Back to Journals » Clinical Ophthalmology » Volume 14
Slowed Progression of Age-Related Geographic Atrophy Following Subthreshold Laser
Authors Luttrull JK , Sinclair SH , Elmann S , Chang DB, Kent D
Received 27 June 2020
Accepted for publication 12 August 2020
Published 1 October 2020 Volume 2020:14 Pages 2983—2993
DOI https://doi.org/10.2147/OPTH.S268322
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Jeffrey K Luttrull,1 Stephen H Sinclair,2 Solly Elmann,3 David B Chang,4 David Kent5
1Ventura County Retina Vitreous Medical Group, Ventura, California, USA; 2Sinclair Retina Associates, Media, Pennsylvania, USA; 3Brooklyn Hospital Medical Center, Brooklyn, New York, USA; 4Retinal Protective Sciences, LLC, Ojai, California, USA; 5The Eye Clinic, Kilkenny, Ireland 6. University of Liverpool, Liverpool, UK
Correspondence: Jeffrey K Luttrull
Ventura County Retina Vitreous Medical Group, 3160 Telegraph Road, Suite 230, Ventura, California 93003, USA
Tel +1 805-650-0664
Email [email protected]
Purpose: To determine the effect of panmacular low-intensity/high-density subthreshold diode micropulse laser (SDM) on age-related geographic atrophy (ARGA) progression.
Methods: The retinal images of all eyes with ARGA in a previously reported database, consisting of all eyes with dry age-related macular degeneration (AMD) active in a vitreoretinal practice electronic medical record (EMR), were identified and analyzed to determine the velocity of radial linear ARGA progression during observation and after panmacular SDM.
Results: Sixty-seven eyes of 49 patients with ARGA, mean age of 86 years were identified as having follow-up both before and after initiation of SDM treatment. All were included in the study. These eyes were followed a mean 910 days (2.5 years) prior to SDM treatment and a mean 805 days (2.2 years) after. Measurement masked to treatment vs observation found the radius of ARGA lesions progressed 1 to 540 μm per year (mean 137μm, SD 107) prior to treatment (controls); and − 44 to 303 μm per year (mean 73μm, SD 59) after initiation of periodic panmacular SDM laser. Thus, the velocity of radial linear progression decreased 47% per year following panmacular SDM (p< 0.0001). There were no adverse treatment effects.
Conclusion: In cohort of eyes with high-risk dry AMD, panmacular SDM slowed linear radial ARGA progression velocity 47% per year (p< 0.0001) without adverse treatment effects. Validated, these findings would constitute an important advance in the prevention of age-related visual loss and a benchmark for future therapies.
Keywords: age-related macular degeneration, laser, micropulse, subthreshold, sublethal, geographic atrophy, progression, heat-shock proteins, reset, prevention, reticular pseudodrusen
Introduction
The designation “advanced” has been applied to the two visually disabling forms age-related macular degeneration (AMD): “wet”, or neovascular AMD, which accounts for about 90% of cases; and the most severe form of “dry”, or non-neovascular AMD, characterized by age-related geographic chorioretinal atrophy (ARGA) involving the macula, accounting for the remaining 10%.1,2 Vascular endothelial growth factor (VEGF) binding drugs have revolutionized the treatment of wet AMD.3 However, there remains no effective treatment for ARGA.4,5
Low-intensity/high-density subthreshold diode laser (SDM) represented a paradigm shift in the conception and performance of laser treatment for chronic progressive retinopathies.6 As the first retinal laser strategy designed to preclude laser-induced retinal damage (LIRD), SDM was first shown to be effective in the treatment of complications of diabetic retinopathy.6–8 As a particular approach to pulsed retinal laser therapy, panmacular SDM is uniquely uniform in its application; employing the identical laser wavelength, laser parameters, treatment technique, treatment field and number of laser spots in every eye, for every indication.6–14
Effective laser treatment absent LIRD led to a new understanding of the mechanism of retinal laser action as a biological “reset” phenomenon.10–13 Reset theory accounts for all known retinal laser effects and has accurately predicted new neuroprotective retinal laser applications not possible with photocoagulation and other forms of damaging retinal laser treatment.6–14 These include reversal of anti-VEGF drug tolerance in wet AMD; improved retinal function by electrophysiology; and improved visual acuity, visual fields, microperimetry, contrast acuity, and mesopic visual function in dry AMD, inherited retinopathies, and open-angle glaucoma; and improved ganglion cell and optic nerve function by electrophysiology in eyes with glaucomatous optic neuropathy.10–15
Recently, a low incidence of choroidal neovascularization (CNV) following panmacular SDM was reported in a large cohort of eyes with high-risk dry AMD.15 This report examines the effect of SDM on ARGA progression in this same group of eyes.
