Back to Journals » Research and Reports in Urology » Volume 10
Simultaneous living donor orthotopic renal transplantation and bilateral nephrectomy for recurrent renal cell carcinoma and renal failure: case report and review of literature
Authors Novotny R , Marada T, Chlupac J , Viklicky O, Fronek J
Received 7 March 2018
Accepted for publication 7 May 2018
Published 11 September 2018 Volume 2018:10 Pages 69—73
DOI https://doi.org/10.2147/RRU.S167507
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jan Colli
Robert Novotny,1 Tomas Marada,1 Jaroslav Chlupac,1 Ondrej Viklicky,2 Jiri Fronek1,3,4
1Transplant Surgery Department, Institute for Clinical and Experimental Medicine, Prague, Czech Republic; 2Nephrology Department, Institute for Clinical and Experimental Medicine, Prague, Czech Republic; 3Second Faculty of Medicine, Charles University, Prague, Czech Republic; 4First Faculty of Medicine, Charles University, Prague, Czech Republic
Background: We report the case of a 43-year-old female patient with systemic lupus erythematosus, class III lupus nephritis, with predialysis creatinine levels around 350 μmol/L (3.95 mg/dL) after partial resection of the left kidney with histologically verified papillary carcinoma in 2010. Preoperative computed tomography of the abdomen revealed a small 8 mm tumor in the left upper kidney pole. The patient was indicated for simultaneous bilateral nephrectomy and orthotopic renal transplantation with the aim to minimize invasiveness of the procedure as well as for curable tumor removal.
Method: The procedure was performed under the full anesthesia trough upper middle laparotomy. As the first step, bilateral transperitoneal nephrectomy was performed. The live donor surgery started in a parallel theater to shorten the cold ischemic time of the graft. The renal graft had singe vessels and ureter; it was placed into the recipient’s right orthotopic position. End-to-end anastomosis of the right renal vein and artery anastomosis were performed; ureter was anastomosed end-to-end using recipient’s ureter.
Results: The postoperative period was uneventful with repeatedly excellent ultrasonography check-up of the graft’s perfusion. The patient was discharged after 13 days with a good renal function of the graft (urea: 15 mmol/L, creatinine 160 µmol/L [1.80 mg/dL]).
Conclusion: Orthotopic renal transplantation is a technically challenging but valid alternative for patients who are unsuitable candidates for heterotopic renal transplantation or in cases where there is a clear benefit of orthotopic renal transplantation.
Keywords: live, donor, renal, transplant, orthotopic, simultaneous
Introduction
The treatment of choice for patients with end-stage renal disease is a renal transplantation.1 The increasing age of recipients combined with the presence of comorbidities had increased the transplant’s complexity from both surgical and medical point of view. Presurgical evaluation of the future renal transplant recipient greatly influences the success of the transplantation (eg, arterial atherosclerosis, unsuitable pelvic veins, pelvic vascular anomaly, caval thrombosis).2 Because of technical ease and success, the heterotopic renal transplantation (HRT) in the right or left iliac fossa is generally preferred over the orthotopic renal transplantation (ORT).3,4 However, in selected patients where HRT is not possible, and so ORT was shown to be a desirable alternative.3
Case presentation
The patient is a 43-year-old female with systemic lupus erythematosus and class III lupus nephritis confirmed by biopsy in 2008. The patient was repeatedly treated with combined immunosuppressive treatment for relapse of systemic lupus erythematosus with cyclophosphamide intermittent intravenous pulse therapy + azathioprine, followed by cyclosporine A+ prednisone therapy. Since September 2012, the patient was continued on triple-combination therapy of Equoral (IVAX Pharmaceuticals Inc., Miami, FL, USA), Myfortic (Novartis, Basel, Switzerland), and prednisone until the pretransplant assessment. Autoantibodies were constantly positive: ANA ++, anti dsDNA ++ (50 kIU/L). The patient’s creatinine levels were around 350 μmol/L (3.95 mg/dL) in the predialysis values. The patient’s medical history revealed the following: partial resection of the left kidney with histologically verified papillary carcinoma in 2010, resection of a benign tumor from the left breast in 2013.
