Back to Journals » Clinical Ophthalmology » Volume 15
Single-Step Transepithelial Photorefractive Keratectomy in Low to Moderate Myopia: A One-Year Follow-Up Study
Authors Abdelwahab SM , Salem MH, Elfayoumi MA
Received 29 June 2021
Accepted for publication 27 July 2021
Published 9 August 2021 Volume 2021:15 Pages 3305—3313
DOI https://doi.org/10.2147/OPTH.S326048
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Video abstract presented by Shereef Abdelwahab.
Views: 1074
Shereef M Abdelwahab, Mohamed Hany Salem, Maha A Elfayoumi
Department of Ophthalmology, Benha University, Benha, Egypt
Correspondence: Shereef M Abdelwahab Email [email protected]
Aim: To evaluate predictability, safety, efficacy, and visual outcome of StreamLight. ™ (SL.), the newly released single-step transepithelial photorefractive keratectomy platform by Alcon WaveLight™ (WL).
Methods: In this prospective cohort study, photorefractive keratectomy (PRK) was conducted on 500 eyes of 250 patients seeking myopic refractive vision correction. The new single-step transepithelial PRK method was applied, using the SL. platform installed in the WL. Ex 500 excimer laser machine. Patients were followed up to monitor intensity and duration of postoperative pain, as well as speed of epithelial healing in the early post-operative period and visual acuity, postoperative refraction and development of postoperative haze for one year post-operatively.
Results: Average pain duration was 1.5 days, and the mean pain intensity score on a scale of 0– 10 was 3.74 + 1.51. Mean postoperative spherical equivalence was 0.01 ± 0.38 D, and the final postoperative uncorrected distance visual acuity (UCDVA) was 20/20 in 98% of eyes included in this study. None of the eyes lost more than one Snellen chart line or developed visually significant postoperative haze during the follow-up period.
Conclusion: The new SL. platform for transepithelial PRK is a safe, accurate platform, offering an easier early post-operative recovery, with no compromise in final visual outcome.
Keywords: transepithelial PRK, SL., photorefractive keratectomy, myopic refractive correction
Introduction
Photorefractive keratectomy (PRK) is a well-known procedure for correction of refractive errors and is one of the primary surgical techniques for laser vision correction.1 PRK involves epithelial removal followed by stromal ablation for laser vision correction. The epithelium can be removed either chemically using alcohol or mechanically using a blade or brush. Alternatively, the epithelium can be removed by laser. Laser epithelial removal is known as phototherapeutic keratectomy (PTK) and when this method was introduced the PRK procedure was conducted in two successive steps: first, PTK, then PRK. Adoption of this two-step technique was limited resulting hyperopic postoperative refractions (hyperopic shifts), caused by a myopic ablation profile.2
A single-step technique involves ablation of both the epithelium and the stroma in one procedure.3 Clinical outcomes of single-step ablation have shown several advantages over the former two-step procedures including less dehydration, no unwanted hyperopic shift, less pain, lower grade of haze and faster reepithelialization.3 The hyperopic shift in the two-step procedure could be minimized by surgical procedure to approximately 50% of the expected level,4 but the need for this correction was eliminated with the single-step procedures.
Single-step transepithelial PRK platforms are available on the Amaris 1050RS (Schwind) and on the Technolas Teneo, (Bausch+Lomb) and the recently introduced SL. on the (WL. EX500.)
The Amaris platform uses a reverse single-step transepithelial PRK procedure, in which the ablation to eliminate the refractive error is performed first followed by another defined ablation to simulate the epithelium thickness profile of a normal population.5 The Technolas platform also combines PRK and PTK and allows for additional PTK after treatment if needed. SL., however, involves epithelial ablation first followed by the refractive correction.
This study is one of the first to evaluate the predictability, safety, efficacy, and visual outcomes of SL.™, the newly released single-step transepithelial PRK platform by Alcon WL. This evaluation is needed to encourage more surgeons to adopt the technique and use the newly released platform with confidence.
During the final steps of preparing our present paper for publication a recent paper (April 2021) studying the difference between Trans-Prk using the SL. platform and PRK28 was published; the study, however, is a short time postoperative study (6 weeks) and evaluated the early outcome of SL. PRK and concluded the procedure to be safe and effective.