Methods
This study was approved by the Western Institutional Review Board and complied with both the Health Insurance Portability and Accountability Act of 1996, and the tenets of the Declaration of Helsinki. As a retrospective review of EMR data, prior written patient consent was neither obtained, nor required by the approving IRB. All data maintained confidentially and anonymized prior to analysis.
The fundus images of all eyes with ARGA in a previously reported database
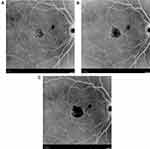
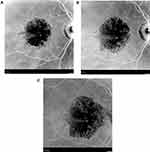
https://datadryad.org/handle/10255/dryad.189065 that included all eyes with dry AMD active in a practice electronic medical records (EMR) system were reviewed.15 As previously described, all patients were managed according to the standard of care including age-related eye disease study (AREDS 1 or 2) nutritional supplements. In addition, they were offered SDM.15 All retinal images were produced with the Heidelberg Spectralis system (Heidelberg Engineering, Heidelberg, Germany) permitting measurement of the ARGA lesions with the Heidelberg micrometer caliper function. Available images extended back through 2008, when the Heidelberg system was first employed in the practice. Images included near-infrared autofluorescence fundus photography (NIR, 790nm exciting wavelength), blue fundus autofluorescence photography (FAF, 488nm exciting wavelength), spectral-domain optical coherence tomography (SD-OCT), and in some cases, fundus fluorescein angiography (FFA). ARGA was identified photographically (in monochrome) according to Age-Related Eye Disease Study criteria.1 Although ARGA of as little as 175µm has been used as the lower limit of size in various studies, the current study used 300µm as the minimum GA lesion diameter for study inclusion. In each case, GA was confirmed by OCT demonstration of chorioretinal atrophy at the locus of well-circumscribed pigment atrophy.1,2,4,17
Only eyes followed and documented before and after initiation of regular periodic panmacular SDM treatment were included for study and statistical analysis. Thus, the pre-treatment course of disease in each treated eye served as the control. Additional inclusion criteria included a minimum of 3 months of follow up (one eye, 81 days); the diagnosis of dry AMD based on clinical examination and macular imaging; high-resolution fundus images of sufficient quality to permit ARGA measurement on the following dates: (1) the earliest photographic documentation of ARGA (Time 1, or T1); (2) the last photographic documentation prior to the first SDM (T2); (3) on the date of initiation of regular periodic SDM (T3); and (4) of the last photographs after initiation of SDM (T4). To maximize the possibility of detecting a treatment effect, eyes were considered treated and included for study only if they had at least 2 consecutive SDM treatments, 6 months or less apart. Thus, T2 and T3 were not always the same. Additional data included patient sex, age, eye, presence or absence of reticular pseudodrusen (RPD), and new CNV or other adverse events. If CNV developed during the course of observation before or after SDM treatment, the final data were taken at the last data prior to the development of CNV. Best-corrected Snellen visual acuity (VA) and ARGA lesion diameters on dates T1-4 were recorded. VAs of less than 20/400 were assigned a value of 20/1000. The days elapsed between dates T1 and T2 (“control”) and dates T3 and T4 (“treated”) were determined using an online calculator (https://www.timeanddate.com/date/duration.html).
Exclusions included eyes with poor quality images, clinical or imaging evidence of current or prior choroidal neovascularization, pigment epithelial detachment, current or prior use of anti-VEGF medications, prior vitreoretinal surgery or macular photocoagulation, other causes of geographic atrophy such as degenerative myopia, and other obfuscating ocular disorders. Peripapillary atrophy was not included in the analysis. Such exclusions thus minimize any confounding effects of local factors on the rate of ARGA progression.18
The velocity of ARGA progression was derived using a simplification of the radius linear model (RLM).16 According to the RLM, the rate of linear expansion of GA in a given eye is constant over time in all meridians and independent of lesion area.16 Typically, this is determined by taking the square root of the measured lesion area to eliminate the artifactual influence of lesion size on apparent lesion growth.15 Determination of ARGA lesion area is complex, difficult, time-consuming, and thus expensive, generally the province of industry-sponsored studies performed by academic reading centers.1,2,16,17 However, independence of the linear expansion velocity from lesion area allows simplification of the method by mathematical elimination of the area factor and determination of the ARGA lesion radius by a direct measure of lesion diameters. As the RLM holds that the velocity of lesion expansion is constant in all directions, the velocity of expansion can be determined by comparing the radius over time in any given meridian in a given eye, provided it is measured along the identical meridian. The change in lesion radius per year prior to panmacular SDM treatment (“control”) was thus calculated by subtracting the lesion diameter at presentation (T1) from the last available lesion diameter prior to treatment (T2) and dividing the result by 2 to obtain the lesion radius, dividing this result by the number of days elapsed between the photographs, and multiplying by 365 to obtain the velocity of progression in µm/year. The same operation was employed to determine the rate of radial linear ARGA progression for treated eyes, using instead the date of treatment (T3) compared to the last image post treatment (T4).
