Back to Journals » Nature and Science of Sleep » Volume 6
Sleep apnea in children with refractory monosymptomatic nocturnal enuresis
Authors El-Mitwalli A, Bediwy AS , Zaher AA, Belal T, Saleh ABM
Received 17 December 2013
Accepted for publication 21 January 2014
Published 13 March 2014 Volume 2014:6 Pages 37—42
DOI https://doi.org/10.2147/NSS.S59317
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Ashraf El-Mitwalli,1 Adel Salah Bediwy,2 Ashraf Ahmed Zaher,1 Tamer Belal,1 Abdel Baset M Saleh3
1Neurology Department, Faculty of Medicine, Mansoura University, Mansoura, 2Chest Department, Faculty of Medicine, Tanta University, Tanta, 3Chest Department, Faculty of Medicine, Mansoura University, Mansoura, Egypt
Background: Children with nocturnal enuresis (NE) are believed to have deep sleep with high arousal threshold. Studies suggest that obstructive sleep apnea–hypopnea syndrome (OSAHS) and NE are common problems during childhood. We sought to assess the prevalence of OSAHS in children with refractory NE and whether its severity is associated with the frequency of bedwetting.
Methods: The study group comprised 43 children with refractory monosymptomatic NE and a control group of 30 children, both aged 6–12 years. All subjects underwent thorough neurological examination, one night of polysomnography only for the patient group, and a lumbosacral plain X-ray to exclude spina bifida.
Results: The groups were well matched. Two subjects of the control group had mild OSAHS. The mean age of the patients was (9.19±2.4 years), 26 were boys, and 67% showed frequent NE (>3 days bedwetting/week). Patients with NE had significantly higher rates of OSAHS (P<0.0001); three patients had mild, 12 had moderate, and eleven showed severe OSAHS. There was no significant statistical difference among patients having OSAHS in relation to age, sex, or family history of NE. The frequency of bedwetting was statistically significantly higher in patients with severe OSAHS (P=0.003).
Conclusion: Patients with refractory NE had a significantly higher prevalence of OSAHS with no sex difference. The frequency of bedwetting was higher in patients with severe OSAHS.
Keywords: nocturnal enuresis, refractory, OSA
Introduction
Nocturnal enuresis (NE) refers to the involuntary loss of urine during the night after the age of 5 years, when children are expected to have achieved full bladder control at night.1 It is classified as primary when the child has never achieved nighttime dryness and secondary when bedwetting occurs after dryness for at least 6 months.2 Moreover, NE is divided into monosymptomatic NE (MNE) with no daytime urinary symptoms and nonmonosymptomatic NE if accompanied by daytime urinary symptoms.3 The prevalence of enuresis (at least 1 night per week) has been reported to be 1.6% to 13.7%, depending on the subject’s age and ethnic and cultural characteristics.2,4,5
Enuretic children suffer from low self-esteem,6 reduced fine motor coordination, visuomotor integration abnormalities,7 attention deficit hyperactivity disorder,8 reading difficulties,9 and there may be an association with migraine.10 Moreover the risk of psychosocial comorbidity is higher in therapy-resistant enuresis.11 Refractory NE is defined as less than 50% improvement in symptoms with an adequate trial of treatment (3 months).12
NE has been related to obstructive sleep-disordered breathing (SDB) in both adults13,14 and children.15,16 The association between the two conditions in pediatric subjects is supported by the decrease or complete resolution of bedwetting after successful treatment of SDB with adenotonsillectomy or intranasal corticosteroids.16,17 Moreover, effective treatment of obstructive sleep apnea–hypopnea in adults by continuous positive airway pressure has improved enuresis.13
Studies have assessed NE in patients with upper airway obstruction.18–23 Nonetheless, to our knowledge none has assessed obstructive sleep apnea (OSA) in patients with NE and whether the frequency of bedwetting is related to the severity of OSA. The aim of our study was to objectively assess the prevalence of OSA using polysomnography in patients with refractory MNE and whether the frequency of bedwetting is related to the severity of OSA.
