Back to Journals » Infection and Drug Resistance » Volume 15
Sinobronchial Syndrome Patients with Suspected Non-Tuberculous Mycobacterium Infection Exacerbated by Exophiala dermatitidis Infection
Authors Watanabe Y , Sano H, Konno S, Kamioka Y, Hariu M, Takano K, Yamada M , Seki M
Received 23 January 2022
Accepted for publication 11 March 2022
Published 20 March 2022 Volume 2022:15 Pages 1135—1141
DOI https://doi.org/10.2147/IDR.S359646
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Yuji Watanabe,1,2 Hirohito Sano,3 Shuichi Konno,3 Yasuhiro Kamioka,1,4 Maya Hariu,1,2 Kazuki Takano,1,2 Mitsuhiro Yamada,3 Masafumi Seki1
1Division of Infectious Diseases and Infection Control, Faculty of Medicine, Tohoku Medical and Pharmaceutical University, Sendai City, Miyagi, Japan; 2Laboratory for Clinical Microbiology, Tohoku Medical and Pharmaceutical University Hospital, Sendai City, Miyagi, Japan; 3Department of Respiratory Medicine, Tohoku University Graduate School of Medicine, Sendai City, Miyagi, Japan; 4Division of Pharmacy, Tohoku Medical and Pharmaceutical University Hospital, Sendai City, Miyagi, Japan
Correspondence: Masafumi Seki, Division of Infectious Diseases and Infection Control, Faculty of Medicine, Tohoku Medical and Pharmaceutical University, 1-15-1 Fukumuro, Miyagino-ku, Sendai City, Miyagi, 983-8612, Japan, Tel +81-22-259-1221, Fax +81-22-290-8956, Email [email protected]; [email protected]
Background: Exophiala dermatitidis is an environmental black fungus that rarely causes respiratory infections, yet its pathophysiological features and treatment regimens have not been established.
Case Series: Two cases of exacerbations of chronic bronchitis and sinusitis due to E. dermatitidis infection in Japan are presented. Both patients were women, and non-tuberculous Mycobacterium (NTM) infection was suspected based on chest radiological findings, but E. dermatitidis was detected from bronchial lavage fluid and nasal mucus, respectively. Both cases were successfully treated by antifungal agents such as liposomal amphotericin B, voriconazole, and itraconazole, but clarithromycin, rifampicin, ethambutol, and sitafloxacin for NTM were not effective.
Conclusion: E. dermatitidis can become a respiratory pathogen, especially in patients with chronic sinobronchial syndrome.
Keywords: black fungus, bronchiectasis, rheumatoid arthritis, bronchoscope
Background
Exophiala dermatitidis (E. dermatitidis) is known as an etiologic fungal agent of subcutaneous, ocular, systemic, and cerebral forms of phaeohyphomycosis.1 It is also regularly isolated from respiratory samples from cystic fibrosis (CF) patients as colonization, with rates varying between 1% and 19%, but it is sometimes diagnosed as the pathogen in similar respiratory Diseases with bronchiectasis.2,3
E. dermatitidis is mainly characterized by its dimorphic character, being able to switch from the yeast-like to the hyphal state, and melanin, part of the cell wall of black yeasts, is one major factor known to contribute to the pathogenicity of E. dermatitidis and its increased resistance against host defense and anti-infective therapies.1 Furthermore, an altered immune status is known to affect the progress of infectious diseases, and the number of cases is steadily increasing in both immunocompromised and immunocompetent people.4 Detailed knowledge regarding infection mechanisms, virulence factors, specific predisposing factors, risk factors, host responses, and treatment methods remains scarce.
In this report, patients with non-cystic fibrosis bronchiectasis with sinusitis in which the fungus were the only organisms recovered from either the lower airways or paranasal sinus infection, respectively, are presented. The infection was ameliorated by administration of antifungal agents, demonstrating a similar role in sinobronchial syndrome (SBS) other than cystic fibrosis.