Analysis of the de-identified subject retinal images was masked to treatment vs observation (SHS) and performed according to the following protocol: The retinal images for each eye were reviewed confirm adequate image quality. The first and final macular images were then examined to appreciate the pattern of ARGA progression (uni- vs multifocal, independent vs coalescent) to aid the selection of the optimal meridian for the measure. In eyes with multifocal lesions, the single ARGA locus with the best-defined margins was chosen for measurement, assuming the same rate of progression for all ARGA lesions in a given eye.4,14,16 Coincidence of the measurement meridians over time was confirmed by extending the micrometer rays through constant anatomic landmarks outside the margins of the ARGA lesions, such as retinal vascular bifurcations. The diameter of ARGA lesions was then measured using the SpectralisTM micrometer caliper function. For a given eye, the same image type was used at each time-point.
SDM Treatment
Following informed consent and pupillary dilation, a topical anesthetic was applied to the cornea followed by a contact lens (Mainster macular contact lens, magnification factor 1.05, Ocular Instruments, Mentor, Ohio). Under minimum slit-lamp illumination, the retina within the major vascular arcades including the area of geographic atrophy was confluently “painted” with 1100–1800 laser spot applications, depending upon the circumscribed area (“panmacular” treatment).11 Identical laser parameters were used in all eyes: 810nm wavelength, 200µm aerial spot size, 5% duty cycle, 1.43 Watt power and 0.15 s spot duration (Oculight SLx, Iris Medical/Iridex Corp, Mountain View, California).10–15 All panmacular SDM treatments were performed by a single surgeon (JKL). Thus, treatment was uniform in all respects in every eye. Informed by long-term prospective retinal and visual function testing in eyes with dry AMD following panmacular SDM showing a return to baseline values 6–9 months following treatment, treatment in the eyes reported here was performed approximately every 3–4 months to maintain the maximum effect.11,17
Statistical Analysis
All data were anonymized prior to statistical analysis. Only eyes followed both before and after SDM treatment were considered for statistical analysis. Frequencies, means, and medians were calculated to summarize the data. The models included fixed eye effects and a random patient intercept to account for inter-eye correlation. Additional hierarchical linear models to explore the association between the difference (post- minus pre-treatment) and pre-treatment values were also performed. Statistical analyses were performed using SAS 9.4 (SAS Institute; Cary, NC).
Results
Demographics
113 eyes of 72 patients (25 male, 47 female) with ARGA were identified in the practice EMR. 15 All had retinal imaging on presentation and in follow up. 2 eyes of 2 patients were excluded due to poor quality photographs yielding 111 eyes of 70 patients with useful images. Of the 84 eyes with untreated observation, 17 were excluded from the treated group due to: development of CNV (2 eyes); central retinal artery occlusion (1 eye), death (4 eyes); relocation out of the practice area (4 eyes); and having only a single initial SDM treatment at the time of data analysis (6 eyes). This yielded 67 eyes of 49 patients with ARGA having follow up both before and after initiation of regular periodic panmacular SDM treatment eligible for study and statistical analysis15 (Figures 1–3).
These eyes were observed 81–2966 days (mean 910, SD 727.1) prior to initiation of routine periodic panmacular SDM treatment and followed 121–1489 days (mean 805, SD 265.2) after initiation of treatment. The number of treatments ranged 3–13 per eye (mean 6.8) for an average inter-treatment interval of 112 days.
VAs ranged 20/20 – 20/1000 throughout the course of the study, decreasing on average with time (Figure 4).
RPD Were Identified as Coincident with ARGA in 52/67 Eyes (78%)
Effect of Regular Periodic Panmacular SDM on ARGA Progression Velocity
Overall, the velocity of linear radial ARGA progression was 137µm/year before treatment (SD 107) and 73µm/year after treatment (SD 59), thus slowing 47% per year following institution of regular periodic panmacular SDM (Figure 5). A linear mixed model analysis comparing the pretreatment radial progression rate (137µm/yr, SE 11.41) to the post-treatment progression rate reduction (64 µm/yr, SE 11.89) found the reduction of ARGA progression following panmacular SDM to be highly significant (p<0.0001). This relation held for eyes with initial lesion diameters of < or > 1000µm as well (p<0.0001 each). A linear mixed model accounting for inter-eye correlation was performed to test the potential difference between eyes with initial lesion diameters of < 1000µm (n=19) or > 1000µm (n=48). Lesion size did not significantly affect rates of progression either before after SDM treatment (pre-treatment continuous measure (p = 0.919), dichotomized scale (p = 0.824); post treatment continuous measure (p = 0.408), dichotomized scale (p = 0.269)).
