Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Skin Microbiota Profiles from Tape Stripping and Skin Biopsy Samples of Patients with Psoriasis Treated with Narrowband Ultraviolet B
Authors Rungjang A, Meephansan J , Payungporn S, Sawaswong V, Chanchaem P, Pureesrisak P , Wongpiyabovorn J, Thio HB
Received 16 May 2022
Accepted for publication 12 August 2022
Published 30 August 2022 Volume 2022:15 Pages 1767—1778
DOI https://doi.org/10.2147/CCID.S374871
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Atiya Rungjang,1 Jitlada Meephansan,1 Sunchai Payungporn,2 Vorthon Sawaswong,2 Prangwalai Chanchaem,2 Purit Pureesrisak,3 Jongkonnee Wongpiyabovorn,4 Hok Bing Thio5
1Division of Dermatology, Chulabhorn International College of Medicine, Thammasat University, Pathum Thani, Thailand; 2The Research Unit of Systems Microbiology, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand; 3Division of Dermatology, Department of Medicine, Rajavithi Hospital, Ministry of Public Health, Bangkok, Thailand; 4Division of Immunology, Department of Microbiology, Faculty of Medicine, Center of Excellence in Immunology and Immune Mediated Diseases, Chulalongkorn University, Bangkok, 10330, Thailand; 5Department of Dermatology, Erasmus University Medical Center, Rotterdam, the Netherlands
Correspondence: Jitlada Meephansan, Division of Dermatology, Chulabhorn International College of Medicine, Thammasat University, Rangsit Campus, Klong Luang, Pathum Thani, 12120, Thailand, Tel +66 0 2564-4444, ext.1535, Fax +66 0 2564-4440, ext.7594, Email [email protected]
Purpose: Although the pathogenesis of psoriasis involves the dermis, most previous studies collected samples using the swab technique. A recent study examining the microbiomes obtained via both skin biopsies and swabs revealed a significant difference in normal skin. We hypothesized that the microbiome profile of patients with psoriasis from tape stripping and skin biopsy might be different. This study sought to contribute to microbiome research on psoriasis by investigating the changes in the microbiome during narrowband ultraviolet B (NBUVB) therapy by comparing the results from the different sampling techniques of tape stripping and skin biopsy.
Patients and Methods: Twenty-three participants, including 14 patients with chronic plaque psoriasis and nine healthy controls, were recruited, and nine patients with psoriasis completed 20-sessions of NBUVB treatment. Skin microbiota from both techniques was analyzed using the 16S rRNA gene at baseline and after treatment.
Results: A clear difference was observed between the results from the two sampling techniques. Alpha diversity of the microbiota obtained from tape stripping was higher than that of the microbiota from skin biopsy, whereas beta diversity was clustered into two groups by sampling technique. The microbiome was altered during NBUVB treatment using both sampling techniques.
Conclusion: Different sampling techniques resulted in different microbiome profiles in patients with psoriasis. Tape stripping and swabs are feasible procedures and are mostly used in psoriasis and other skin microbiome studies; however, skin biopsy may also expand our understanding of psoriasis and other skin diseases that pathophysiology involves deeper to the dermis or subcutaneous tissue.
Keywords: skin microbiome, psoriasis, sampling techniques, NBUVB
Introduction
Psoriasis is a condition diagnosed in approximately 2–3% of the world’s population and is an immune-mediated skin disease that causes inflammation and discomfort.1 The disease is multifactorial and has an etiology comprising an underlying genetic predisposition as well as the immune-mediated condition itself, which can be triggered by environmental factors.
Human skin is home to a vast number of fungi, viruses, protozoa, and bacteria that form the skin microbiota.2 These microorganisms that inhabit the skin have a vital role in protecting the body from pathogens, which might otherwise lead to infection. They also boost the immune system and play a similar role as microorganisms in the human gut by helping to decompose natural matter.3 Many common skin diseases are caused by changes in skin microbiota.4 There is evidence to suggest that the onset of both human and animal psoriasis might be associated with changes in the skin or gut microbiota influencing host homeostasis and immune response, particularly in Th17 cell development.5–9 It is still unclear whether changes in the microbial community and its context are correlated with clinical appearance and treatment response.9 One standard form of psoriasis treatment is narrowband ultraviolet B (NBUVB) irradiation, which can influence the innate immune system, as well as the adaptive cellular immune response.10 Assarsson et al11 reported that NBUVB treatment affects the skin microbiota in swab samples of patients with psoriasis and suggested that these findings are associated with treatment response.