This study was approved by the Committee for Clinical Scientific Research of Tohoku Medical and Pharmaceutical University Hospital on February 10, 2017 (No. ID 2020-2-11), and the patients whose specimens were used provided written, informed consent to have their case details and any accompanying images published.
Case Series
Case 1
A 65-year-old woman who had a history of sinusitis and Mycobacterium avium complex (MAC)-related nodular and bronchiectasis (NB) had been maintained on erythromycin (EM) 200 mg/day orally for SBS from 2017 to 2019. The cultures of MAC in her sputum changed to negative two or more times, and the anti-GPL-core IgA antibody was negative. However, in June 2020, her cough and sputum were slightly increased, and some infiltration shadows were found on her chest X-ray and CT (Figure 1A and B). She had no other immunocompromised history including HIV infections and diabetic status, and the laboratory data shows that white blood cells (WBC) count, 3.6 ×103/μL and C-reactive protein (CRP), 1.11 mg/dL, but anti-aspergillus antigens, anti-CryptococcusCryptococcus antibody, and beta-d-glucan were negative in her serum. Exacerbation of MAC was suspected, and clarithromycin (CAM) 600 mg/day, rifampicin (RIP) 450 mg/day, ethambutol 750 mg/day, and sitafloxacin (SFTX) 100 mg/day orally for one year were started.
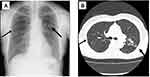 |
Figure 1 Chest X-ray (A) and computed tomography (CT) (B) findings of patient 1. Arrows indicate the abnormal small nodules, infiltrate shadows, and bronchiectasis. |
The infiltration shadows remained in part, and brown to black sputum was found. Thus, bronchoscopy was performed in June 2021, and not MAC and other pathogenic bacteria and fungus, but black fungus was detected from the bronchial lavage fluid: At 24-hour culture using by potato-dextrose agar with chloramphenicol at 35°C, the colonies and filamentous form fungi were found (Figure 2A and B), and unique annelloconidia were found using by phenol-cotton blue staining after 48 hours of incubation of the small colonies at 24 hours using by Sabouraud broth with chloramphenicol at 35°C with human leucocytes as the stimulator (Figure 2C). They became the yeast-like form at 72-hour incubation (Figure 2D and E) and showed high viscosity (Figure 2F). Matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry (MS) analysis using by yeast-like form fungi at 72 hours culture showed a high-score match with E. dermatitidis by the smear method protocol (Figure 3). The patient was started on voriconazole (VRCZ) 300 mg/day orally, and the black sputum and infiltration shadows improved.
Case 2
A 47-year-old woman who was on methotrexate for rheumatoid arthritis (RA) had been maintained on CAM 200 mg/day orally for chronic sinusitis and MAC-related bronchiectasis from 2016 because anti-GPL-core IgA antibody was positive and she had extended yellow serous nasal discharge. She had no other immunocompromised history including HIV infections and diabetic status.Her chest X-ray and CT improved and showed only small nodules and slight bronchiectasis (Figure 4A and B), however in May 2021, she reported impaired the sense of smell and her nasal discharge changed to black mucus. The laboratory data shows that WBC count, 6.3 ×103/μL and CRP, 3.07 mg/dL, but anti-aspergillus antigens, anti-Cryptococcus antibody, and beta-d-glucans were negative in her serum. Her right paranasal cavity became slightly cloud and narrow, and a small amount of fluid was suggested (Figure 4C and D). E. dermatitidis with neutrophils was isolated from her nasal mucous although we had not detected E. dermatitidis before the culture of her nasal discharge. We also did not isolate the other pathogens, including bacteria and fungus, and treatment by the antibiotics: SFTX 100mg/day for two weeks orally were not effective. We then thought E. dermatitidis as the pathogen, but not the colonization, and treatment was started with liposomal amphotericin B (L-Amp B) 5 mg/kg/day and VRCZ 6 mg/day intravenously for a week, subsequently by oral itraconazole (ITCZ) 200 mg/day. Her black nasal mucus and impaired the sense of smell have been improved, and E. dermatitidis has been disappeared in the following cultures.