Materials and methods
Patients
Included in the study were consecutive children with primary MNE, aged 6–12 years, and medication resistant, who were recruited from the neurology outpatient clinic in the Department of Neurology at Mansoura University Hospital in Mansoura, Egypt over a 3-year period (2010–2013). A control group of healthy children, not complaining of symptoms related to urinary tract or ear, nose, and throat conditions, were selected to be similar with respect to age and sex. They were recruited through the personnel of our hospital and their acquaintances. The study was approved by the medical ethics committee of Mansoura University, and informed consent was given by the parents of each child in both groups.
Inclusion criteria for the patient group were age from 6–12 years, presence of MNE (with no daytime urinary symptoms), and refractory MNE (either no response or less than 50% symptom improvement despite more than 3 months of continuous medication [compliance with medication had been assured by the parents]).
Exclusion criteria for the patient group were clinical, laboratory, or radiological signs suggestive of an underlying neurological disease other than MNE; psychological problems; diabetes mellitus and diabetes insipidus; ear, nose, and throat problems; daytime urinary symptoms such as incontinence, urgency, and frequency (defined as >10 voidings per day).
Inclusion criteria for the control group were age-, sex-, and body mass index-matched normal healthy children without NE.
Exclusion criteria for the control group were neurological disease; psychological problems; diabetes mellitus and diabetes insipidus; ear, nose, and throat problems; presence of urinary symptoms, either NE or daytime symptoms.
Methods
All patients were subjected to a thorough history taking, including the frequency of bedwetting per week, compliance with medication, snoring, seizures, perianal itching, vulvovaginitis, excessive thirst, nighttime drinking (diabetes mellitus and diabetes insipidus), and family history of NE. Patients underwent anteroposterior plain X-ray of the spine to detect and exclude individuals with spina bifida occulta.
A single night of attended polysomnography (Compumedics Limited, Melbourne, VIC, Australia) was performed on each subject at the sleep laboratory, and no drugs were used to induce sleep. Two channel electroencephalogram, electrooculogram, and tibialis and chin electromyogram were registered using standard methods. Oronasal airflow was recorded by thermistor, and thoracic and abdominal respiratory efforts were measured by impedance plethysmography. Body position was recorded using body position sensor. Oxygen saturation was measured by finger pulse oximetry (ResMed Model 305A; San Diego, CA, USA) and the electrocardiogram from a precardial lead. Sleep data were staged manually according to standard criteria.24 An apnea was defined as cessation of airflow or reduction of thermistor signal to less than 10% of the normal flow, with a duration of at least 10 seconds and oxygen desaturation of >4%; and hypopnea was defined as a discernible reduction of airflow to between 50% and 75% of at least 10 seconds duration, followed by either an arousal or a desaturation of ≥4%.25
The data were coded and entered into a computer using Statistical Package for Social Sciences (SPSS), version 16.0 (IBM Corporation, Armonk, NY, USA). Data are expressed as mean ± standard deviation unless otherwise stated. Student’s t-test was used to ascertain the significance of differences between mean values of two continuous variables, and the Mann–Whitney test was used for nonparametric distribution. Chi-square analysis was performed to test for differences in proportions of categorical variables between two or more groups. The level of P<0.05 was considered as the cut-off value of significance.
Results
Between January 2010 and May 2013, a total of 43 children with primary monosymptomatic, pharmacologically-resistant NE and 30 age- and sex-matched children were enrolled in our study. Thirty nine (90%) patients had been initially on imipramine (25–50 mg/night) for 1 to 4 months without improvement and eventually shifted to desmopressin while only four were already on desmopressin. A total of 22 patients (51%) continued on desmopressin (nasal: 20–40 μg/night or oral: 0.2–0.4 mg) alone while the remaining 21 patients have been on combined desmopressin and oxybutynin (5–10 mg/night) for 9.4±5.8 months.