Although it is theorized that psoriasis pathogenesis involves the dermis, Grice et al, compared the microbiome obtained by swabs, scrapes and biopsies. They reported a comparable microbial composition from all the employed sampling techniques.12 From then, a high proportion of studies have utilized samples obtained by swabbing the skin surface. However, a recent study by Prast-Nielsen examining the microbiomes obtained via both skin biopsies and swabs revealed stark differences in the context of normal skin.13 When sampling approaches vary, the composition of the microbiota may also differ, as studies involving mucosal or gut luminal samples have confirmed.14 A study by Fahlen et al,15 however, presented variation in study outcomes when the skin from psoriasis patients was sampled using biopsies compared with the observed microbiome from other studies using swabs. Therefore, it is essential that the sampling technique employed allows a genuinely representative sample to be obtained.12,16 To further illuminate the consequences of different sampling approaches, this study sought to contribute to microbiome research by investigating the changes in the microbiome during NBUVB therapy and compare the results from tape stripping and skin biopsies, two different sampling techniques. This study may provide valuable insights regarding microbiome study.
Patients and Methods
Study Participants
This study focused on 23 Thai citizens aged 18 years or older. Of these patients, 14 presented with chronic plaque psoriasis, whereas nine served as healthy controls. All recruitment was conducted as a cross-sectional study at baseline.
Patients who were excluded were those with cancer, psoriatic arthritis, or autoimmune disease, along with those in immunocompromised states, and those using oral antibiotics, immunomodulators, systemic anti-inflammatory agents, probiotics, or those who had been dieting to a significant extent during the month before the commencement of the study. Patients who were undergoing additional treatment for psoriasis, those who had undergone topical therapy during the previous two weeks, and those who had undergone systemic therapy within the previous month were also excluded. Participants with contraindications to NBUVB were also excluded, thus eliminating those with xeroderma pigmentosum or hereditary conditions associated with UV-induced carcinogenesis, skin cancer, or photodermatosis. Photosensitive participants were also excluded, along with pregnant or lactating women. The healthy control group was required to be free of psoriasis and dermatosis, and to have no first-degree relatives diagnosed with psoriasis.
The protocols employed for the study were approved by the Human Research Ethics Committee of Thammasat University (COA: 188/2563), and the research was performed at the OPD Dermatology, Benjakitti Park Hospital, from August to December 2020.
All the participants provided written informed consent. The history of each participant was recorded, including age, sex, BMI, skin type, occupation, smoking and drinking habits, diet, sleep patterns, exposure to sunlight, underlying health conditions, current medications, family health history, and personal background. A physical check was also performed, and psoriasis severity was assessed using the Psoriasis Area Severity Index (PASI).
Sample Collection
For two weeks prior to the study, the participants were permitted to use only very mild liquid soaps and moisturizing creams, and washing was prohibited during the final 24 h before the samples were taken. Bacterial colonization of the skin relies on skin physiology;17 therefore, the samples were all drawn from skin regions considered dry microenvironments. Skin without lesions was determined to be normal and was located no less than 5 cm from the psoriatic lesions. Because psoriasis has a symmetrical appearance, it was not possible to find a non-lesional match using the matching skin site on the contralateral side of the body.
Skin microbiota samples of all participants were collected using tape stripping. In patients with psoriasis, an area of 3×3 cm of a target lesion, which was fully developed and not located on the sun-exposed area, and a nearby normal skin area were selected. Tape-stripped samples were drawn from the healthy control group at baseline from the matching sites. To obtain sufficient DNA, the use of 2 cm-diameter adhesive tape discs sterilized via ultraviolet radiation, sticking, and peeling was performed in 50 cycles.
In the psoriasis group, a 3 mm punch biopsy was performed at both the target lesion site and non-lesional skin from areas adjacent, but not overlapping, to the tape stripping sites. Before biopsy sampling was performed, 2% Xylocaine local anesthetic was used, but no antiseptics were applied prior to sample collection. Once obtained, all samples from both techniques were frozen at a temperature of −80℃.
NBUVB was then performed in line with the clinical protocol, and further samples were obtained via both biopsy and tape stripping following either 20 NBUVB sessions or an improvement of 75%. New samples were taken from the same sites, although the new biopsy process did not overlap with the existing wound from the previous biopsy.
NBUVB Protocol
A Waldmann 5002 cabin (Waldmann Medizintechnik, Villingen-Schwenningen, Germany) was used to provide the NBUVB (311 nm) therapy. The initial dose was 0.3 J/cm,2 which was subsequently increased in 20% increments per visit under the condition that the patient was able to tolerate the previous dose.