Subjects and Methods
This study enrolled patients with a manifest spherical refractive error ranging from −0.25 to −6.5 diopters (D) with up to 4 D of astigmatism. The study followed the tenets of the Declaration of Helsinki and was approved by the medical ethics committee of Benha University Faculty of Medicine. All patients were given an informed consent form to read and sign before participation in the study. Two hundred and fifty patients were selected, surgery was conducted between November 2019 and February 2020 and patients were followed up for one year post-operatively. All surgeries were performed and followed up by three well experienced refractive surgeons (SMA, MHS, MAE).
Before surgery, all patients underwent slit lamp examination including applanation tonometry, both uncorrected and best corrected visual acuity were recorded, and manifest and subjective refractions were conducted and recorded. Corneal topography was conducted using the WL. Oculyzer Tm II corneal topographer and data were transmitted via the WaveNet™ to the WL. refractive Suite (Alcon Laboratories, Fort Worth TX, USA.)
Inclusion criteria were stable refraction over the preceding year of less than −7 D spherical equivalent with astigmatism of not more than −4 D, normal corneal topography and a calculated residual stromal bed of at least 300 µm. Patients with pre-operative central corneal thickness of less than 470 µm, collagen vascular diseases, history of keratoconus, or any prior ocular surgery were excluded. To study the predictability of this new platform, we compared the patient’s final visual postoperative outcome with his best corrected preoperative visual acuity.
Surgical Technique
After sterilization and draping, 2–3 drops of benoxinate hydrochloride 0.4% were instilled, a lid speculum was inserted, and the patient was instructed to look at a green fixation light in the straight-ahead position. The SL.™ platform requires the surgeon to select the depth of epithelium to be ablated. The selected depth setting for PTK in all cases was based on the SL.™ recommendations of 45–65 µm. The SL. platform allows selection of epithelium ablation depth in 5µm steps, allowing the surgeon to customize this depending on the mode of ablation, depth of ablation or optical coherence tomography examination (Alcon Communication).
The eye was washed with chilled balanced salt solution (BSS), adding a cooling effect to the cornea before laser ablation, then dried with a wet micro sponge. The surgeon then pressed on the centration pedal to activate the pupil centration and eye tracker, then on the laser firing pedal to start the epithelial ablation, tracking its progress on a green progression bar visible in the surgeon’s microscope (yellow bar on WL. computer screen). The bar is divided into two parts to track the epithelial ablation progress and the stromal ablation progress, respectively (Figure 1).
Once tracking indicates the end of epithelial ablation the surgeon releases pressure from the pedal and waits for 10 s allowing the cornea to cool down, after which the pedal is depressed again to begin stromal laser ablation.
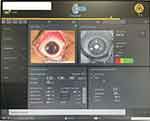 |
Figure 1 The SL. platform computer display, showing the yellow bar denoting the end of epithelial ablation (yellow arrowhead) and beginning of stromal ablation. |
Immediately after ablation, the eye was washed again with chilled BSS. Following surgery, a bandage contact lens (BCL) soaked in 0.45% ketorolac for 20 minutes was applied and the procedure was repeated in the fellow eye.
0.02% Mitomycin C (MMC) was used when the calculated ablation depth was more than 60µ and was applied for 20 seconds.
Patients were instructed to use non-steroidal anti-inflammatory drops (Ketorolac tromethamine 0.45%) three times daily for one-week, antibiotic eye drops (Moxifloxacin hydrochloride 0.5%) four times daily for one-week, topical steroid eye drops (dexamethasone 0.1%) four times a day for one week then tapered and stopped after eight weeks, lubricant eye drops at least four times daily for two months post-operatively. Vitamin C 1000 milligram (once daily for one month) was also prescribed. Patients were asked to wear their UV protective sunglasses outdoors in sunlight throughout the follow-up period.
Patients were followed up on the first postoperative day, then on the third day, and daily thereafter until the epithelium was completely healed, if not healed by the third day. The patients were seen after one week, one month, three months, six months and one year. On the first visit, patients were asked about the severity of the post-operative pain according to a pain scale diagram graded from zero (no pain) to 10, by marking the level of their pain on the scale. In the first follow-up visits, epithelial healing was monitored by slit lamp examination, and once completely healed, the time of healing was recorded and the BCL was removed. Patients were seen again on day 7 and after one month when refraction and uncorrected distance visual acuity (UCDVA) were evaluated, and the anterior eye was examined for corneal clarity and development of post-operative haze. Corneal haze was evaluated using the Heitzmann grading system from 0 to 5. This system was introduced in 1993 and includes the following grades: 0=clear cornea; 0.5=haze slightly detectable on slit lamp examination; 1=reticular haze, easily detectable on slit lamp examination; 2 clinically significant haze with areas of focal confluency; 3=clinically significant haze with areas of diffuse confluency; 4=confluent and diffuse haze leading to difficulties in examining the iris; 5=corneal opacity preventing iris examination.6 At three and six months postoperatively, refraction and UCDVA were recorded. At one-year, patients’ UCDVA was evaluated, and corneal haze was graded.