The E. dermatitidis isolated from the above two patients had relatively high susceptibility/low minimum inhibitory concentration (MIC) to AmpB, ITCZ, and VRCZ but lower susceptibility/higher MIC to micafungin, caspofungin, flucytosine, fluconazole, and miconazole in vitro (Table 1).
 |
Table 1 Minimum Inhibitory Concentrations (MICs) of Various Antifungal Agents for the Exophiala dermatitidis Isolated from the Two Patients |
Discussion
E. dermatitidis is one of the environmental and clinical black yeasts, and the number of studies dealing with E. dermatitidis as a human pathogen has increased in recent years, highlighting the importance of E. dermatitidis in medical mycology.1 E. dermatitidis is a frequent colonizer of the respiratory tract of CF patients, but it causes exacerbations in CF patients, and E. dermatitidis is also known to be a cause of central nervous system infections in otherwise healthy, immunocompetent patients.1,5 Although studies have reported that E. dermatitidis respiratory infections occurred mostly in CF patients, some cases were reported in non-CF patients. Most of the non-CF cases were from Asia, where CF is rare, and the patients were more often female and had bronchiectasis.3,6,7
In this report, both patients were female and had bronchiectasis and a history of sinusitis. Thus, they were diagnosed as having SBS and received long-term macrolide therapy. One patient had RA, which might have been the cause of bronchiectasis, and both patients were suspected to have exacerbations of mycobacterial infections, especially MAC infection. However, anti-MAC drugs, including CAM, RIP, EB, and fluoroquinolones were not effective, and the symptoms, such as black sputum and nasal mucus, and the shadows on nasal and chest X-ray findings continued. Bronchiectasis may be one of the key factors in respiratory infection by E. dermatitidis because thin and enlarged bronchioles may be suitable for E. dermatitidis to settle, similar to MAC, and be more important than the immunological status, such as whether immunocompetent or immunocompromised, respectively. In our two case, they are not so compromised, but both of the patients like gardening, and suggested to have the chance to expose the E. dermatitidis from the environment.
On microbiological analysis and diagnosis, the diagnosis of invasive fungal infections is often delayed, and delayed treatment, even if appropriate, may worsen patients’ outcomes.8 Thus, appropriate methods for species-specific identification are necessary. However, no species-specific standardized diagnostic tools are available for E. dermatitidis. The symptoms, such as the black sputum and nasal discharge may be characteristic and we should positively suspect the E. dermatitidis infection if we find these symptoms in the patients although we could suspect the aspergillus infection routinely in the CF and similar diseases, including SBS.
Morphological identification of E. dermatitidis is restricted by its slow-growing behavior, and there is therefore a risk of bacterial overgrowth, eg by P. aeruginosa and Burkholderia cepacia complex organisms, especially in CF patients.9,10 Sabouraud agar is the most commonly used agar for isolation and cultivation of fungal species. However, for E. dermatitidis isolation, Burkholderia cepacia-selective agar, potato-dextrose agar with rose-bengal and chloramphenicol, erythritol-chloramphenicol agar, or Sabouraud gentamicin-chloramphenicol agar is recommended, with all of them leading to increased isolation rates compared to Sabouraud media alone by inhibiting bacterial growth.1,9,10
In this study, potato-dextrose agar with chloramphenicol was used and successfully isolated E. dermatitidis as small and large colonies as 24-hour and 72-hour cultures. Since E. dermatitidis is known to be able to switch from the yeast like to hyphal state, filamentous and yeast-like forms were found in 24 and 72-hour cultured colonies, respectively. In addition, the colonies had high viscosity, which may be one of the key microbiological characteristics in the diagnosis of E. dermatitidis.11
Furthermore, MALDI-TOF MS has recently been used for the diagnosis of E. dermatitidis in addition to genetic ribosomal DNA internal transcribed spacer (ITS) sequencing.1,4,12 This protein level analysis was also used in the present study, providing accurate results. It is rapid and easy and should be very supportive to identify E. dermatitidis in combination with conventional microbiological methods, as in the bacterial identification we previously reported.13,14 However, the fungus were correctly identified by the MALDI-TOF MS in the yeast-like form, but not the filamentous form. Therefore, we should take of the form of the isolated fungus and the colony isolated time after cultures were started.