There were no adverse treatment effects, including LIRD, in any eye.
Discussion
In a recently reported cohort of all eyes with dry AMD active in a vitreoretinal practice EMR system, augmentation of standard dry AMD management with panmacular SDM was followed by an age-adjusted annual incidence of new CNV 93–98% lower than expected compared to the AREDS.15 Included in this cohort were eyes with ARGA. In that study, ARGA progression was not a study endpoint. The current study focuses on the eye with ARGA, finding that panmacular SDM also significantly slowed the radial velocity of ARGA progression in eyes with pre-existing ARGA.
The demographics and macular characteristics of the eyes reported here with ARGA are consistent with prior studies; including reduced VA, advanced age, bilateral occurrence, female preponderance, and associated RPD.1,2,4,17–21 Reflecting the association of both advanced age and ARGA with RPD, 78% of eyes identified with ARGA in the current study (average and median age 86 years) had coincident RPD, compared to 39% of eyes in the overall dry AMD database (average age 82, median 84 years).15 Despite advanced high-risk dry AMD, panmacular SDM slowed, rather than accelerated, disease progression.25 This slowing of radial linear velocity of ARGA progression of 47% per year following panmacular SDM was highly significant (p<0.0001).
A recent meta-analysis by Shen, et al, reviewed the results of 25 ARGA progression studies including 2942 eyes. Comparing the various current methods of ARGA progression measurement, including the area linear model in which the lesion area changes linearly with time; and the area exponential model in which the lesion area changes exponentially with time; Shen et al concluded that the radius linear model (RLM, in which the lesion radius derived from the square root transformation of the lesion area grows linearly with time) provided the best fit to the data available from the meta-analysis, and thus provided the most accurate measure of lesion progression.16 The meta-analysis found that the velocity of linear ARGA lesion expansion was constant in a given eye over time, and independent of lesion area.16 Independence of the velocity of radial linear ARGA expansion from time and lesion size permits simplification of ARGA progression determination, by mathematical elimination of the area factor (and thus the need for often difficult and complex lesion area determination) in favor of the simpler process of direct measurement of lesion diameters.4,15,16 Despite this simplification, we note that the velocity of linear radial ARGA expansion for the untreated control eyes measured in the current study (mean 137 µm per year, median 115) is in the range of rates reported for untreated eyes in prior studies of 53–264µm/year, and the Shen metanalysis finding of 158–167µm/year.1,2,4,16–22
Challenging the meta-analysis of Shen et al are two recent studies.16,20,21 Uji and associates reported 24 eyes comparing the square root of ARGA lesion area with linear radial growth velocity measured by a novel method of directional kinetics analysis.20 The authors found variable velocities in different meridians; faster growth toward the fovea; and faster growth of smaller lesions. In the current study, ARGA diameter measurements were taken in the meridians with the most clearly defined photographic margins over time to achieve the most precise measurements. Thus, the lesion diameter measurements were random with respect to particular axes or polarities, thereby minimizing any potential confounding effect of directional bias in growth or measurement. In another study of 126 eyes followed an average 3.1 years, Mones and Biarnes used conventional square root analysis derived by manual border-tracing of ARGA lesions followed by application Heidelberg Engineering Region FinderTM software (Heidelberg Engineering, Heidelberg, Germany) to determine lesion areas, followed by square root conversion.21 Like Uji, they found more rapid progression of smaller lesions.21 While “smaller” was not defined by Uji, et al, Mones and Biarnes used AREDS categories and tertiles to delineate baseline lesion sizes.20,21 Our findings instead agree with the larger Shen meta-analysis.16 In the current study, comparison of eyes with baseline lesion diameters < or > 1mm found no difference in progression rates based on lesion diameter in control eyes or after SDM treatment (despite a significant slowing of progression following SDM). Thus, the slowing of ARGA progression following treatment we report does not appear to be an artifact of any natural slowing of progression with time. Finally, the influence of any such confounding variables would almost certainly be insignificant in comparison to the magnitude of the treatment effect we find in the current study (47% annual slowing of progression, p<0.0001).