We managed to follow up all our 250 patients for the one-year follow-up period, we had to contact them sometimes several times to come for their scheduled follow-ups specially after the six months visit when all our patients had stable clear vision, we had no dropouts and did not need to exclude any data in our statistical analysis.
Statistical Analysis
Pre- and post-operative UCDVA, preoperative best spectacle corrected distance visual acuity (BCDVA) and postoperative UCDVA at final follow-up as well as mean manifest refractive spherical equivalence at different postoperative stages were compared within individuals using the paired Student’s t-test.
Results
This study included 500 eyes of 250 patients (110 male and 140 female). The mean age of patients included in the study was 27.3+6.1 years (range: 18–49 years). All patients were low to moderate myopes with or without astigmatism Mean preoperative spherical error was −1.84+0.86D (range: −0.25 to −6.5 D), mean preoperative astigmatism was −0.55+0.53D (range: 0–2.25 D) and the mean preoperative spherical equivalence (SE) was −2.12+0.83D. (range: −0.87 to −6.5 D)
The mean size of the ablated epithelial zone was 8.2+0.92 mm (range: 6.5–9 mm); this was calculated by the SL.™ platform based on the manually entered ablation zone size. In all cases, the selected epithelial ablation depth was 55 µ.
The mean depth of stromal ablation was 35 µ+13 µ (range: 16–94 µ). Mitomycin C (MMC) was used for 20 s in only 19 eyes with calculated ablation depth over 60 µ.
Figure 2 shows the refractive and visual acuity results at six months postoperatively. Because the three and six month and one year refractive and visual acuity results were almost identical, only the 6-month data were plotted. The mean postoperative SE was 0.01+0.38D and was significantly less than the preoperative value (P < 0.005). Interestingly, in 94% of eye astigmatism was within+0.5 D cylinder. All patients achieved a postoperative unaided visual acuity equal to or better than their preoperative best corrected visual acuity (P < 0.005) indicating efficacy of the platform, and best corrected distance Snellen visual acuity was not reduced postoperatively in any of the patients, suggesting safety.
On day one, the mean pain score was 3.74+1.51 (range: 1–8) on the 0–10 pain scale, the mean pain duration was 1.5+1.2 days (range: 1–6 days). After epithelial healing and BCL removal, none of the patients reported any eye pain throughout the follow-up period.
When the epithelium was completely healed the BCL was removed, and the timing of this occurrence was recorded. In 90.6% of the eyes included in this study (453 eyes) complete epithelial healing was reached by day three, the remaining 9.4% of eyes needing a BCL replacement on day three. All (100%) of the eyes were completely healed by day six.
Postoperative corneal haze in all the eyes included in this study throughout the one-year follow-up was never graded higher than 0.5 on the Heitzmann grading system. A grade 0.5 of corneal haze was seen on slit lamp examination in 17% of eyes (85 eyes) on the follow-up visit at three and six months postoperatively. It was not affecting the patients’ visual acuity, nor was it a source of complaint from any patient. By the last follow-up visit, all patients had a corneal haze score of zero.
Discussion
Transepithelial PRK has been used for refractive laser correction since it was introduced in the early 1990s, and became more popular after studies conducted by Aron-Rosa et al7 and Gimbel et al.8 It is an effective and safe technique and is well tolerated by patients.9 Published research10–12 has compared clinical results between different methods of epithelial removal, including the transepithelial method using the Schwind Amaris laser platform or other two-step procedures.
To our knowledge, no published studies to date have focused on the new SL. Alcon WL.EX500 single-step transepithelial PRK platform.
In 2019 Alcon added the SL. Platform to its EX500 Excimer laser. The present study assessed the safety, efficacy, and reliability of the platform as well as the patients’ satisfaction in terms of postoperative pain and final visual outcome at the end of the follow-up period. Comparison between this and other single-step transepithelial PRK platforms was beyond the scope of the study.