In treatment, although several studies investigated resistance and susceptibility of both environmental and clinical isolates of E. dermatitidis to various anti-infective agents, there are currently no available standardized broth microdilution methodologies or validated MIC breakpoints for in vitro resistance testing for E. dermatitidis.1 However, VRCZ, ITCZ, and posaconazole have been shown to be active against E. dermatitidis, and almost all tested strains were susceptible to Amp B (MIC = 0.25–2 μg/mL), 5-fluorocytosine (MIC =0.25–1 μg/mL), ITCZ (MIC = 0.25–8 μg/mL), fluconazole (MIC = 8–64 μg/mL), and VRCZ (MIC = 0.25–1 μg/mL) in several reports.15,16 In contrast, the echinocandins, such as caspofungin (MIC50 = 4 μg/mL) and anidulafungin (MIC50 = 2 μg/mL) demonstrated weak activity against E. dermatitidis.17 An MIC of 8 μg/mL micafungin against three different clinical isolates of E. dermatitidis was detected, also indicating weak activity of micafungin against the fungus.1,18 The present isolated E. dermatitidis also showed similar susceptibility patterns, and the conditions of the patients improved with VRCZ, L-AmpB, and ITCZ. However, the duration of antifungal therapy may be critical, because some cases were reported to be recurrent although the patient received VRCZ for 11 months.1,2,4 More than 2 years of treatment may be necessary depending on the patients’ status.2 Further studies of prolonged therapy are needed.
In conclusion, two E. dermatitidis infected cases with SBS were reported. One case was the patient with pulmonary symptoms and findings, and the other patient was s showed mainly nasal symptoms and findings. Both patients were not so compromised, and suspected by characteristic symptoms, such as black sputum and nasal discharge. Diagnosis were performed by detailed conventional examinations and MALDI- TOF MS analysis. Their symptoms improved and E. dermatitidis disappeared with antifungal agents, such as VCRZ, L-AmpB, and ITCZ. These case may be not so novel, however, we could recognize the importance of sharing the experience of such a rare infectious disease because the detailed pathogenesis, diagnostic strategy, drug susceptibility, and antifungal chemotherapy, including duration of therapy remain unclear.
For Review
We present two cases of exacerbated sinobronchial syndrome due to Exophiala dermatitidis infection in Japan. Both patients were suspected to have non-tuberculous Mycobacterium infection. However, following a short period of hospitalization and combination therapy with intravenous liposomal amphotericin B and voriconazole, both cases were successfully treated as outpatients with oral itraconazole.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kirchhoff L, Olsowski M, Rath PM, Steinmann J. Exophiala dermatitidis: key issues of an opportunistic fungal pathogen. Virulence. 2019;10:984–998. doi:10.1080/21505594.2019.1596504
2. Sekiguchi R, Urabe N, Sakamoto S, Sasaki M, Homma S, Kishi K. Exophiala dermatitidis pneumonia with bronchiectasis required prolonged voriconazole treatment. Respirol Case Rep. 2021;9:e00783. doi:10.1002/rcr2.783
3. Mukaino T, Koga T, Oshita Y, Narita Y, Obata S, Aizawa H. Exophiala dermatitidis infection in non-cystic fibrosis bronchiectasis. Respir Med. 2006;100:2069–2071. doi:10.1016/j.rmed.2006.03.003