Pretransplant computed tomography (CT) of the abdomen had revealed a small 8 mm tumor in the left upper kidney pole. This lesion was suspected to be a renal malignancy recurrence. There were no other lesions found on CT. If this was tumor recurrence, it would be stated as cT1a (c = clinical = preoperative). In such cases, we would proceed with renal transplantation or listing the patient on a renal transplant waiting list immediately after the native kidney nephrectomy. Because of this reasoning, we decided to proceed with bilateral nephrectomy and live donor kidney transplantation at the same time. The kidney was donated from a living donor – the recipient’s mother. Preoperative immunology was as follows: PRA 30% with DTT 17%, CM (CDS, FACS) negative with mother, Luminex negative, 4 matches in HLA (1× A, 1× B, 2× DR).
The procedure was performed under general anesthesia trough upper middle laparotomy 15 cm in length. The abdomen was fully assessed for potential malignancy spread, including intraoperative ultrasonography of the liver. There were no suspicious lesions found, and no enlarged lymph nodes were observed.
Left-sided nephrectomy
The colon was dissected and moved medially, Gerota’s fascia was cut, and the left kidney together with the perinephric fat was dissected from the surrounding anatomical structures. The ureter was tied off and cut at the level of common iliac vessels; renal artery (RA) and vein were cut with vascular stapler, and kidney was removed together with the surrounding fat. The specimen was sent for pathology assessment. Well-to-moderately differentiated light-cell carcinoma, ~8 mm in size, limited to the kidney with staging pT1a, pN0, pMX, was observed, and lymph nodes showed no metastasis.
Right-sided nephrectomy
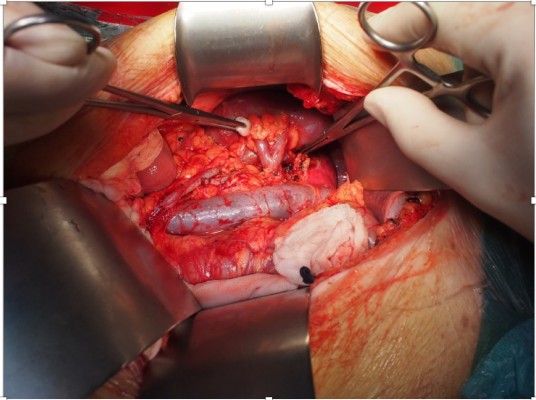
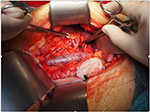
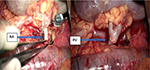
The colon was dissected and moved medially, Gerota’s fascia cut, and vascular pedicle and ureter were dissected carefully with the aim to preserve enough length for orthotopic graft anastomosing. The native ureter was clamped, ligated, and cut ~3 cm above the right common iliac artery. The RA was clamped close to the aorta and ligated just above the first bifurcation. The right renal vein (RV) was lamp close to its join with the inferior vena cava, ligated in the kidney hilum; a roughly 15 mm long stump was left. The right kidney was removed and sent for pathology assessment. Surgical swabs were placed in the operative field on both sides, retractor was released, and open wound covered on both sides; the patient was kept under general anesthesia on the table in theater. In the meantime, the donor’s right kidney was removed using hand-assisted retroperitoneoscopic live donor nephrectomy technique. After the removal of the kidney from the donor was completed, the graft was placed into to recipient’s right orthotopic position (Figure 1). RA was anastomosed end-to-end with the use of Prolene 7/0; RV was anastomosed end-to-end using 6/0 Prolene (Figure 2). Both anastomoses were performed with use of surgical microscope. The kidney graft started passing urine immediately after reperfusion. Ureters were anastomosed end-to-end using V-shape cut ~10 mm in length with PDS 6/0. Anastomosis was placed just above the common iliac artery bifurcation with an insertion of a 24 cm French JJ stent (Figure 3). After the transplantation, the graft was well perfused with no ischemic patches. The transplanted kidney was placed into the original right-sided kidney bed and Gerota’s fascia was closed with 3 interrupted absorbable stitches (Figure 4). Laparotomy was closed layer by layer, and both retroperitoneal spaces were drained. The patient was moved to the intensive care unit. The postoperative period was uneventful with good perfusion reported on daily ultrasound scans and excellent graft function. The patient was discharged after 13 days with urea: 15 mmol/L and creatinine 160 μmol/L (1.80 mg/dL).