There were no statistically significant differences in demographic characteristics between patients and controls (Table 1). Two subjects (6.6%) of the control group showed mild OSAHS. Sixty seven percent of the patients showed frequent NE (>3 days bedwetting/week). Twenty six (60%) out of the 43 patients showed OSAHS. Compared with controls, patients showed significantly higher rates of OSAHS (P<0.0001); three patients had mild, 12 had moderate, and eleven had severe OSAHS (Table 2). The frequency of bedwetting was statistically significantly higher in patients with severe OSAHS (P=0.03) (Table 3). There was no significant statistical difference among patients having OSAHS regarding the sex (12 females and 14 males; P=0.098) or the family history of NE (out of the 26 patients, eleven had a positive family history of NE; P=0.52).
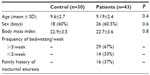  | Table 1 Demographic characteristics of the studied groups |
  | Table 2 Polysomnographic criteria of the studied groups |
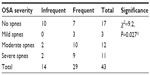  | Table 3 Relation between OSAHS and frequency of bedwetting |
Discussion
NE in children is generally benign but can cause emotional stress to children and their parents.26 The pathogenesis of enuresis may be based on a mutual balance of three basic mechanisms: bladder capacity, nocturnal production of urine, and the threshold of arousability.27,28 Among NE children, sleep could be strongly altered, thus helping to affirm the hypothesis that NE tends to alter sleep architecture, or it could itself be the consequence of an abnormal sleep structure.29
The prevalence of OSAHS in children ranges from 0.7% to 3% in different epidemiological studies.30,31 The incidence peak was found in preschool children within the age in which tonsil hypertrophy and adenoid are more common.32 In the present study, two subjects of the control group showed OSAHS; only one (3%) showed clinically significant OSA (AHI >1). The incidence of NE among the children with upper airway obstruction was 34.5%,33 and the prevalence of NE in OSA patients is 47% compared to17% for non-OSA children.15 The frequency of NE in Chinese children with OSA was 51.6% compared to 15.8% in primary snorers.34
To the best of our knowledge, no study has evaluated the prevalence of OSA in patients with refractory NE. Our study showed significantly higher rates of OSAHS in patients with refractory NE compared with controls. Sixty percent of the patients showed OSAHS, which is still higher than prevalence of NE in patients with OSA; this may reflect the selection of a specific group of therapy-resistant patients or the reverse method used for assessment.
The current study showed no significant statistical difference among patients having OSAHS regarding the sex or the family history of NE. Some studies revealed a higher prevalence of SDB symptoms among boys,35–39 and others showed no difference by sex40–48 while a single study reported a higher prevalence of snoring in girls.49 Prevalence of NE was increased with increasing severity of OSA in girls.50 In our study, 67% of the OSA patients showed frequent NE (>3 days bedwetting/week), and frequency of bedwetting was statistically significantly higher in patients with severe OSAHS.
The treatment protocol for NE recommended by the International Children’s Continence Society is initially lifestyle modification, followed by alarm treatment, and pharmacotherapy.51 Despite the high percentage of the adherence to enuresis treatment which is more than 70%,52 a substantial number of enuretics do not become dry in response to desmopressin.53 Moreover, tolterodine in monotherapy had no proven effect. Imipramine was better than placebo, but side effects were common.54 Oxybutynin alone was not effective in management of NE55 but in combination with desmopressin gave significantly faster and more cost-effective results.56 Alarm therapy was effective for cases refractory to pharmacotherapy, and nonresponders to alarm therapy were also refractory to pharmacotherapy.57 In patients with refractory MNE, a combined approach may improve enuresis,58–60 and methods such as posterior tibial nerve stimulation,61 interferential electrical stimulation therapy,62 acupuncture treatment,63 abdominal and pelvic floor muscle retraining,64 and assumption of unusual posture during sleep, in particular legs retracting or crossing during sleep to enlarge the diaphragmatic excursion and promote alveolar gas exchanges,65 have been tried in refractory primary MNE. The identification of SDB may open new therapeutic pathways, in terms of surgery for the upper obstructed airway for those patients with NE who do not respond to standard treatment.66
Patients with NE had significantly higher positive SDB than controls.67 NE is associated with OSA and appears to improve or resolve following adenotonsillectomy.15,16,67–69 In children diagnosed with SDB, the preoperative prevalence of enuresis was 31% and the postoperative was 16% in a review of 14 studies.70 The acquisition of urinary continence seems to be complex and is probably not yet completely understood.71 Increased brain natriuretic peptide levels may account for the increased prevalence of enuresis in the context of SDB;67 children with refractory NE showed abnormal sleep architecture, high incidences of periodic limb movements in sleep, and increased cortical arousability, leading to awakening.72
The vast majority of the published studies have investigated NE among children with SDB, and to the best of our knowledge no study has investigated OSA and its severity in relation to the frequency of NE among children with pharmacologically-resistant NE. Our study shows higher frequency of OSA in children with refractory NE compared with the controls and a positive correlation between frequency of NE and the severity of OSA. In conclusion, patients with refractory NE had a significantly higher prevalence of OSAHS with no sex difference. The frequency of bedwetting was higher in patients with severe OSAHS. These finding may open up further investigative pathways and treatment modalities for patients with refractory NE with OSA.