The SL.™ Trans-epithelial PRK removes corneal epithelium and stroma in a single step. In this technique, the laser removes corneal epithelial tissue based on a preset thickness of a normal corneal epithelium (45–65 μ), surgeons’ preference and Alcon WL. recommendations.
Since one epithelial ablation algorithm is used for all eyes in transepithelial PRK, regardless of the actual epithelial topometry, more stroma might be ablated than necessary in eyes with a thin epithelium, whereas in eyes with a thick epithelium the refractive part of the ablation might begin with some epithelium remaining on the surface.13 Reinstein et al14 found that the mean epithelial thickness at the corneal vertex was 53.4±4.6 μ, and that the epithelium is thicker inferiorly than superiorly (5.9 μ at the 3-mm radius, P < 0.001) and thicker nasally than temporally. The thinnest part of the epithelium was at a mean location of 0.33 mm temporal and 0.90 mm superior to the corneal vertex. Using spectral-domain anterior-segment optical coherence tomography, Kanellopoulos and Asimellis15 found mean epithelial thickness at the pupil center to be 53.28±3.34 μ, superiorly 51.86±3.78 μ, and inferiorly 53.81±3.44 μ. These findings suggest that it may not be safe to assume that the epithelium thickness map is rotationally symmetrical, and in fact both studies found high inter-individual variability of the central epithelial thickness and 3-dimensional epithelial maps. These variations may affect the predictability, safety, and efficacy of transepithelial PRK ablations in comparison to the standard PRK procedures in which the surgeon manually removes and visually confirms epithelial removal.
SL.™, however, uses special epithelial lists (EPI List) which are a set of corrections or algorithms that provide a uniform ablation of the epithelium. The Lists are calculated individually for each patient so that epithelium thickness variations over the corneal surface are taken in account and a complete removal of the epithelium can be expected. The lists have been calculated to consider the difference in ablation behavior between epithelium and stroma, as stated by the manufacturer (Alcon Communication), yet further research studies are needed to document this.
The single-step treatment profile in which both PTK and PRK are conducted sequentially with only one centration required, enhances precision. The PTK zone is automatically adjusted to the required optical zone for the consecutive refractive correction – As small as possible, as big as necessary. (Alcon communication)
In this study, the selected PTK depth was 55 µ in all eyes, based on calculated wave front optimized optical ablation zone and PTK calculated optical zone, based on the Alcon supplied table for selection of PTK depth.
The development of corneal haze is one of the limits of PRK.16 Postoperative haze may appear 1–3 months after surgery and then disappear after one year, or may appear three months after surgery (late-onset corneal haze) and persist for 2–3 years.17 Corneal haze after transepithelial PRK is attributed to the higher total excimer laser energy load during PTK. Most of the laser energy is delivered to the epithelium but causes an increase in temperature of the stromal tissue, which is the main risk factor for haze formation after surface ablation. Studies on high degrees of myopia reported some degree of corneal haze in the first postoperative months, with values ranging between 5% and 20%.18
Luger et al19 compared one year postoperative results after myopic correction with a transepithelial method used on one eye and an alcohol-assisted PRK used on the fellow eye. They found slightly hyperopic (+0.07±0.23D) postoperative mean spherical equivalent in transepithelial PRK and results of 0.01±0.27 with alcohol assisted PRK. They used the Schwind Amaris system and included 66 eyes of 33 patients with a range of preoperative myopic spherical equivalence from −1.0 to −9.38. In the present study, the mean postoperative spherical equivalent was 0.01+0.38 which appears to be slightly lower and could be attributed to our lower range of preoperative myopic spherical equivalence, larger sample, as well as use of the SL.™ optimized ablation profile.