DNA Extraction
Total DNA was extracted using the GenUP™ gDNA extraction kit (Biotechrabbit, Germany) with a modified protocol for the sample processing steps. For tissue (biopsy) samples, the tissue was homogenized with 400 µL LYSIS LG buffer in a 2 mL microtube with a tissue lyzer (50 Hz for 5 min). The suspension was then centrifuged for 5 min at 15,000 × g. The supernatant was treated with 25 µL proteinase K and 3 µL RNase A, and subsequently extracted according to the manufacturer’s guidelines. For the tape strip, the strip was cut into eight equal parts and directly incubated with 400 µL Buffer LYSIS LG, 25 µL Proteinase K, and 3 µL RNase A at 50 ℃ for 24 h. The supernatants were then collected and extracted according to the manufacturer’s standard protocol.
PCR Amplification
Amplification of the V3-V4 region of bacterial 16S rDNA obtained from the skin samples was carried out with primers that contained overhang Illumina Nextera adapters (341F:5’-TCGTCGGCAGCGTCAGATGTG TATAAGAGACAGCCTACGGGNGGCWGCAG −3’ and 805R: 5’- TCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC −3’). The 25 µL PCR mixture contained 10 ng of DNA template, 0.2 µM of each primer, and AccuStart™ II PCR SuperMix (Quantabio, USA). Amplification of this reaction was conducted under the following conditions: 98 ℃ for 2 min, 25 cycles of 98 ℃ for 20s, 60 ℃ for 30s, 72 ℃ for 30s, and 72 ℃ for 1 min. A second PCR was then carried out to attach the multiplexing indices and Illumina adapters using the Nextera XT Index Kit (Illumina, USA) in line with the regular protocol. The final overall length of the DNA library reached 580 bp, allowing examination using 2% agarose gel electrophoresis, followed by purification using AMPure XP beads (Beckman Coulter, USA).
Next-Generation Sequencing
The KAPA absolute quantification kit (KAPA Bioscience, Germany) was used to determine the concentrations of the purified products by first pooling the samples at concentrations equal to 2 nM prior to denaturation and loading them into a MiSeq reagent v2 cartridge, which had an eventual concentration of 6 pM. The library was then sequenced for paired-end 2×250 cycles on the Illumina MiSeq platform (Illumina, USA) using 20% spiked-in PhiX control, in accordance with the manufacturer’s guidelines. This process was performed at the Omics Sciences and Bioinformatics Center (Chulalongkorn University, Thailand).
Data Analysis
Demultiplexing of the raw sequencing data was performed using MiSeq reporter software (version 2.6.2.3), whereas the FASTQ files were analyzed using the QIIME2 pipeline (version 2019.7).18 The paired-end sequences were then joined and trimmed based on their quality score (< Q30). The merged reads underwent deduplication and clustering with 97% similarity using VSEARCH.19 The UCHIME algorithm was employed to filter out chimeric sequences,20 whereupon the filtered reads could be classified on the basis of the 16S Greengenes database (version 13.5)21 using the VSEARCH algorithm. Plugins in QIIME2 were used to carry out alpha diversity analysis, whereas the analysis of differential abundance made use of the linear discriminant analysis effect size (LEfSe).22 Finally, the Wilcoxon matched-pairs test results were analyzed using GraphPad Prism version 6.01.
Results
Study Cohort
This study included 23 participants at baseline. Of these, nine patients with psoriasis underwent a full course of NBUVB treatment. Nine healthy control participants did not undergo NBUVB treatment because exposure to UV radiation is inadvisable for healthy individuals. There was no significant difference in baseline characteristics between the controls and patients with psoriasis. All participants were Thai, aged between 26–64 years old, BMI range between 20.05–35.02 kg/m2, with Fitzpatrick’s skin type 4, living in Bangkok province. The participants reported no smoking, no alcoholic drinking, no pets, showers twice a day with normal soap, sun exposure 1–2 h/day, and sleep 6.30–8 h/day.
NBUVB Treatment of Patients with Psoriasis
Among the patients with psoriasis, nine completed 20 NBUVB sessions. Following treatment, two patients exhibited worsening skin lesions. Prior to treatment, the PASI scores were 18.76 ± 15.71, which declined to 13.37 ± 17.36 once the treatment was complete. The cumulative dosage of NBUVB was 21.28 ± 4.46 mJ/cm.2
Sequence Output from Tape Stripping and Skin Biopsy Samples
To carry out further analysis, the FASTQ output from samples that achieved at least 5000 total reads per sample was collected. The rarefaction plots exhibited an asymptotic state, confirming that there was an adequate sequencing depth to show the rich diversity of the bacterial community from tape stripping and skin biopsy. Among the various groups, the mean number of raw reads was in the range of 39,147.89 ± 15,103.66 up to 86,682.00 ± 154,158.36 from tape stripping sample, and 68,237.83 ± 16,990.58 up to 83,587.00 ± 39,443.80 from biopsy sample, with more than 99% of those raw reads meeting quality control standards and subsequently undergoing classification using the QIIME2 pipeline (Tables S1 and S2).