4. Usuda D, Higashikawa T, Hotchi Y, et al. Exophiala dermatitidis. World J Clin Cases. 2021;9:7963–7972. doi:10.12998/wjcc.v9.i27.7963
5. Li DM, Li RY, de Hoog GS, Sudhadham M, Wang DL. Fatal Exophiala infections in China, with a report of seven cases. Mycoses. 2011;54(Fatal Exophiala infections in China, with a report of seven cases):e136–42. doi:10.1111/j.1439-0507.2010.01859.x
6. Bulloch MN. The treatment of pulmonary Wangiella dermatitidis infection with oral voriconazole. J Clin Pharm Ther. 2011;36:433–436. doi:10.1111/j.1365-2710.2010.01214.x
7. Mukai Y, Nureki S, Hata M, et al. Exophiala dermatitidis pneumonia successfully treated with long-term itraconazole therapy. J Infect Chemother. 2014;20:446–449. doi:10.1016/j.jiac.2014.02.006
8. Wickes BL, Wiederhold NP. Molecular diagnostics in Medical mycology. Nat Commun. 2018;9(1):5135. doi:10.1038/s41467-018-07556-5
9. Kondori N, Gilljam M, Lindblad A, Jönsson B, Moore ER, Wennerås C. High rate of Exophiala dermatitidis recovery in the airways of patients with cystic fibrosis is associated with pancreatic insufficiency. J Clin Microbiol. 2011;49:1004–1009. doi:10.1128/JCM.01899-10
10. Raclavsky V, Novotny R. Burkholderia cepacia selective agar can be useful for recovery of Exophiala dermatitidis from sputum samples of cystic fibrosis patients. J Cyst Fibros. 2016;15:e19. doi:10.1016/j.jcf.2015.12.021
11. Masoud-Landgraf L, Badura A, Eber E, Feierl G, Marth E, Buzina W. Modified culture method detects a high diversity of fungal species in cystic fibrosis patients. Med Mycol. 2014;52:179–186. doi:10.3109/13693786.2013.792438
12. Kondori N, Erhard M, Welinder-Olsson C, Groenewald M, Verkley G, Moore ER. Analyses of black fungi by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS): species-level identification of clinical isolates of Exophiala dermatitidis. FEMS Microbiol Lett. 2015;362:1–6. doi:10.1093/femsle/fnu016
13. Hariu M, Watanabe Y, Oikawa N, Seki M. Usefulness of matrix-assisted laser desorption ionization time-of-flight mass spectrometry to identify pathogens, including polymicrobial samples, directly from blood culture broths. Infect Drug Resist. 2017;10:115–120. doi:10.2147/IDR.S132931
14. Hariu M, Watanabe Y, Oikawa N, Manaka T, Seki M. Evaluation of blood culture broths with lysis buffer to directly identify specific pathogens by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry methods. Infect Drug Resist. 2018;11:1573–1579. doi:10.2147/IDR.S169197
15. Gao L, Sun Y, He C, Zeng T, Li M. Synergistic effects of tacrolimus and azoles against Exophiala dermatitidis. Antimicrob Agents Chemother. 2017;61:e00948–17. doi:10.1128/AAC.00948-17
16. Niweze EI, Ezute S. Isolation and antifungal susceptibility of Exophiala dermatitidis isolates from human stool samples in Nigeria. Mycopathologia. 2010;169:201–206. doi:10.1007/s11046-009-9244-2
17. Badali H, de Hoog GS, Sudhadham M, Meis JF. Microdilution in vitro antifungal susceptibility of Exophiala dermatitidis, a systemic opportunist. Med Mycol. 2011;49:819–824. doi:10.3109/13693786.2011.583285
18. Kirchhoff L, Olsowski M, Zilmans K, et al. Biofilm formation of the black yeast-like fungus Exophiala dermatitidis and its susceptibility to antiinfective agents. Sci Rep. 2017;17:42886. doi:10.1038/srep42886
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.