Variations in preoperative epithelial thickness might potentially also contribute to post-operative refractive error. In our study, choice of the 55µ depth and inclusion of de-novo eyes with no previous ocular surgery or corneal pathology assured a full thickness epithelial removal in all cases avoiding encroachment on the stroma during PTK. Reinstein et al20 found a difference in epithelial behavior after myopic correction for low, moderate and high grades of myopia, and reported mean epithelial thickening at the corneal vertex of 7.41 1.09µ 9.29
1.09µ 9.29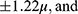 12.33
12.33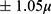 respectively. This change in behavior may explain the low variation in our study in which most eyes were in the low to moderate myopia range rather than moderate to high. The pause between PTK and PRK which is marked both visually and audibly on the SL.™ platform is intended to allow time for the eye to cool down before proceeding with the planned PRK and gives the surgeon time to inspect and visually confirm full epithelial removal before continuing. In the present study, we observed no residual epithelium after PTK and none of the cases required further surgical intervention prior to PRK. All eyes treated in this study were in the low to moderate range of myopia with or without astigmatism, so choosing an epithelial ablation depth in the mid-range of the manufacturer’s recommendations and visually confirming the complete epithelial removal before proceeding could also favor our results.
respectively. This change in behavior may explain the low variation in our study in which most eyes were in the low to moderate myopia range rather than moderate to high. The pause between PTK and PRK which is marked both visually and audibly on the SL.™ platform is intended to allow time for the eye to cool down before proceeding with the planned PRK and gives the surgeon time to inspect and visually confirm full epithelial removal before continuing. In the present study, we observed no residual epithelium after PTK and none of the cases required further surgical intervention prior to PRK. All eyes treated in this study were in the low to moderate range of myopia with or without astigmatism, so choosing an epithelial ablation depth in the mid-range of the manufacturer’s recommendations and visually confirming the complete epithelial removal before proceeding could also favor our results.
Haze was evaluated by slit lamp examination and was graded according to the Heitzmann grading system on a scale from 0 to 5. We found no significant haze in any of our cases. A grade 0.5 haze was found in 17% of eyes (n=85), which did not affect the patient’s visual acuity and was not a source of patient complaint. This slit lamp finding was observed between the 3rd and 6th months postoperatively and disappeared from all eyes by the final one-year follow-up visit. The lack of significant haze could be attributed to our preoperative patient selection criteria, with cases of low to moderate myopia, with or without astigmatism, being eligible to participate, and the use of MMC whenever the calculated stromal depth was more than 60µ.
Transepithelial platforms result in less heat generation at the corneal surface.21 Effective control of the maximum temperature rise during laser ablation could reduce the incidence of postoperative haze. The baseline ocular surface temperature, immediately prior to beginning excimer laser, ranges from 32°C to 34.9°C. The maximum ocular surface temperature during epithelial ablation ranges from 35.2°C to 39.7°C, and during high and low fluence laser stromal ablation ranges from 32.9°C to 36.5°C and from 34.4°C to 37.7°C, respectively. De Ortueta et al21 concluded that limiting the maximum temperature to below the safety limit of 40°C reported in the literature could decrease the occurrence of postoperative corneal haze. The routine usage of chilled BSS before and immediately after laser ablation, and the time gap of about 10 s between PTK and PRK add a cooling effect to the cornea compensating for heat generated by the excimer laser. The new technological advances in the SL. platform reduce the likelihood of thermally induced postoperative corneal haze. The SL.™ EPI lists as well as the wave front optimized (WFO) lists are calculated using an algorithm that minimizes the thermal side effects of ablation. Each pulse is directed at a position on the corneal surface which has had time to cool since a previous pulse at that location. A 10 second pause between EPI and WFO lists as well as cooling by chilled BSS supports temperature control reducing the incidence of postoperative haze development (Alcon communication).
0.02% Mitomycin C (MMC) was used when the calculated ablation depth was more than 60µ, and was applied for 20 seconds when the cornea was at high risk of developing postoperative haze, based on previous findings that haze is more likely to occur when planned ablation depth is >75 µ.22
Transepithelial PRK, PRK, and LASIK significantly decrease the levels of ascorbic acid in tear film.23 The highest concentration of vitamin C levels in corneal tissue is found in the corneal epithelium,24 so its removal decreases the ascorbic acid concentration in the corneal tissue and the harmful effects of superoxide radicals may be more evident in PRK and transepithelial PRK. Postoperative administration of systemic vitamin C may improve the concentration of the ascorbic acid in the corneal tissue reducing the incidence of post-operative haze.