Historically, macular laser has been the mainstay of treatment for dry AMD.22–30 This reflected the desire to eliminate the hallmark clinical feature of AMD, drusen.22–30 While early reports found laser-induced drusen reduction was beneficial, subsequent studies found either no benefits, or worsened outcomes.23–30 This was attributed to the observation that the efficiency of drusen reduction paralleled (higher) retinal burn intensity and increased retinal damage, which in turn increased the incidence of subsequent CNV and visual loss.28–30 In an attempt to improve these results, short-pulsed continuous wave (SPCW) lasers designed to better limit LIRD to the outer retina and RPE have been studied. Possibly reflecting reduced LIRD to Bruch’s membrane, SPCW lasers have not been found to notably increase the risk of CNV.22,23 However, they have caused rapid acceleration and marked worsening of high-risk dry AMD and ARGA.22,23 Treatment directed at the intact margins of ARGA lesions by microsecond CW laser (“selective retinal treatment”, or SRT; Lutronic, Seoul, S. Korea) increased the rate of ARGA progression by 50%, bringing early closure to a prospective clinical trial.22 Likewise, nanosecond CW laser (2RT; Ellex, Adelaide, Australia) treatment of dry AMD in the LEAD study also caused rapid worsening of high-risk dry AMD, particularly by development and progression of ARGA in eyes with RPD, such as the eyes in the current study, 78% of which had coincident RPD.23 While nanosecond CW laser treatment is described as “non-damaging” (https://www.ellex.com/), it is inherently photodisruptive to the RPE, at minimum.9,23 The contrast between the results of the current study of panmacular SDM, wholly sublethal to the RPE; and laser modes inherently damaging to the retina, such as microsecond (SRT) and nanosecond (2RT) CW lasers, is noteworthy. Panmacular SDM, preserving the RPE and normalizing retinal function, results in significant slowing of ARGA progression in eyes with the highest-risk advanced dry AMD and reticular pseudodrusen; while RPE-lethal and retinal damaging short-pulse CW lasers cause rapid worsening in these same eyes. This is consistent with current concepts of modern retinal laser therapy, illustrating the importance of distinguishing between laser treatment sublethal to the RPE, and treatment simply “subthreshold” to visibility at a given level of scrutiny, but damaging to the retina nonetheless.9,31 The phenotypic hallmarks of advanced AMD indicate compromise and stress of the compensatory mechanisms designed to maintain macular health and function.4 Superimposition of LIRD in this already tenuous setting may accelerate the disease process by further stressing these mechanisms, leading to decompensation and consequently more rapid disease progression.22,23,33 The slowing, rather than speeding, of ARGA we report in the current study thus likely reflects the absence of tissue damage associated with SDM, compared to micro- and nano-second CW lasers.6–15,31–33 Further study will show if more frequent treatment with panmacular SDM might result in an even greater slowing of ARGA progression.
The only known effects of SDM, sublethal to the RPE, are therapeutic. There are no known adverse treatment effects associated with SDM clinically, in vitro or in vivo.6–15,31,32 This is because therapeutic retinal laser effects arise from living cells affected, but not killed by laser exposure; and adverse retinal laser effects arise from LIRD, which is, at minimum, lethal to the RPE.6–15,34–50 The effects of thermal laser effects sublethal to the RPE are multivalent, catalytic, reparative, restorative and functionally normalizing to the retina.6–15,34–50
The response to SDM represents a physiologic “reset” phenomenon.6–15,34–50 This is because the currency of cellular dysfunction, induced by virtually any stressor, including ageing and all chronic progressive retinopathies, is protein misfolding. Because HSP-mediated correction of protein misfolding and consequent normalization of cell function is agnostic to the cause of protein misfolding, SDM acts as a non-specific trigger of disease-specific repair; much like the “reset” function common to electronic devices.
As AMD is a neurodegenerative disorder, such laser effects are by definition neuroprotective.11–14 These include down-regulation of VEGF and up-regulation of pigment epithelial-derived factor (PEDF); RPE heat-shock protein (HSP) activation and acceleration HSP-mediated protein repair in unhealthy cells; improved mitochondrial function; inhibition of apoptosis; reduced indicators of degenerative chronic and increased indicators of reparative acute inflammation; decreased reactive oxygen species and increased nitrous oxide and superoxide dismutase; improved Mueller cell function; reparative local and systemic immunomodulation and stem cell activation; modulation of tissue matrix metalloproteinases; and normalized RPE cytokine, chemokine and interleukin expression and response, improved retinal autoregulation. These are reflected in improvements in electrophysiology, multiple measures of visual function including microperimetry, mesopic visual acuity, contrast visual acuity, and reduced neovascular conversion following SDM in AMD.11,14,34–50 These SDM-induced changes occur as the result of functional normalization of the RPE/retina and represent re-establishment of normalized physiologic levels and relations in a global, rather than selective, response to treatment. We believe that SDM-induced normalization of retinal function, reflected by factors such as those listed above, is responsible for the slowing of ARGA reported in this study. Just as one would expect normalized cardiac and pulmonary function to decrease the likelihood of cardiac and pulmonary failure, it would seem reasonable to expect normalized macular function to decrease the risk of macular failure; in this case, progression of age-related geographic atrophy.
