Back to Journals » Therapeutics and Clinical Risk Management » Volume 14
Significant improvement of bone mineral density by denosumab treatment in Japanese osteoporotic patients following breast cancer treatment
Authors Nakamura Y, Kamimura M, Morikawa A, Taguchi A , Suzuki T, Kato H
Received 8 November 2017
Accepted for publication 3 January 2018
Published 14 March 2018 Volume 2018:14 Pages 543—549
DOI https://doi.org/10.2147/TCRM.S156466
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Yukio Nakamura,1,2 Mikio Kamimura,3 Akio Morikawa,4 Akira Taguchi,5 Takako Suzuki,1 Hiroyuki Kato1
1Department of Orthopaedic Surgery, Shinshu University School of Medicine, Matsumoto, 2Department of Orthopedic Surgery, Showa-Inan General Hospital, Komagane, 3Center for Osteoporosis and Spinal Disorders, Kamimura Orthopaedic Clinic, Matsumoto, 4Department of Surgery, Showa-Inan General Hospital, Komagane, 5Department of Oral and Maxillofacial Radiology, School of Dentistry, Matsumoto Dental University, Shiojiri, Japan
Background: The aim of this study was to evaluate the effects of denosumab in patients with osteoporosis (OP) and non-metastatic breast cancer following treatment of 1) surgery, 2) surgery and aromatase inhibitors, and 3) surgery, aromatase inhibitors, and anti-cancer agents, compared with those in primary OP patients.
Patients and methods: In this retrospective 24-month study, patients were divided into the primary OP group (34 cases) or OP receiving breast cancer treatment group (breast cancer group; 17 cases). We measured serum calcium, whole parathyroid hormone (PTH), 1,25OH2D3, bone alkaline phosphatase (BAP), tartrate-resistant acid phosphatase-5b (TRACP-5b), and bone mineral density (BMD) of the lumbar 1–4 vertebrae (L-BMD) and bilateral total hips (H-BMD) for 24 months.
Results: The percent changes of serum calcium in the breast cancer group were significantly lower than those in the primary OP group at 1 week, 1 and 12 months. The percent changes of whole PTH in the primary OP group were significantly lower than those in the breast cancer group at 2 and 4 months. Significant differences were found between the groups at 18 months (-34.5% in the primary OP group and -52.6% in the breast cancer group, respectively) for the percent changes of BAP. Significant differences were found between the groups at 12, 18, and 24 months (-39.7% in the primary OP group and -64.0% in the breast cancer group at 24 months, respectively) for the percent changes of TRACP-5b. The percent changes of L-BMD and H-BMD were significantly increased at 12, 18, and 24 months in both the primary OP group (7.0% and 4.7% at 24 months, respectively) and breast cancer group (8.0% and 5.4% at 24 months, respectively), compared with pre-treatment levels. Significant differences were not found between the groups for the percent changes of L-BMD and H-BMD.
Conclusion: Denosumab significantly increased L-BMD and H-BMD to comparable degrees in both groups; therefore, it represents a good therapeutic option for OP receiving breast cancer treatment as well as primary OP. Also, vitamin D supplementation is required due to the potential hypocalcemia, and estrogen may be responsible for the decrease of serum calcium in the breast cancer patients.
Keywords: bone mineral density, bone turnover markers, breast cancer, denosumab, osteoporosis
Introduction
Breast carcinoma is one of the most common malignancies among women worldwide, with over 3,000,000 breast cancer survivors living in the USA alone.1 It is considered that estrogen stimulates the proliferation of breast cancer cells.2 Estrogen is produced mainly in the ovaries before menopause. Ovary function decreases in postmenopausal women, which reduces the expression of estrogen. In response to this, androgen is secreted from the adrenal glands and estrogen is made by aromatase existing in adipose tissues.3
Aromatase inhibitors have now replaced tamoxifen (AstraZeneca K.K., Osaka, Japan) as the treatment of choice for hormone-responsive breast cancer in most postmenopausal women due to their higher efficacy and fewer serious side effects, such as the induction of uterine cancers and thromboembolic events.4 Stratton et al5 have reported that aromatase inhibitors are commonly used as adjuvant therapy in postmenopausal women with breast cancer. The survival rate in receptor-positive breast cancer has markedly improved by these advancements.6
However, there are numerous reports of aromatase inhibitors causing bone loss and predisposing patients to osteoporosis (OP) and fracture.4–7 OP has become a serious issue in aging breast cancer patients, thus the establishment of appropriate treatments for OP is needed.