Disclosure
The authors report no conflicts of interest in this work.
References
Byrd RS, Weitzman M, Lanphear NE, Auinger P. Bed-wetting in US children: epidemiology and related behavior problems. Pediatrics. 1996;98(3 Pt 1):414–419. | |
Hjalmas K, Arnold T, Bower W, et al. Nocturnal enuresis: an international evidence based management strategy. J Urol. 2004;171(6 Pt 2):2545–2561. | |
Butler RJ, and Holland P. The three systems: A conceptual way of understanding nocturnal enuresis. Scand J Urol Nephrol. 2000;34:270–277. | |
Lee SD, Sohn DW, Lee JZ, Park NC, Chung MK. An epidemiological study of enuresis in Korean children. BJU Int. 2000;85(7):869–873. | |
Gümüş B, Vurgun N, Lekili M, Işcan A, Müezzinoğlu T, Büyuksu C. Prevalence of nocturnal enuresis and accompanying factors in children aged 7–11 years in Turkey. Acta Paediatr. 1999;88(12):1369–1372. | |
Hägglöf B, Andrén O, Bergström E, Marklund L, Wendelius M. Self-esteem before and after treatment in children with nocturnal enuresis and urinary incontinence. Scand J Urol Nephrol Suppl. 1997; 183:79–82. | |
Esposito M, Gallai B, Parisi L, et al. Visuomotor competencies and primary monosymptomatic nocturnal enuresis in prepubertal aged children. Neuropsychiatr Dis Treat. 2013;9:921–926. | |
Duel BP, Steinberg-Epstein R, Hill M, Lerner M. A survey of voiding dysfunction in children with attention deficit-hyperactivity disorder. J Urol. 2003;170(4 Pt 2):1521–1523; discussion 1523–1524. | |
Esposito M, Carotenuto M, Roccella M. Primary nocturnal enuresis and learning disability. Minerva Pediatr. 2011;63(2):99–104. | |
Carotenuto M, Esposito M, Pascotto A. Migraine and enuresis in children: an unusual correlation? Med Hypotheses. 2010;75(1):120–122. | |
von Gontard A, Mauer-Mucke K, Plück J, Berner W, Lehmkuhl G. Clinical behavioral problems in day- and night-wetting children. Pediatr Nephrol. 1999;13(8):662–667. | |
Nevéus T, von Gontard A, Hoebeke P, et al. The standardization of terminology of lower urinary tract function in children and adolescents: report from the Standardisation Committee of the International Children’s Continence Society. J Urol. 2006;176(1):314–324. | |
Steers WD, Suratt PM. Sleep apnoea as a cause of daytime and nocturnal enuresis. Lancet. 1997;349(9065):1604. | |
Kramer NR, Bonitati AE, Millman RP. Enuresis and obstructive sleep apnea in adults. Chest. 1998;114(2):634–637. | |
Brooks LJ, Topol HI. Enuresis in children with sleep apnea. J Pediatr. 2003;142(5):515–518. | |
Weider DJ, Sateia MJ, West RP. Nocturnal enuresis in children with upper airway obstruction. Otolaryngol Head Neck Surg. 1991;105(3):427–432. | |
Alexopoulos EI, Kaditis AG, Kostadima E, Gourgoulianis K. Resolution of nocturnal enuresis in snoring children after treatment with nasal budesonide. Urology. 2005;66(1):194. | |
Guilleminault C. Obstructive sleep apnea. The clinical syndrome and historical perspective. Med Clin North Am. 1985;69(6):1187–1203. | |
Maddern BR. Snoring and obstructive sleep apnea syndrome. In: Bluestone CD, Stool SE, Scheetz MD, editors. Pediatric Otolaryngology. Philadelphia, PA: WB Saunders Company;1990:927–934. | |