Bacterial Diversity from Tape Stripping and Skin Biopsy Samples
Alpha diversity indices were used to evaluate the diversity of bacteria within the samples. Chao1 revealed the richness of bacterial taxa, whereas Shannon showed the richness and evenness of the taxa. The different approaches resulted in higher Chao1 and Shannon diversities from tape stripping than from skin biopsy samples (Figure 1). Wilcoxon matched-pairs signed rank test of Chao1 and Shannon index were used to compare the alpha diversity of psoriasis patients before and after NBUVB treatment. The changes in microbial diversity from both sampling techniques were not correlated with each other.
From tape stripping, when comparing the psoriasis lesional skin, non-lesional skin, and healthy control skin, no significant differences were found in the microbial diversity, either at baseline or when evaluated following the NBUVB treatment process (Figure 1A–F). From skin biopsy, Chao1 and Shannon diversity (Figure 1G–K) revealed no significant difference between lesional and non-lesional skin before and after treatment. A significant decrease in Shannon diversity was detected in psoriasis lesions after NBUVB treatment (p = 0.03) (Figure 1L).
Beta diversity (PERMANOVA) revealed the variability of microbial communities in different groups. The present study showed that beta diversity was clustered into two groups by sampling techniques: clear clustering was shown for both lesional and non-lesional skin and before and after NBUVB treatment (PERMANOVA test, p < 0.001), and no significant difference among groups from the same technique was detected as determined by Jaccard dissimilarity (Figure 2).
Difference in Sampling Technique Revealed Differences in Microbial Abundance
The most frequently observed phyla and genera in skin biopsy and tape stripping were different (Figure 3, Table S3). From skin biopsies, the most observed phyla in patients with psoriasis were Proteobacteria, followed by Bacteroidetes and Actinobacteria (Figure 3A). In contrast, from tape stripping, the most abundant phyla in patients with psoriasis included Actinobacteria, Proteobacteria, Firmicutes, Bacteroidetes, and Cyanobacteria, and the most abundant phyla in healthy controls were Proteobacteria (Figure 3B). At the genus level, the most abundant genera from biopsies were Sediminibacterium and the order Rhizobiales (Figure 3C), whereas from tape stripping, Corynebacterium was found to be the predominant among the groups (Figure 3D). We further examined the dominant taxa (those with mean relative abundance ≥1%). Results from ANOVA and the Bonferroni post-hoc test confirmed greatly enriched Propionibacterium in the healthy control when compared with psoriasis lesional and non-lesional skin (Table S3B).
LEfSe Analysis from Tape Stripping and Biopsy
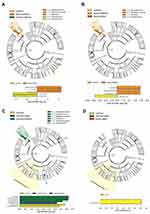
Using tape stripping, LEfSe analysis was employed to determine the differences at baseline between patients with psoriasis and the healthy control group. The cladogram presents the relative abundance differences for five levels of taxa: phylum, class, order, family, and genus. It was observed that there was greater enrichment in Alishewanella and Pseudomonas in the healthy controls, whereas lesional skin notably contained the order Bacillales, family Staphylococcaceae, and genus Staphylococcus (Figure 4A).
Following NBUVB treatment, patients with psoriasis were found to have significantly lower bacterial abundance of the order Bacillales family Staphylococcaceae and genus Staphylococcus, which were observed in both lesional skin and non-lesional skin (Figure 4B). Furthermore, the family Oxalobacteraceae increased in abundance following NBUVB treatment in non-lesional skin (Figure 4C). In addition, there were no dominant distinct taxa when comparing lesional skin and non-lesional skin, and the abundance of order Bacillales family Staphylococcaceae and genus Staphylococcus was no longer predominant in the lesion (Figure 4D).
From skin biopsy, we examined the changes in microbiota during NBUVB treatment and found that lesional skin was more enriched with the genera Bacteroides, family Bacteroidaceae, genus Odoribacter, family Odoribacteraceae, genus Prevotella, family Prevotellaceae, class Bacteroidia, family Enterobacteriaceae, and order Enterobacteriales at baseline, and no predominant taxa after treatment (Figure S1A). Non-lesional skin was more enriched with the genus Sphingomonas, family Sphingomonadaceae, order Sphingomonadales, and genus Acinetobacter at baseline. After NBUVB treatment, genus Faecalibacterium, family Ruminococcaceae, and genus Kaistobacter and Enhydrobacter were more dominant (Figure S1B). We compared lesional and non-lesional skin at baseline, and microbial communities of lesions had dominant class Gammaproteobacteria, genus Faecalibacterium, family Ruminococcaceae, family Rikenellaceae, and order Bacteroidales (Figure S1C). After NBUVB, the genus Acinetobacter and the order Caulobacterales were more enriched in lesions, whereas the family Bradyrhizobiaceae was dominant in non-lesional skin (Figure S1D).