One of the main drawbacks of PRK surgery is significant postoperative pain secondary to removal of epithelium and exposure of the nerve endings.25 This pain usually continues for the first 2–3 days until corneal surface re-epithelialization occurs. Topical NSAID drugs are among the most popular medications to control post-PRK pain. Diclofenac and Ketorolac have been approved by the FDA for pain control after surface ablations.25 Pre- and post-operative cooling in PRK effectively reduces post-operative pain without any additional adverse effect.26 The use of a bandage contact lens soaked in Ketorolac 0.45% solution acts as a repository for Ketorolac, releasing it on the ocular surface over time providing postoperative pain relief immediately after photorefractive surgery.26,27
The limitations of our study include the fact that only low and moderate grades of myopia, with or without astigmatism, were studied. Further research is needed to evaluate this approach in higher degrees of myopia and in treatment of hyperopia. We also did not study the effect of the SL. transepithelial PRK on the development of postoperative aberrations, and a separate study is needed to further evaluate these.
In a recently published clinical observational study by Harald, C Gaekle,28 his results on short-term follow-up of trans-PPK cases done using the SL. platform showed that it is a safe and effective method for correction of low to moderate myopia with or without astigmatism and considered it a good option for patients who refuse or are not eligible for femto-LASIK and demand a fast and more comfortable recovery time than that of PRK.
In conclusion, the new SL.™ is a safe and reliable platform for performing transepithelial PRK. Patients were happy to finish their surgical procedure rapidly and with no surgical instruments other than the eye speculum and the laser beam touching their eyes.
Acknowledgments
The authors have equally shared in the study, performing the surgeries, follow up of patients, data collection and preparing the article for publication. None of the authors have any financial disclosures, and no financial support was received for this study.
Disclosure
None of the authors has a financial or proprietary interest in any material or method mentioned. The authors report no conflicts of interest for this work.
References
1. Shetty R, Narasimhan R, Dadachanji Z, et al. Early corneal and epithelial remodeling differences identified by OCT imaging and artificial intelligence between two transepithelial PRK platforms. J Refract Surg. 2020;36(10):678–686. doi:10.3928/1081597X-20200730-03
2. Arba-Mosquera S, de Ortueta D. Geometrical analysis of the loss of ablation efficiency at non-normal incidence. Opt Express. 2008;16(6):3877–3895. doi:10.1364/OE.16.003877
3. Adib-Moghaddam S, Soleyman-Jahi S, Sanjari Moghaddam A, et al. Efficacy and safety of transepithelial photorefractive keratectomy. J Cataract Refract Surg. 2018;44(10):1267–1279. doi:10.1016/j.jcrs.2018.07.021
4. Muller LT, Candal EM, Epstein RJ, Dennis RF, Majmudar PA. Transepithelial phototherapeutic keratectomy/photorefractive keratectomy with adjunctive mitomycin-C for complicated LASIK flaps. J Cataract Refract Surg. 2005;31(2):291–296. doi:10.1016/j.jcrs.2004.04.044
5. Aslanides I, Padroni S, Arba-Mosquera S. Comparison of single-step reverse transepithelial All-Surface Laser Ablation (ASLA) to alcohol-assisted photorefractive keratectomy. Clin Ophthalmol. 2012;973–980. doi:10.2147/OPTH.S32374
6. Heitzmann J, Binder PS, Kassar BS, Nordan LT. The correction of high myopia using the excimer laser. Arch Ophthalmol. 1993;111(12):1627–1634. doi:10.1001/archopht.1993.01090120049021
7. Aron-Rosa DS, Colin J, Aron B, et al. Clinical results of excimer laser photorefractive keratectomy: a multicenter study of 265 eyes. J Cataract Refract Surg. 1995;21(6):644–652. doi:10.1016/S0886-3350(13)80560-3
8. Gimbel HV, DeBroff BM, Beldavs RA, Van Westenbrugge JA, Ferensowicz M. Comparison of laser and manual removal of corneal epithelium for photorefractive keratectomy. J Refract Surg. 1995;11(1):36–41. doi:10.3928/1081-597X-19950101-10
9. Ang EK, Couper T, Dirani M, Vajpayee RB, Baird PN. Outcomes of laser refractive surgery for myopia. J Cataract Refract Surg. 2009;35(5):921–933. doi:10.1016/j.jcrs.2009.02.013
10. Shapira Y, Mimouni M, Levartovsky S, et al. Comparison of three epithelial removal techniques in PRK: mechanical, alcohol assisted, and transepithelial laser. J Refract Surg. 2015;31(11):760–766.