Timms DJ. Rapid maxillary expansion in the treatment of nocturnal enuresis. Angle Orthod. 1990;60(3):229–233. | |
Grundfast KM, Wittich DJ Jr. Adenotonsillar hypertrophy and upper airway obstruction in evolutionary perspective. Laryngoscope. 1982;92(6 Pt 1):650–656. | |
Guilleminault C, Eldridge FL, Tilkian A, Simmons FB, Dement WC. Sleep apnea syndrome due to upper airway obstruction: a review of 25 cases. Arch Intern Med. 1977;137(3):296–300. | |
Nowak KC, Weider DJ. Pediatric nocturnal enuresis secondary to airway obstruction from cleft palate repair. Clin Pediatr (Phila). 1998;37(11):653–657. | |
Rechtschaffen A, and Kales A. A Manual of Standardized Terminology Techniques and Scoring System for Sleep Stages of Human Subjects. Los Angeles, CA; UCLA Brain Information Service/Brain Research Institute;1968. | |
Whyte KF, Allen MB, Jeffrey AA, Gould GA, Douglas NJ. Clinical features of the sleep apnea/hypopnea syndrome. Q J Med. 1989;72(269):659–666. | |
Moffatt ME. Nocturnal enuresis: psychologic implications of treatment and nontreatment. J Pediatr. 1989;114(4 Pt 2):697–704. | |
Wolfish NM, Pivik RT, Busby KA. Elevated sleep arousal thresholds in enuretic boys: clinical implications. Acta Paediatr. 1997;86(4):381–384. | |
Wolfish NM. Sleep/arousal and enuresis subtypes. J Urol. 2001;166(6):2444–2447. | |
Esposito M, Gallai B, Parisi L, et al. Primary nocturnal enuresis as a risk factor for sleep disorders: an observational questionnaire-based multicenter study. Neuropsychiatr Dis Treat. 2013;9:437–443. | |
Brunetti L, Rana S, Lospalluti ML, et al. Prevalence of obstructive sleep apnea syndrome in a cohort of 1,207 children of southern Italy. Chest. 2001;120(6):1930–1935. | |
Anuntaseree W, Rookkapan K, Kuasirikul S, Thongsuksai P. Snoring and obstructive sleep apnea in Thai school-age children: prevalence and predisposing factors. Pediatr Pulmonol. 2001;32(3):222–227. | |
Bower CM, Buckmiller L. What’s new in pediatric obstructive sleep apnea? Curr Opin Otolaryngol Head Neck Surg. 2001;9:352–358. | |
Cinar U, Vural C, Cakir B, Topuz E, Karaman MI, Turgut S. Nocturnal enuresis and upper airway obstruction. Int J Pediatr Otorhinolaryngol. 2001;59(2):115–118. | |
Xu Z, Cheuk DK, Lee SL. Clinical evaluation in predicting childhood obstructive sleep apnea. Chest. 2006;130(6):1765–1771. | |
Chng SY, Goh DY, Wang XS, Tan TN, Ong NB. Snoring and atopic disease: a strong association. Pediatr Pulmonol. 2004;38(3):210–216. | |
Liu X, Ma Y, Wang Y, et al. Brief report: an epidemiologic survey of the prevalence of sleep disorders among children 2 to 12 years old in Beijing, China. Pediatrics. 2005;115(Suppl 1):266–268. | |
Kaditis AG, Finder J, Alexopoulos EI, et al. Sleep-disordered breathing in 3,680 Greek children. Pediatr Pulmonol. 2004;37(6):499–509. | |
Ng DK, Kwok KL, Cheung JM, et al. Prevalence of sleep problems in Hong Kong primary school children: a community-based telephone survey. Chest. 2005;128(3):1315–1323. | |
Ersu R, Arman AR, Save D, et al. Prevalence of snoring and symptoms of sleep-disordered breathing in primary school children in Istanbul. Chest. 2004;126(1):19–24. | |