Discussion
Our study used a tape stripping technique due to its feasibility, the fact that epidermal disruption may trigger psoriasis, and a previous report showing comparable microbial composition between samples obtained by swabs, scrapes and biopsies.12 We revealed results comparable to those of swab testing, which most previous studies have used to evaluate the surface microbiota. However, psoriasis pathogenesis begins within the dermis, commencing with the appearance of CD4-activated lymphocytes, which then make their way to the epidermis.23 Therefore, a sampling approach that focuses solely on the surface of the skin may prove inadequate for evaluating the skin microbiota. Recently, Prast-Nielsen emphasized the difference between microbiota profiles from different sampling techniques: swabs and biopsies from normal skin. However, our study is the first to draw comparisons between microbiome changes during the course of NBUVB therapy via tape stripping which represents surface microbiota and skin biopsy which represents microbiota of the full skin layer.
In line with Prast-Nielsen, our data revealed that skin biopsy and tape stripping showed significant differences in alpha diversity, higher Chao1 and Shannon diversities from tape stripping than from skin biopsy samples, beta diversity that showed a clustering into two groups by sampling techniques, and microbial communities that reported a different kind of microbiota captured from tape stripping and biopsy. A potential explanation for the reported differences in the bacterial biota when using tape stripping in comparison to biopsies include bacteria being internalized within cells; this is thought to be feasible given that this takes place in the tonsils with β-hemolytic streptococci.24,25 Therefore, it may be the case that psoriasis patients harbor additional bacteria in their dermal and epidermal cells. Nakatsuji et al16 noted that a different microbiota may be present in the dermis, epidermis, and superficial adipose tissue. The pilosebaceous unit hosts many bacteria, which will only appear on the skin surface under the application of pressure26 it would not be possible for tape stripping or swabs to detect such bacteria. The bacteria living in the squames of the stratum corneum might not be the same as those on the skin surface. Indeed, parts of the skin below the surface have been shown to host bacteria that differ from those on the surface,16 and therefore it is unlikely that bacteria discovered through the use of tape stripping or swabs alone would represent anything more than a small fraction of the entire skin microbiota.
Changes in microbial abundance during NBUVB treatment were detected from both sampling techniques. This may suggest that not only the surface microbiota but also the microbiota in the deeper layer was altered after NBUVB treatment. These changes could be related to the treatment and may affect the clinical response. Future studies with a larger number of participants and including skin biopsies from patients with psoriasis are needed to provide robust data for analysis.
Previous studies using the swab technique reported inconsistent results for microbial diversity,5,27–30 whereas no significant difference was detected with the biopsy technique.15 The inconsistency in these findings might be attributable to differences in the methods applied, such as the sampling approaches used, the possibility of heightened variation between individuals, or encountering certain niches within the various sampling locations. In this study, we sought to minimize the variability that can result from differences in intra- and inter-individual microbiota profiles. For example, we used both psoriatic and normal skin samples taken from ecologically dry regions of the same individuals, as well as tape-stripped skin samples from healthy control patients. However, our small sample size may not be sufficient to reveal the diversity of differences. Alternatively, psoriasis may not have specific patterns of taxonomical richness, evenness, or community variability but could be more closely connected with the relative abundance of microbiota and the existence of specific microbes.