  | Figure 1 Renal graft placed into to recipient’s right orthotopic position. |
  | Figure 2 End-to-end anastomosis of donor’s and host’s right RVs and end-to-end anastomosis of donor’s and host’s right RAs. Abbreviations: RA, renal artery; RV, renal vein. |
  | Figure 3 End-to-end anastomosis of donor’s and host’s ureters with inserted 24 cm JJ stent. |
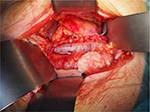  | Figure 4 Closed Gerosta’s fascia with 3 interrupted absorbable stitches. |
Because there are no established screening protocols for kidney cancer with the exception of patients with certain hereditary conditions, the patient will undergo an abdomen and chest CT check-up in 6- and 12-month time interval after the surgery. Also, the renal graft will be assessed with ultrasound on a regular basis. Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Discussion
The treatment of choice in patients with end-stage renal disease is a renal transplant. Conditions such as atherosclerosis, previously occupied iliac fossa, caval thrombosis, and arterial or venous malformation are contraindications for the HRT.1 The choice between HRT vs ORT in most cases leads to selection of HRT due to its relative technical ease and outstanding success rate.2,3 However, this approach is not suitable for all patients. ORT presents the surgeons with certain technical challenges that arise from placing a graft in its “physiological” position.
In the Hevia et al’s3 review, no difference in graft and patient survival was observed between ORT vs HRT. This suggests that the outcome of ORT is comparable to the technically less challenging HRT. A paper published by Musquera et al4 4 years also later supported this finding.
Only very few case reports describing ORT can be found in the literature.5–7 Even more sporadic are published series with ORTs. One of the most important published series were by the inventor of ORT, Gil-Vernet et al,8,9 and date back to 1978 and 1989. With the exception of Musquera et al’s4 series published in 2010, only very small series with couple of patients, such as Paduch et al’s11 in 2001, Blanco et al’s12 in 2009, and Izquierdo et al’s10 in 2010, have been published lately.3,13 No large series dealing with ORT has been published in decades. This lack of data dose not allows us to compare HRT vs ORT patient series in terms of graft function and urological complications.
The transmission of malignancy from a donor’s organ to a recipient is rare, well known but frequently unavoidable complication of a renal transplantation.14 The incidence or renal cell carcinoma (RCC) in renal transplant patients without nephrectomy ranges from 0.3% to 4.8%. Therefore, in patients with RCC, radical nephrectomy combined with renal transplantation represents an optimal treatment option in order to remove the risk of RCC infiltration into the transplanted kidney.15
In the modern era of medicine, laparoscopy had become the preferred approach in surgical practice due to its multiple benefits. In recent years, renal transplants by laparoscopy or robotic techniques have been explored.13 Thus, experimental animal and human cadaver models dealing with the idea of laparoscopist ORT are the next stages in the exploration of this technique’s new possibilities.16–18 Experimental models on animals are used today to perfect the ORT in the pursuit of reducing the warm ischemia time.19 Despite the high promise of these new techniques and improvements, extensive research is needed to embed this techniques and improvements into the medical practice.
Conclusion
ORT is a technically challenging but valid alternative for patients who are unsuitable candidates for HRT or in cases where there is a clear benefit of ORT. Complications arising from ORT are mostly of a surgical nature given by the technical difficulty of placing the graft into the “physiological” position. Graft and patient survival are identical to a conventional HRT. In our case, the ORT made the procedure less invasive – 1 incision only – and led to prompt kidney graft functioning. In selected patients, where bilateral nephrectomy is needed at the time of transplantation, ORT should be strongly considered as an alternative option.