Bidad K, Anari S, Aghamohamadi A, Gholami N, Zadhush S, Moaieri H. Prevalence and correlates of snoring in adolescents. Iran J Allergy Asthma Immunol. 2006;5(3):127–132. | |
Owens JA, Spirito A, McGuinn M, Nobile C. Sleep habits and sleep disturbance in elementary school-aged children. J Dev Behav Pediatr. 2000;21(1):27–36. | |
Corbo GM, Fuciarelli F, Foresi A, De Benedetto F. Snoring in children: association with respiratory symptoms and passive smoking. BMJ. 1989;299(6714):1491–1494. | |
Goodwin JL, Babar SI, Kaemingk KL, et al; Tucson Children’s Assessment of Sleep Apnea Study. Symptoms related to sleep-disordered breathing in white and Hispanic children: the Tucson Children’s Assessment of Sleep Apnea Study. Chest. 2003;124(1):196–203. | |
Sogut A, Altin R, Uzun L, et al. Prevalence of obstructive sleep apnea syndrome and associated symptoms in 3–11-year-old Turkish children. Pediatr Pulmonol. 2005;39(3):251–256. | |
Zhang G, Spickett J, Rumchev K, Lee AH, Stick S. Snoring in primary school children and domestic environment: a Perth school based study. Respir Res. 2004;5:19. | |
Lu LR, Peat JK, Sullivan CE. Snoring in preschool children: prevalence and association with nocturnal cough and asthma. Chest. 2003;124(2):587–593. | |
Gislason T, Benediktsdóttir B. Snoring, apneic episodes, and nocturnal hypoxemia among children 6 months to 6 years old. An epidemiologic study of lower limit of prevalence. Chest. 1995;107(4):963–966. | |
Owen GO, Canter RJ, Robinson A. Snoring, apnoea and ENT symptoms in the paediatric community. Clin Otolaryngol Allied Sci. 1996;21(2):130–134. | |
Smedje H, Broman JE, Hetta J. Parents’ reports of disturbed sleep in 5–7-year-old Swedish children. Acta Paediatr. 1999;88(8):858–865. | |
Su MS, Li AM, So HK, Au CT, Ho C, Wing YK. Nocturnal enuresis in children: prevalence, correlates, and relationship with obstructive sleep apnea. J Pediatr. 2011;159(2):238–242. e1. | |
Nevéus T, Eggert P, Evans J, et al; International Children’s Continence Society. Evaluation of and treatment for monosymptomatic enuresis: a standardization document from the International Children’s Continence Society. J Urol. 2010;183(2):441–447. | |
Baeyens D, Lierman A, Roeyers H, Hoebeke P, Walle JV. Adherence in children with nocturnal enuresis. J Pediatr Urol. 2009;5(2):105–109. | |
Tullus K, Bergström R, Fosdal I, Winnergård I, Hjälmås K. Efficacy and safety during long-term treatment of primary monosymptomatic nocturnal enuresis with desmopressin. Swedish Enuresis Trial Group. Acta Paediatr. 1999;88(11):1274–1278. | |
Nevéus T, Tullus K. Tolterodine and imipramine in refractory enuresis; a placebo-controlled crossover study. Pediatr Nephrol. 2008;23(2):263–267. | |
Lovering JS, Tallett SE, McKendry JB. Oxybutynin efficacy in the treatment of primary enuresis. Pediatrics. 1988;82(1):104–106. | |
Lee T, Suh HJ, Lee HJ, Lee JE. Comparison of effects of treatment of primary nocturnal enuresis with oxybutynin plus desmopressin, desmopressin alone or imipramine alone: a randomized controlled clinical trial. J Urol. 2005;174(3):1084–1087. | |