11. Naderi M, Jadidi K, Mosavi S, Daneshi S. Transepithelial photorefractive keratectomy for low to moderate myopia in comparison with conventional photorefractive keratectomy. J Ophthalmic Vis Res. 2016;11(4):358–362. doi:10.4103/2008-322X.194070
12. Zarei-Ghanavati S, Shandiz JH, Abrishami M, Karimpour M. Comparison of mechanical debridement and trans-epithelial myopic photorefractive keratectomy: a contralateral eye study. J Curr Ophthalmol. 2019;31(2):135–141. doi:10.1016/j.joco.2019.01.003
13. Sin S, Simpson TL. The repeatability of corneal and corneal epithelial thickness measurements using optical coherence tomography. Optoml Vis Sci. 2006;83(6):360–365. doi:10.1097/01.opx.0000221388.26031.23
14. Reinstein DZ, Archer TJ, Gobbe M, Silverman RH, Coleman DJ. Epithelial thickness in the normal cornea: three-dimensional display with artemis very high-frequency digital ultrasound. J Refract Surg. 2008;24(6):571–581.
15. Kanellopoulos AJ, Asimellis G. In vivo three-dimensional corneal epithelium imaging in normal eyes by anterior-segment optical coherence tomography: a clinical reference study. Cornea. 2013;32(11):1493–1498. doi:10.1097/ICO.0b013e3182a15cee
16. Spadea L, Giovannetti F. Main complications of photorefractive keratectomy and their management. Clin Ophthalmol. 2019;13:2305–2315. doi:10.2147/OPTH.S233125
17. Meyer JC, Stulting RD, Thompson KP, Durrie DS. Late onset of corneal scar after excimer laser photorefractive keratectomy. Am J Ophthalmol. 1996;121(5):529–539. doi:10.1016/S0002-9394(14)75427-3
18. Taneri S, Weisberg M, Azar DT. Surface ablation techniques. J Cataract Refract Surg. 2011;37(2):392–408. doi:10.1016/j.jcrs.2010.11.013
19. Luger MHA, Ewering T, Arba-Mosquera S. Consecutive myopia correction with transepithelial versus alcohol-assisted photorefractive keratectomy in contralateral eyes: one-year results. J Cataract Refract Surg. 2012;38(8):1414–1423. doi:10.1016/j.jcrs.2012.03.028
20. Reinstein DZ, Srivannaboon S, Gobbe M, et al. Epithelial thickness profile changes induced by myopic LASIK as measured by artemis very high-frequency digital ultrasound. J Refract Surg. 2009;25(5):444–450.
21. De Ortueta D, Arba-Mosquera S, Magnago T. High-speed recording of thermal load during laser trans-epithelial corneal refractive surgery using a 750 Hz ablation system. J Optom. 2019;12(2):84–91. doi:10.1016/j.optom.2018.05.002
22. Pandit RT
23. Bilgihan A, Bilgihan K, Toklu Y, Konuk O, Ö Y, Hasanreisoǧlu B. Ascorbic acid levels in human tears after photorefractive keratectomy, transepithelial photorefractive keratectomy, and laser in situ keratomileusis. J Cataract Refract Surg. 2001;27(4):585–588. doi:10.1016/S0886-3350(00)00877-4
24. Ringvold A, Anderssen E, Kjønniksen I. Ascorbate in the corneal epithelium of diurnal and nocturnal species. Invest Ophthalmol Vis Sci. 1998;39(13):2774–2777.
25. Abri Aghdam K, Aghaei H, Shokrollahi S, et al. Comparison of the effect of cycloplegic versus NSAID eye drops on pain after photorefractive keratectomy. J Curr Ophthalmol. 2015;27(3–4):87–91. doi:10.1016/j.joco.2015.11.006
26. Shetty R, Dalal R, Nair AP, Khamar P, D’Souza S, Vaishnav R. Pain management after photorefractive keratectomy. J Cataract Refract Surg. 2019;45(7):972–976. doi:10.1016/j.jcrs.2019.01.032
27. Sánchez-González JM, López-Izquierdo I, Gargallo-Martínez B, De-hita-cantalejo C, Bautista-Llamas MJ. Bandage contact lens use after photorefractive keratectomy. J Cataract Refract Surg. 2019;45(8):1183–1190. doi:10.1016/j.jcrs.2019.02.045
28. Gaekle HC. Early clinical outcomes and comparison between trans-PRK and PRK, regarding refractive outcome, wound healing, pain intensity and visual recovery time in real-world setup. BMC Ophthalmol. 2021;20(1):1–9.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