We describe the principle of improving retinal function as “homeotrophy”, or “normalizing”.11,12 The importance of the physiologic nature of the SDM treatment response may be illustrated by the following: Selective deletion of VEGFA in mice has been found to induce retinal atrophy and visual loss.51 This is because physiologic levels of VEGF are necessary to maintain normal retinal function. By contrast, SDM-induced VEGF reduction results in normalization of VEGF levels from pathologic to physiologic. In addition, SDM normalizes the physiologic balance of VEGF with many other factors, such as PEDF, by normalizing RPE chemokine expression and response in general, via the reset phenomenon. Along with improved RPE function and reduced markers of chronic inflammation, we expect that these laser-induced improvements in macular function may account for the inhibition ARGA progression noted in the current study.11,14,34–51
This study has limitations and weaknesses common to retrospective studies and should be read with the appropriate and customary caution. However, several aspects mitigate these weaknesses. Analysis included all eyes with ARGA in an EMR database, and all eligible eyes for study. Study endpoints and analysis techniques were simple, uniform, objective, and masked52,53 Treatment was uniform in all respects, employing the identical laser settings, treatment technique (confluent coverage of the retina), treatment area (panmacular), and number of spot applications in each treated eye; also safe, simple, and easily replicated. Follow up of both pre- and post-treatment is long. Despite the small size, the findings statistically robust and consistent with all prior studies of SDM, natural history studies of ARGA progression, and the low incidence of new CNV reported in eyes with high-risk AMD following panmacular SDM, another key indicator of AMD progression.15 As a critical test of scientific findings is reproducibility, we note that the methods employed in the current study are easily replicated.52,53 As ARGA is an important and otherwise untreatable cause of visual loss for millions worldwide, further study of prevention by panmacular SDM is indicated.1,2,4 Appearing highly effective and without adverse effects, SDM might, if confirmed, contribute significantly to the reduction of visual loss and disability due to AMD.
Funding
There is no funding to report.
Disclosure
Jeffrey K Luttrull reports equity for Replenish, Inc, and management and equity for Ojai Retinal Technologies, LLC, and Retinal Protection Sciences, LLC, during the conduct of the study. The authors report no other potential conflicts of interest for this work.
References
1. Age-Related Eye Disease Study Research Group. The age-related eye disease study system for classifying age-related macular degeneration from stereoscopic color fundus photographs: the Age-Related Eye Disease Study Report Number 6. Am J Ophthalmol. 2001;132(5):668–681.
2. Klein R, Meuer SM, Knudtson MD, Klein BE. The epidemiology of progression of pure geographic atrophy: the Beaver Dam Eye Study. Am Ophthalmol. 2008;146:692–699.
3. Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K, SEVEN-UP Study Group. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP). Ophthalmology. 2013;120:2292–2299.
4. Schmitz-Valckenberg S, Sadda S, Straugenghi G, et al. Geographic atrophy semantic considerations and literature review. Retina. 2016;36(12):2250–2264.
5. Boyers LN, Karimkhani C, Hilton J, Richheimer W, Dellaville RP. Global burden of eye and vision disease as reflected in the Cochrane Database of Systematic Reviews. JAMA Ophthalmol. 2015;133(1):25–31.
6. Luttrull JK, Musch MC, Mainster MA. Subthreshold diode micropulse photocoagulation for the treatment of clinically significant diabetic macular edema. Br J Ophthalmol. 2005;89(1):74–80.
7. Luttrull JK, Spink CJ. Serial optical coherence tomography of subthreshold diode laser micropulse photocoagulation for diabetic macular edema. Ophthalmic Surg Lasers Imag. 2006;37:370–377.
8. Luttrull JK, Spink CJ, Musch DA. Subthreshold diode micropulse panretinal photocoagulation for proliferative diabetic retinopathy. Eye. 2008;22(5):607–612.
9. Chhablani J, Roh YJ, Jobling AI, et al. Restorative retinal laser therapy: present state and future directions. Surv. Ophthalmol. 2017;4:
10. Luttrull JK, Chang DB, Margolis BWL, Dorin G, Luttrull DK. Laser re-sensitization of medically unresponsive neovascular age-related macular degeneration: efficacy and implications. Retina. 2015;35(6):1184–1194.