Kawauchi A, Naitoh Y, Yoneda K, et al. Refractory enuresis related to alarm therapy. J Pediatr Urol. 2006;2(6):579–582. | |
Vermandel A, de Wachter S, Wyndaele JJ. Refractory monosymptomatic nocturnal enuresis: a combined stepwise approach in childhood and follow-up into adolescence, with attention to the clinical value of normalizing bladder capacity. BJU Int. 2005;96(4):629–633. | |
Austin PF, Ferguson G, Yan Y, Campigotto MJ, Royer ME, Coplen DE. Combination therapy with desmopressin and an anticholinergic medication for nonresponders to desmopressin for monosymptomatic nocturnal enuresis: a randomized, double-blind, placebo-controlled trial. Pediatrics. 2008;122(5):1027–1032. | |
Ahmed AF, Amin MM, Ali MM, Shalaby EA. Efficacy of an enuresis alarm, desmopressin, and combination therapy in the treatment of saudi children with primary monosymptomatic nocturnal enuresis. Korean J Urol. 2013;54(11):783–790. | |
Raheem AA, Farahat Y, El-Gamal O, et al. Role of posterior tibial nerve stimulation in the treatment of refractory monosymptomatic nocturnal enuresis: a pilot study. J Urol. 2013;189(4):1514–1518. | |
Lee HE, Park K. Efficacy of salvage interferential electrical stimulation therapy in patients with medication-refractory enuresis: a pilot study. Int Neurourol J. 2013;17(3):139–144. | |
El Koumi MA, Ahmed SA, Salama AM. Acupuncture efficacy in the treatment of persistent primary nocturnal enuresis. Arab J Nephrol Transplant. 2013;6(3):173–176. | |
Zivkovic V, Lazovic M, Vlajkovic M, et al. Diaphragmatic breathing exercises and pelvic floor retraining in children with dysfunctional voiding. Eur J Phys Rehabil Med. 2012;48(3):413–421. | |
Carotenuto M, Gimigliano F, Fiordelisi G, Ruberto M, Esposito M. Positional abnormalities during sleep in children affected by obstructive sleep apnea syndrome: the putative role of kinetic muscular chains. Med Hypotheses. 2013;81(2):306–308. | |
Waleed FE, Samia AF, Samar MF. Impact of sleep-disordered breathing and its treatment on children with primary nocturnal enuresis. Swiss Med Wkly. 2011;141:w13216. | |
Stone J, Malone PS, Atwill D, McGrigor V, Hill CM. Symptoms of sleep-disordered breathing in children with nocturnal enuresis. J Pediatr Urol. 2008;4(3):197–202. | |
Ahmadi MS, Amirhassani S, Poorolajal J. The effect of adenotonsillectomy on pediatric nocturnal enuresis: a prospective cohort study. Iran J Otorhinolaryngol. 2013;25(70):37–40. | |
Firoozi F, Batniji R, Aslan AR, Longhurst PA, Kogan BA. Resolution of diurnal incontinence and nocturnal enuresis after adenotonsillectomy in children. J Urol. 2006;175(5):1885–1888; discussion 1888. | |
Jeyakumar A, Rahman SI, Armbrecht ES, Mitchell R. The association between sleep-disordered breathing and enuresis in children. Laryngoscope. 2012;122(8):1873–1877. | |
Naseri M, Hiradfar M. Monosymptomatic and non-monosymptomatic nocturnal enuresis: a clinical evaluation. Arch Iran Med. 2012;15(11):702–706. | |
Dhondt K, Raes A, Hoebeke P, Van Laecke E, Van Herzeele C, Vande Walle J. Abnormal sleep architecture and refractory nocturnal enuresis. J Urol. 2009;182(Suppl 4):1961–1965. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