Acknowledgment
The authors would like to thank Dr Jiri Fronek, Transplant Surgery Department, Institute for Clinical and Experimental Medicine, Prague, Czech Republic, for intraoperative pictures and also critical reviews on this report.
Disclosure
The authors report no conflicts of interest in this work.
References
Levin A, Hemmelgarn B, Culleton B, et al. Guidelines for the management of chronic kidney disease. CMAJ. 2008;179(11):1154–1162. | ||
Catalá V, Martí T, Diaz JM, et al. Use of multidetector CT in presurgical evaluation of potential kidney transplant recipients. Radiographics 2010;30(2):517–531. | ||
Hevia V, Gómez V, Álvarez S, Díez-Nicolás V, Fernández A, Burgos FJ. Orthotopic kidney transplant: a valid surgical alternative for complex patients. Curr Urol Rep. 2015;16(1):470. | ||
Musquera M, Peri LL, Alvarez-Vijande R, Oppenheimer F, Gil-Vernet JM, Alcaraz A. Orthotopic kidney transplantation: an alternative surgical technique in selected patients. Eur Urol. 2010;58(6):927–933. | ||
Sasaki H, Nakazawa R, Iwata T, et al. Orthotopic kidney transplantation in an elderly patient with various severe comorbid conditions: a case report. Transplant Proc. 2017;49(10):2388–2391. | ||
Markić D, Valencić M, Maricić A, et al. Orthotopic kidney transplantation – a case report. Lijec Vjesn. 2014;136(3–4):87–89. Croatian. | ||
Nghiem DD. Orthotopic kidney retransplantation in simultaneous pancreas kidney transplant patients with renal failure. Transplant Proc. 2008;40(10):3609–3610. | ||
Gil-Vernet JM, Gil-Vernet A, Caralps A, et al. Orthotopic renal transplant and results in 139 consecutive cases. J Urol. 1989;142(2 Pt 1):248–252. | ||
Gil-Vernet JM, Caralps A, Ruano D. New approach to the splenic vessels. J Urol. 1978;119(3):313–315. | ||
Izquierdo L, Peri L, Piqueras M, et al. Third and fourth kidney transplant: still a reasonable option. Transplant Proc. 2010;42(7):2498–2502. | ||
Paduch DA, Barry JM, Arsanjani A, Lemmers MJ. Indication, surgical technique and outcome of orthotopic renal transplantation. J Urol. 2001;166(5):1647–1650. | ||
Blanco M, Medina J, Gonzalez E, et al. Third kidney transplantation: a permanent medical-surgical challenge. Transplant Proc. 2009;41(6):2366–2369. | ||
Tsai MK, Lee CY, Yang CY, Yeh CC, Hu RH, Lai HS. Robot-assisted renal transplantation in the retroperitoneum. Transpl Int. 2014;27(5):452–457. | ||
Dhakal P, Giri S, Siwakoti K, Rayamajhi S, Bhatt VR. Renal cancer in recipients of kidney transplant. Rare Tumors. 2017;9(1):6550. | ||
di Capua Sacoto C, Lujan Marco S, Bahilo Mateu P, Budía Alba A, Pontones Moreno JL, Jiménez Cruz JF. [De novo urological neoplasms in kidney transplant patients: experience in 1,751 patients]. Actas Urol Esp. 2010;34(1):88–94. Spanish. | ||
He B, Musk GC, Mou L, Waneck GL, Delriviere L. Laparoscopic kidney orthotopic transplant: preclinical study in the pig model. Transplant Proc. 2013;45(5):1776–1779. | ||
Han X, Zhang B, Yan W, et al. Feasibility of laparoscopic orthotopic kidney transplantation: initial research with a pig model. Ann Transplant. 2013;18:342–348. | ||
He B, Mou L, Delriviere L, Hamdorf J. A human cadaver model for laparoscopic kidney transplant. Exp Clin Transplant. 2014;12(1):21–24. | ||
Jin ZD, Xue LN, Peng LS. Orthotopic kidney transplantation in the rat with the use of a sleeve arterial anastomosis method and a modified stenting technique for renal veins. Transplant Proc. 2017;49(8):1942–1946. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