Denosumab is a fully human monoclonal antibody against receptor activator of nuclear factor-kappa B ligand that selectively inhibits osteoclastogenesis. Consequently, denosumab abrogates bone resorption, increases bone mineral density (BMD), and prevents fragility fracture.8,9 The 1-year open-label extension of the FREEDOM study demonstrated that the prevalence of non-vertebral fracture decreased for up to 10 years after denosumab treatment and BMD increased linearly.10 We recently reported that denosumab could increase BMD even in bisphosphonate (BP)-unresponsive cases.11 Thus, denosumab is considered to be a good therapeutic agent for OP with respect to BMD increase, improvement in bone turnover markers, and prevention of fracture.
Several reports have shown denosumab as highly effective drug for the bone loss induced by aromatase inhibitors.12,13 Gnant et al12 described that denosumab significantly increased BMD in OP of non-metastatic breast cancer female patients with adjuvant aromatase inhibitors. In their randomized, double-blinded, placebo-controlled trial, denosumab reduced the risk of clinical fracture and the major side effects of adjuvant breast cancer treatment, suggesting that this combination should be considered for OP with breast cancer.12 However, no studies exist on comparisons of: 1) detailed calcium (Ca) metabolism and 2) changes of BMD values after denosumab therapy between primary and secondary OP with non-metastatic breast cancer.
This study investigated if denosumab treatment exerted different effects on markers of bone metabolism and BMD between Japanese primary and secondary OP patients with breast cancer once breast cancer treatment has finished.
Patients and methods
Seventeen OP patients, who had received treatment for breast cancer (breast cancer group), were recruited from Shinshu University School of Medicine and Showa-Inan General Hospital between June 2014 and January 2016. Thirty-four primary OP patients (primary OP group), whose background was matched with that of the breast cancer group, were also enrolled. All patients were diagnosed as having OP based on the Japanese Society of Bone and Mineral Research.14 The inclusion criteria for the study were OP patients with low T-score in total hip BMD (H-BMD; ie, less than −2.5 SD). The exclusion criteria in this study were patients with chronic renal failure (estimated glomerular filtration rate <40 [mL/min/1.73 m2]) with bone metabolic disorder or diabetes mellitus that could affect OP. All consecutive patients meeting the criteria were retrospectively enrolled. Thirteen of 34 patients in the primary OP group and 6 of 17 patients in the breast cancer group had received BPs prior to denosumab treatment (Table 1). All subjects received denosumab (Pralia® 60 mg once every 6 months subcutaneously; Amgen Inc., Thousand Oaks, CA, USA). In the primary OP group, 12 patients took vitamin D or Ca for at least 3 months and stopped it thereafter due to the occurrence of adverse effects, such as gastrointestinal symptoms. In the remaining 22 patients, 12 took vitamin D and Ca supplementation (Denotas® chewable combination tablets; Nitto Pharmaceutical Industries, Ltd., Kyoto, Japan), 4 took alfacalcidol (Onealfa® Tablets 1.0 μg per day; Teijin Pharma Ltd, Tokyo, Japan), 6 took eldecalcitol (Edirol® capsule 0.75 μg per day; Chugai Pharmaceutical Co., Ltd., Tokyo, Japan). In the breast cancer group, 6 patients took vitamin D or Ca for at least 3 months and stopped it thereafter period due to the occurrence of adverse effects, such as gastrointestinal symptoms. In the remaining 11 patients, 5 took vitamin D and Ca supplementation (Denotas chewable), 3 took alfacalcidol, 3 took eldecalcitol. The mean (standard error [SE]) period from surgery to the start of denosumab administration in the breast cancer group was 6.8 (1.6) years. Three patients underwent surgery only, 5 received surgery and adjuvant aromatase inhibitor therapy, and 9 received surgery, adjuvant aromatase therapy, and chemotherapy. Prior to denosumab treatment, all of the patients had completed aromatase therapy and/or chemotherapy, and the mean (SE) period from the end of aromatase inhibitor therapy and/or chemotherapy was 2.1 (0.8) and 3.5 (0.7) years, respectively.
