte recovery in individuals with advanced HIV–1 infection receiving potent antiretroviral therapy for 4 years: the Swiss HIV Cohort Study. Arch Intern Med. 2003;163(18):2187–2195. doi:10.1001/archinte.163.18.2187
42. Kipp AM, Pungrassami P, Nilmanat K, et al. Socio-demographic and AIDS-related factors associated with tuberculosis stigma in southern Thailand: a quantitative, cross-sectional study of stigma among patients with TB and healthy community members. BMC Public Health. 2011;11(1):675. doi:10.1186/1471-2458-11-675
43. Maqutu D, Zewotir T, North D, et al. Determinants of optimal adherence over time to antiretroviral therapy amongst HIV positive adults in South Africa: a longitudinal study. AIDS Behav. 2011;15(7):1465–1474. doi:10.1007/s10461-010-9688-x
44. Florence E, Lundgren J, Dreezen C, et al. Factors associated with a reduced CD4 lymphocyte count response to HAART despite full viral suppression in the Euro SIDA study. HIV Med. 2003;4(3):255–262. doi:10.1046/j.1468-1293.2003.00156.x
45. Ebonyi AO, Agbaji OO, Anejo-Okopi JA, et al. Factors associated with a low CD4 count among HIV–1 infected patients at enrolment into HAART in Jos Nigeria. Br J Med Med Res. 2014;4(13):2536. doi:10.9734/BJMMR/2014/8469
46. Smith CJ, Sabin C, Youle M, et al. Factors influencing increases in CD4 cell counts of HIV-positive persons receiving long-term highly active antiretroviral therapy. J Infect Dis. 2004;190(10):1860–1868. doi:10.1086/425075
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