11. Luttrull JK, Margolis BWL. Functionally guided retinal protective therapy as prophylaxis for age-related and inherited retinal degenerations. A pilot study. Invest Ophthalmol Vis Sci. 2016;7(1):265–275. doi:10.1167/iovs.15-18163
12. Luttrull JK. Improved retinal and visual function following subthreshold diode micropulse laser (SDM) for retinitis pigmentosa. Eye. 2018. doi:10.1038/s41433-018-0017-3
13. Luttrull JK, Samples JR, Kent D, Lum BJ. Panmacular subthreshold diode micropulse laser (SDM) as neuroprotective therapy in primary open-angle glaucoma. Glaucoma Res. 2018;281–294.
14. Luttrull JK, Kent D. Modern retinal laser for neuroprotection in open-angle glaucoma. In: Samples JR, Ahmed IIK, editors. New Concepts in Glaucoma Surgery1. Amsterdam, Kugler Publications; 2017.
15. Luttrull JK, Sinclair SH, Elmann S, Glaser BM. Low incidence of choroidal neovascularization following subthreshold diode micropulse laser (SDM) for high-risk AMD. PLoS One. 2017;13(8):e0202097. doi:10.1371/journal.pone.0202097
16. Shen L, Liu F, Nardini HG, Del Priore LV. Natural history of geographic atrophy in untreated eyes with non exudative age-related macular degeneration. A systematic review and met-analysis. Ophthalmol Retina;2016. doi:10.1016/j.oret.2018.01.019
17. Keenan TD, Agrón E, Domalpally A, et al. Progression of geographic atrophy in age-related macular degeneration. AREDS report number 16. Ophthalmol. 2018;125(12):1913–1928.
18. Pau M, Moller PT, Kunzel SH, et al. Type 1 choroidal neovascularization is associated with localized progression of atrophy in age-related macular degeneration. Ophthalmol Retina. 2020;3:238–248.
19. Holz FG, Sadda SR, Busbee B, et al. Efficacy and Safety of Lampalizumab for geographic atrophy due to age-related macular degeneration: chroma and Spectri Phase 3 randomized clinical trials. JAMA Ophthalmol. 2018;136(6):666–677.
20. Uji A, Nittala NM, Hariri A, Velaga SB, Sadda SR. Directional kinetics analysis of the progression of geographic atrophy. Graefe’s Arch Clin Exp Ophthalmol;2010:1.
21. Monés J, Biarnés M. The rate of progression of geographic atrophy decreases with increasing baseline lesion size even after the square root transformation. Transl Vis Sci Technol. 2018;7(6):40.
22. Roider J, Brinkmann R, Wirbelauer C, Laqua H, Birngruber R. Subthreshold (retinal pigment epithelium) photocoagulation in macular diseases: a pilot study. Br J Ophthalmol. 2000;84:40–47.
23. Guymer RH, Wu Z, Hodgson LAB, et al. Subthreshold nanosecond laser intervention for age-related macular degeneration: the LEAD randomized controlled clinical trial. Ophthalmology. 2018;20:
24. Virgili G, Michelessi M, Parodi MB, Bacherini D, Evans JR. Laser treatment of drusen to prevent progression to advanced age-related macular degeneration. Syst Rev. 2015;10:CD006537. doi:10.1002/14651858.CD006537.pub3
25. Complications of Age-Related Macular Degeneration Prevention Trial Research Group. Laser treatment in patients with bilateral large drusen: the complications of age-related macular degeneration prevention trial. Ophthalmology. 2006;113(11):1974–1986.
26. Ho AC, Maguire MG, Yoken J, et al. Laser-induced drusen reduction improves visual function at 1 year. Choroidal Neovascularization Prevention Trial Research Group. Ophthalmology. 1999;106(7):1367–1373.
27. Owens SL, Bunce C, Brannon AJ, et al. Prophylactic laser treatment hastens choroidal neovascularization in unilateral age-related maculopathy: final results of the drusen laser study. Am J Ophthalmol. 2006;141(2):276–281.
28. Rodanant N, Friberg TR, Cheng L, et al. Predictors of drusen reduction after subthreshold infrared (810 nm) diode laser macular grid photocoagulation for nonexudative age-related macular degeneration. Am J Ophthalmol. 2002;134(4):577–585.
29. Kaiser RS, Berger JW, Maguire MG, Ho AC, Javornik NB. Choroidal Neovascularization Prevention Trial Study Group. Laser burn intensity and the risk for choroidal neovascularization in the CNVPT Fellow Eye Study. Arch Ophthalmol;2020.
30. Owens SL, Bunce C, Brannon AJ, Wormald R, Bird AC. Drusen Laser Study Group. Prophylactic laser treatment appears to promote choroidal neovascularisation in high-risk ARM: results of an interim analysis. Eye. 2003;17(5):623–627.
31. Luttrull JK, Dorin G. Subthreshold diode micropulse laser photocoagulation (SDM) as invisible retinal phototherapy for diabetic macular edema: a review. Curr Diabetes Rev. 2012;8:274–284.