We also subdivided the breast cancer group into the following 3 groups for further analysis: the surgery only group, which had not been treated previously with aromatase therapy or chemotherapy (group 1), the surgery and aromatase therapy group (group 2), and the surgery, aromatase therapy, and chemotherapy group (group 3).
Serum albumin and Ca levels were measured using the arsenazo III method. Serum bone alkaline phosphatase (BAP) was measured as a bone-formation marker using a chemiluminescent enzyme immunoassay and antibody radioimmunoassay with a coefficient of variation (CV) of 2.5%. Serum tartrate-resistant acid phosphatase (TRACP)-5b (Osteomark®; Osteox International, Seattle, WA, USA) was measured as a marker of bone resorption using an enzyme-linked immunosorbent assay with a CV of 3.2%. Percent changes in serum levels of parathyroid hormone (PTH) and the active form of vitamin D (1,25(OH)2D3) were determined by immunoradiometric assays. Each marker was measured just before denosumab administration and at 1 week and 1, 2, 4, 6, 8, 12, 15, 18, 21, and 24 months of denosumab treatment. After overnight fasting, serum and first-void urine samples were collected between 08:30 and 10:00 am for testing. Immunoassays were carried out by SRL (Tokyo, Japan).
BMD was measured using a dual-energy X-ray absorption fan-beam bone densitometer (Lunar Prodigy; GE Healthcare, Waukesha, WI, USA) at the lumbar 1–4 levels of the posteroanterior spine and bilateral total hips. The CVs of BMD measurements at the lumbar spine (L-BMD) and bilateral total hips (H-BMD) were 0.6% and 0.5%, respectively. The study protocol was approved by the ethics committees of Shinshu University School of Medicine (Matsumoto, Japan) and Showa-Inan General Hospital (Komagane, Japan). This investigation was carried out in accordance with the ethical standards set forth in the Declaration of Helsinki (2014 revision). Written informed consent was obtained from all patients.
Results were expressed as the mean ± SE. For both groups, we compared the changes in markers, L-BMD, and H-BMD, at each time point compared with pre-treatment levels using the Bonferroni correction method for multiple comparisons. Comparisons of markers, L-BMD, and H-BMD between the 2 groups (the primary OP group and breast cancer group) at each measurement point were performed using Welch’s t-test. Also, comparisons of serum Ca, BAP, and TRACP-5b among the 3 groups (group 1–3) were performed by the Kruskal–Wallis test. Differences were considered statistically significant at P<0.05.
Results
No significant differences were found in patient background between the primary OP and breast cancer groups (Table 1). No serious adverse events, such as hypocalcemia, occurred during the observation period.
Serum albumin-corrected Ca
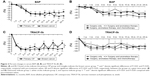
The percent changes of serum Ca significantly decreased at 1 week in the breast cancer group, but not in the primary OP group during the observational period, compared with pre-treatment levels. Significant differences were found between the groups at 1 week, 1 month, and 12 months of treatment (Figure 1A). On the other hand, there was a significant difference among the groups 2 and 3 at 8 months of therapy (P<0.05; Figure 1B).
Serum whole PTH and 1,25(OH)2D3
The percent changes of whole PTH were increased during the early stages of the study period in the breast cancer group but hovered around baseline in the primary OP group. Significant differences were noted at 2 and 4 months between the groups (Figure 1C).
The percent changes of 1,25(OH)2D3 were increased in both groups during the early stages of the study period (Figure 1D).