Using the tape stripping technique, our findings revealed a difference in bacterial abundance between psoriasis and control and between lesional and non-lesional skin. At the phylum level, Proteobacteria were prominent in healthy controls, whereas patients with psoriasis exhibited dominant Actinobacteria. As topographic characteristics of the human body affect the skin microbiota, there are three main ecological areas: sebaceous, moist, and dry. We collected all samples from dry areas that were shown to be inhabited by a mixture of species, although Proteobacteria was dominant. We found Actinobacteria to be dominant in psoriatic skin, in line with Alekseyenko et al28 who proposed that the microbiota of psoriatic lesions display enrichment with cutaneotype 2. This may correspond to the disease’s influence being greater extent than that of the topographical effect. At the genus level, Corynebacterium was found to be the most abundant in patients with psoriasis and healthy controls, whereas Propionibacterium was dominant in the healthy controls. Earlier research found that Corynebacterium and Propionibacterium are imbalanced in psoriatic patients with lesional skin; the abundance of Corynebacterium was higher, whereas that of Propionibacterium was lower. Two further studies reported lower abundance of Propionibacterium in the skin with lesions.31,32 The abundance of Propionibacterium is much greater in healthy skin than in hidradenitis suppurativa skin,33 suggesting that Propionibacterium and its strain populations might provide protective benefits. Moreover, psoriatic lesions are typically dry, which would not be an ideal setting for Propionibacterium as this type favors greater humidity in sebaceous skin, whereas Corynebacterium does not fare well in acidic settings and is less frequently observed in sebaceous skin.34
We found that Staphylococcus was significantly more abundant on lesional skin, in accordance with the results of Tett et al29 using shotgun metagenomics, whereas Assarsson et al11 found significantly lower levels of Staphylococcus prior to treatment in lesional skin than in non-lesional skin, which is in contrast to a previous study. Staphylococcus is a diverse genus. Some species, such as the commensal S. epidermidis, appear to enhance the innate immune barrier and limit pathogen invasion,35 whereas others, such as S. aureus, evoke a pathogenic Th17 response.36 However, the abundance of S. aureus increased, whereas that of S. epidermis decreased when examined in patients with psoriatic lesions.37,38
Following NBUVB treatment, significantly reduced Staphylococcus abundance was observed in both lesional and non-lesional skin suggesting that NBUVB affects the growth of this bacteria and possibly affects treatment response. These findings were consistent with previous studies showing that UVR inhibits S. aureus growth in vivo and in vitro39–41 and lowers the generation of super-antigens, which are understood to have the capacity to induce immune responses.42
The skin biopsy findings showed that the most abundant phylum in cases of psoriasis was Proteobacteria, followed by Bacteroidetes and Actinobacterium, which differs from Fahlen et al,15 who reported that the most common phylum in controls and psoriasis lesions was Firmicutes, followed by Proteobacteria and Actinobacteria. This could result from the different locations of skin sampling. After NBUVB, the genus Acinetobacter and the order Caulobacterales were more enriched in lesions, whereas the family Bradyrhizobiaceae was dominant in non-lesional skin.
Changes in skin microbiota after NBUVB from tape stripping and biopsy may be explained by NBUVB directly affecting epidermal and dermal microbiota by DNA and membrane damage, or indirectly through changes of surface microbiota which subsequently shape the dermal microbiota, or by a local/systemic immunological effect of NBUVB. Although it is clear that a microbiota comprising diverse residents exists, the details of its functioning in the skin remain to be determined, along with the question of which inhabitants play helpful, harmful, or neutral roles. Additionally, if and how changes in skin microbiota correlate with psoriasis and treatment response are still unknown.
This study had certain limitations, such as its small sample size. Thus, a study with more participants is needed to confirm our findings. The skin biopsy group did not include healthy controls owing to ethical issues. Although we tried to control for inter- and intra-personal variation, other factors, such as known or unknown underlying diseases and medications, may also affect the skin microbiota.
Conclusions
Our study revealed that different sampling techniques (tape stripping and skin biopsy) result in different microbiome profiles in patients with psoriasis. In addition, the microbiome was altered during NBUVB treatment using both sampling techniques. Skin surface sampling, such as tape stripping and swabs, is a feasible procedure and can demonstrate that microbial changes still play a major role in psoriasis skin microbiome studies; however, skin biopsy may also expand our understanding of psoriasis pathophysiology and treatment response and other skin diseases that involve not only the skin surface.
Data Sharing Statement
All data generated or analyzed during this study are included in this article and its supplementary material files. Further inquiries can be directed to the corresponding author.
Ethics Approval and Informed Consent
This study protocol was reviewed and approved by the Human Research Ethics Committee of Thammasat University COA: 188/2563 and conducted at the outpatient department of Dermatology, Benjakitti Park Hospital from August 2020 to December 2020. Written informed consent was obtained from all participants. All experiments were performed in accordance with relevant guidelines and regulations and complied with the Declaration of Helsinki.
Acknowledgments
The authors gratefully acknowledge the financial support provided by Chulabhorn International College of Medicine contract no T1/2563 and Bualuang ASEAN Chair Professorship Fund. We would like to thank Editage (www.editage.com) for English language editing.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The authors gratefully acknowledge the financial support provided by the Chulabhorn International College of Medicine (contract no. T1/2563) and the Bualuang ASEAN Chair Professorship Fund.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Perera GK, Di Meglio P, Nestle FO. Psoriasis. Annu Rev Pathol. 2012;7:385–422. doi:10.1146/annurev-pathol-011811-132448
2. Byrd AL, Belkaid Y, Segre JA. The human skin microbiome. Nat Rev Microbiol. 2018;16(3):143–155. doi:10.1038/nrmicro.2017.157
3. Scharschmidt TC, Fischbach MA. What lives on our skin: ecology, genomics and therapeutic opportunities of the skin microbiome. Drug Discov Today Dis Mech. 2013;10(3–4):e83–e89. doi:10.1016/j.ddmec.2012.12.003
4. Iebba V, Totino V, Gagliardi A, et al. Eubiosis and dysbiosis: the two sides of the microbiota. New Microbiol. 2016;39(1):1–12.