32. Luttrull JK, Sinclair SH. Safety of transfoveal subthreshold diode micropulse laser for fovea-involving diabetic macular edema in eyes with good visual acuity. Retina. 2014;34:2010–2020.
33. Rosenfeld PJ, Feuer WJ. Warning: do not treat intermediate AMD with laser therapy. Am J Ophthalmol. 2019;125(6):839–840.
34. Kregel K. Invited Review: heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J Appl Physiol. 2002;5(92):2177–2186.
35. Karu T. Photobiology of low-power laser effects. Rev Health Phys. 1989;56(5):691–704.
36. Lund DJ, Sliney DH. A new understanding of multiple-pulsed laser-induced retinal injury thresholds. Health Phys. 2014;106(4):505–515.
37. Lavinsky D, Wang J, Huie P, et al. Nondamaging Retinal Laser Therapy: rationale and Applications to the Macula. Invest Ophthalmol Vis Sci. 2016;57(6):2488–2500.
38. Luttrull JK, Sramek C, Palanker D, Spink CJ, Musch DC. Long-term safety, high-resolution imaging, and tissue temperature modeling of subvisible diode micropulse photocoagulation for retinovascular macular edema. Retina. 2012;32(2):375–386.
39. Gao X, Xing D. Molecular mechanisms of cell proliferation induced by low power laser irradiation. J Biomed Sci. 2009;16:4.
40. Karu TI, Kolyakov SF. Exact action spectra for cellular responses relevant to phototherapy. Photomed Laser Surg. 2005;23:355–361.
41. Inagaki K, Shuo T, Katakura K, Ebihara N, Murakami A, Ohkoshi K. Sublethal Photothermal Stimulation with a Micropulse Laser Induces Heat Shock Protein Expression in ARPE-19 Cells. J Ophthalmol. 2015;2015:729792.
42. Chang DB, Luttrull JK. Comparison of subthreshold 577nm and 810nm micropulse laser effects on heat-shock protein activation kinetics: implications for treatment efficacy and safety. Trans Vis Sci Tech. 2020:1.
43. Midena E, Bini S, Martini F, et al. Changes of aqueous humor Muller cells’ biomarkers in human patients affected by diabetic macular edema after subthreshold micropulse laser treatment. Retina. 2018;8. doi:10.1097/IAE.0000000000002356.
44. Iwami H, Pruessner J, Shariaki K, Brinkmann R, Miura Y. Protective effect of a laser-induced sub-lethal temperature rise on RPE cells from oxidative stress. Exp Eye Res. 2014. doi:10.1016/j.exer.2014.04.014
45. Hattenbach LO, Beck KF, Pfeilschifter J, Koch F, Ohrloff C, Schake W. Pigment epithelium- derived factor is up regulated in photocoagulated human retinal pigment epithelial cells. Ophthalmic Res. 2005;37:341–346.
46. Caballero S, Kent DL, Sengupta N, et al. Bone Marrow-Derived Cell Recruitment to the Neurosensory Retina and Retinal Pigment Epithelial Cell Layer Following Subthreshold Retinal Phototherapy. Invest Ophthalmol Vis Sci. 2017;58(12):5164–5176. doi:10.1167/iovs.16-20736
47. De Cilla S, Vezzola D, Farruggio S, et al. The subthreshold micropulse laser treatment of the retina restores the oxidant/antioxidant balance and counteracts programmed forms of cell death in the mice eyes. Acta Ophthalmol. 2018. doi:10.1111/aos.13995
48. Flaxel C, Bradle J, Acott T, Samples JR. Retinal pigment epithelium produces matrix metalloproteinases after laser treatment. Retina. 2007;27:629–634.
49. Subrizi A, Toropainen E, Ramsay E, et al. Oxidative stress protection by exogenous delivery of rhHsp70 chaperone to the retinal pigment epithelium (RPE), a possible therapeutic strategy against RPE degeneration. Pharm Res. 2015;32(1):211–221.
50. Kannan R, Sreekumar PG, Hinton DR. Alpha crystallins in the retinal pigment epithelium and implications for the pathogenesis and treatment of age-related macular degeneration. Biochim Biophys Acta. 2016;1860(1 Pt B):258–268.
51. Kurihara T, Westenskow PD, Bravo S, et al. Targeted deletion of Vegfa in adult mice induces vision loss. J Clin Invest. 2012;122(11):4213–4217.
52. Bacon F. Novum Organnum. In: Devey J, editor. New York: P. F. Collier & Sons; 1902.
53. Nature Publishing; Policies: Availability of data, materials, code and protocols. https://www.nature.com/authors/policies/availability.html#data. Accessed August 17, 2020.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.