5. Chang HW, Yan D, Singh R, et al. Alteration of the cutaneous microbiome in psoriasis and potential role in Th17 polarization. Microbiome. 2018;6(1):154. doi:10.1186/s40168-018-0533-1
6. Nemoto Y, Kanai T, Takahara M, et al. Th1/Th17-mediated interstitial pneumonia in chronic colitis mice independent of intestinal microbiota. J Immunol. 2013;190(12):6616–6625. doi:10.4049/jimmunol.1202930
7. Geem D, Medina-Contreras O, McBride M, Newberry RD, Koni PA, Denning TL. Specific microbiota-induced intestinal Th17 differentiation requires MHC Class II but not GALT and mesenteric lymph nodes. J Immunol. 2014;193(1):431–438. doi:10.4049/jimmunol.1303167
8. Bellone M, Brevi A, Huber S. Microbiota-Propelled T Helper 17 cells in inflammatory diseases and cancer. Microbiol Mol Biol Rev. 2020;84(2). doi:10.1128/MMBR.00064-19
9. Fry L, Baker BS, Powles AV, Fahlen A, Engstrand L. Is chronic plaque psoriasis triggered by microbiota in the skin? Br J Dermatol. 2013;169(1):47–52. doi:10.1111/bjd.12322
10. Rácz E, Prens EP, Kurek D, et al. Effective treatment of psoriasis with narrow-band UVB phototherapy is linked to suppression of the IFN and Th17 pathways. J Invest Dermatol. 2011;131(7):1547–1558. doi:10.1038/jid.2011.53
11. Assarsson M, Duvetorp A, Dienus O, Söderman J, Seifert O. Significant changes in the skin microbiome in patients with chronic plaque psoriasis after treatment with narrowband ultraviolet B. Acta Derm Venereol. 2018;98(4):428–436. doi:10.2340/00015555-2859
12. Grice EA, Kong HH, Renaud G, et al. A diversity profile of the human skin microbiota. Genome Res. 2008;18(7):1043–1050. doi:10.1101/gr.075549.107
13. Prast‐Nielsen S, Tobin AM, Adamzik K, et al. Investigation of the skin microbiome: swabs vs. biopsies. Br J Dermatol. 2019;181(3):572–579. doi:10.1111/bjd.17691
14. Zmora N, Zilberman-Schapira G, Suez J, et al. Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell. 2018;174(6):1388–1405.e21. doi:10.1016/j.cell.2018.08.041
15. Fahlén A, Engstrand L, Baker BS, Powles A, Fry L. Comparison of bacterial microbiota in skin biopsies from normal and psoriatic skin. Arch Dermatol Res. 2012;304(1):15–22. doi:10.1007/s00403-011-1189-x
16. Nakatsuji T, Chiang HI, Jiang SB, Nagarajan H, Zengler K, Gallo RL. The microbiome extends to subepidermal compartments of normal skin. Nat Commun. 2013;4:1431. doi:10.1038/ncomms2441
17. Grice EA, Kong HH, Conlan S, et al. Topographical and temporal diversity of the human skin microbiome. Science. 2009;324(5931):1190–1192. doi:10.1126/science.1171700
18. Bolyen E, Rideout JR, Dillon M, et al. QIIME 2: reproducible, interactive, scalable, and extensible microbiome data science. Peer J. 2018. doi:10.7287/peerj.preprints.27295
19. Rognes T, Flouri T, Nichols B, Quince C, Mahé F. VSEARCH: a versatile open source tool for metagenomics. PeerJ. 2016;4:e2584. doi:10.7717/peerj.2584
20. Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27(16):2194–2200. doi:10.1093/bioinformatics/btr381
21. DeSantis TZ, Hugenholtz P, Larsen N, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006;72(7):5069–5072. doi:10.1128/AEM.03006-05
22. Segata N, Izard J, Waldron L, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12(6):R60. doi:10.1186/gb-2011-12-6-r60
23. Baker BS, Laman JD, Powles A, et al. Peptidoglycan and peptidoglycan-specific Th1 cells in psoriatic skin lesions. J Pathol. 2006;209(2):174–181. doi:10.1002/path.1954
24. McFadden JP, Baker BS, Powles AV, Fry L. Psoriasis and streptococci: the natural selection of psoriasis revisited. Br J Dermatol. 2009;160(5):929–937. doi:10.1111/j.1365-2133.2009.09102.x
25. Österlund A, Engstrand L. An intracellular sanctuary for Streptococcus pyogenes in human tonsillar epithelium – studies of asymptomatic carriers and in vitro cultured biopsies. Acta Oto-Laryngol. 1997;117(6):883–888. doi:10.3109/00016489709114219
26. Snelling AM, Saville T, Stevens D, Beggs CB. Comparative evaluation of the hygienic efficacy of an ultra-rapid hand dryer vs conventional warm air hand dryers. J Appl Microbiol. 2011;110(1):19–26. doi:10.1111/j.1365-2672.2010.04838.x
27. Gao Z, Tseng CH, Strober BE, Pei Z, Blaser MJ. Substantial alterations of the cutaneous bacterial biota in psoriatic lesions. PLoS One. 2008;3(7):e2719. doi:10.1371/journal.pone.0002719
28. Alekseyenko AV, Perez-Perez GI, De Souza A, et al. Community differentiation of the cutaneous microbiota in psoriasis. Microbiome. 2013;1(1):31. doi:10.1186/2049-2618-1-31
29. Tett A, Pasolli E, Farina S, et al. Unexplored diversity and strain-level structure of the skin microbiome associated with psoriasis. Npj Biofilms Microbiomes. 2017;3(1):14. doi:10.1038/s41522-017-0022-5
30. Loesche MA, Farahi K, Capone K, et al. Longitudinal study of the psoriasis-associated skin microbiome during therapy with ustekinumab in a randomized phase 3b clinical trial. J Invest Dermatol. 2018;138(9):1973–1981. doi:10.1016/j.jid.2018.03.1501
31. Tauch A, Fernández-Natal I, Soriano F. A microbiological and clinical review on Corynebacterium kroppenstedtii. Int J Infect Dis. 2016;48:33–39. doi:10.1016/j.ijid.2016.04.023
32. Quan C, Chen XY, Li X, et al. Psoriatic lesions are characterized by higher bacterial load and imbalance between Cutibacterium and Corynebacterium. J Am Acad Dermatol. 2020;82(4):955–961. doi:10.1016/j.jaad.2019.06.024
33. Ring HC, Thorsen J, Saunte DM, et al. The follicular skin microbiome in patients with hidradenitis suppurativa and healthy controls. JAMA Dermatol. 2017;153(9):897–905. doi:10.1001/jamadermatol.2017.0904
34. Rozas M, Hart de Ruijter AH, Fabrega MJ, et al. From dysbiosis to healthy skin: major contributions of Cutibacterium acnes to skin homeostasis. Microorganisms. 2021;9(3):628. doi:10.3390/microorganisms9030628
35. Naik S, Bouladoux N, Linehan JL, et al. Commensal-dendritic-cell interaction specifies a unique protective skin immune signature. Nature.2015;520(7545):104–108. doi:10.1038/nature14052
36. Schirmer M, Smeekens SP, Vlamakis H, et al. Linking the human gut microbiome to inflammatory cytokine production capacity. Cell. 2016;167(4):1125–1136.e8. doi:10.1016/j.cell.2016.10.020
37. Ng CY, Huang YH, Chu CF, Wu TC, Liu SH. Risks for Staphylococcus aureus colonization in patients with psoriasis: a systematic review and meta-analysis. Br J Dermatol. 2017;177(4):967–977. doi:10.1111/bjd.15366
38. Liu SH, Yu HY, Chang YC, Chung-Yee Hui R, Huang YC, Huang YH. Host characteristics and dynamics of Staphylococcus aureus colonization in patients with moderate-to-severe psoriasis before and after treatment: a prospective cohort study. J Am Acad Dermatol. 2019;81(2):605–607. doi:10.1016/j.jaad.2018.05.031
39. Jekler J, Bergbrant IM, Faergemann J, Larkö O. The in vivo effect of UVB radiation on skin bacteria in patients with atopic dermatitis. Acta Derm Venereol. 1992;72(1):33–36.
40. Thyssen JP, Zirwas MJ, Elias PM. Potential role of reduced environmental UV exposure as a driver of the current epidemic of atopic dermatitis. J Allergy Clin Immunol. 2015;136(5):1163–1169. doi:10.1016/j.jaci.2015.06.042
41. Yoshimura M, Namura S, Akamatsu H, Horio T. Antimicrobial effects of phototherapy and photochemotherapy in vivo and in vitro. Br J Dermatol. 1996;135(4):528–532. doi:10.1111/j.1365-2133.1996.tb03825.x
42. Yoshimura-Mishima M, Akamatsu H, Namura S, Horio T. Suppressive effect of ultraviolet (UVB and PUVA) radiation on superantigen production by Staphylococcus aureus. J Dermatol Sci. 1999;19(1):31–36. doi:10.1016/S0923-1811(98)00046-2
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.