Bone turnover markers
Markers of bone formation
The percent changes of BAP were significantly decreased from 2 to 24 months in both groups, compared with pre-treatment levels. At 18 months, the percent changes of BAP in the breast cancer group were significantly lower than those in the primary OP group (Figure 2A). There were no significant differences of the percent changes of BAP among the groups 1, 2, and 3. However, there were significant differences from 2 to 24 months in the group 1, while there were no significant differences in the group 2 or 3, compared with pre-treatment levels (Figure 2B).
Markers of bone resorption
In the breast cancer group, the percent changes of TRACP-5b were significantly decreased than in the primary OP group at 12, 18, and 24 months. In both groups, the percent changes of TRACP-5b were significantly decreased at every time point, compared with baseline (Figure 2C). There were no significant differences of the percent changes of TRACP-5b among the groups 1, 2, and 3. However, there were significant differences at any time point in the group 1 and 3 (except for 12 months in the group 3), while there were no significant differences in the group 2, compared with pre-treatment levels (Figure 2D).
L-BMD and H-BMD
The percent changes of L-BMD increased steadily for 24 months in both the primary OP group (7.0% increase at 24 months) and breast cancer group (8.0% increase at 24 months). These changes were comparable during the observational period in both groups. In the primary OP group, significant differences were found in L-BMD at 8, 12, 18, and 24 months versus baseline values. We noted similar differences at 12, 18, and 24 months in the breast cancer group . There were no significant differences between the groups (Figure 3A).
The percent changes of H-BMD increased steadily for 24 months in both the primary OP group (4.7% increase at 24 months) and breast cancer group (5.4% increase at 24 months). These changes were also comparable during the observational period in both groups. Significant differences were found at 12, 18, and 24 months in both groups, compared with pretreatment values. There were no significant differences between the groups (Figure 3B).
Discussion
This study reports for the first time comparative data on denosumab therapy for primary OP patients and OP patients with breast cancer. In the breast cancer group, denosumab administration produced: 1) significantly decreased serum Ca in the early treatment phase, 2) significantly suppressed bone metabolism, and 3) greatly improved BMD which was comparable with that in the primary OP group.
Fourteen of 17 breast cancer patients had received adjuvant aromatase inhibitor therapy prior to denosumab treatment. Estrogen is a key regulator of bone metabolism, thus estrogen replacement decreases bone-acting cytokines to inhibit bone resorption.15 As previously reported, aromatase inhibitors also affect bone metabolism,4,12,13 as may chemotherapy. Since all of the patients in the breast cancer group had ceased aromatase inhibitor therapy and/or chemotherapy prior to this study, it was unlikely that these therapies directly affected bone metabolism.
Hypocalcemia is one of the most common adverse effects in denosumab treatment for OP.16,17 Several studies have advised that Ca and vitamin D be taken together with denosumab.16,17 We recently demonstrated that Ca and vitamin D supplementation was necessary during denosumab therapy in primary OP to prevent hypocalcemia and increase BMD.17 Thus, we first prescribed Ca and vitamin D supplementation to all of the patients; however, due to adverse effects, such as constipation, approximately one third of patients in each group did not take Ca or vitamin D supplementation. However, all patients had taken vitamin D and Ca supplementation for at least 3 months, thus it was considered that the cause of hypocalcemia would not have been due to the non-intake of vitamin D and Ca supplementation. Hypocalcemia occurred even in the surgery group in this study, suggesting the unlikeliness that aromatase inhibitors or anti-cancer agents had caused hypocalcemia. Also, serum Ca in the breast cancer group was significantly decreased at 1 week and 1 month, compared with those in the primary OP group, confirming the potential benefit of vitamin D and Ca intake when undergoing denosumab treatment for OP with breast cancer.
The percent changes of serum Ca in the breast cancer group were significantly decreased at the early phase compared with those in the primary OP group. On the other hand, Ellis et al7 have reported that in breast cancer patients with aromatase inhibitors plus denosmuab treatment, there was no hypocalcemia like in placebo patients. Taken together, our findings and Ellis et al7 suggest that under denosumab therapy, the decrease of serum Ca observed in breast cancer patients might have been suppressed by aromatase inhibitors, thus, estrogen may be responsible for the decrease of serum Ca in breast cancer patients. Thus, we speculate that one of the factors altering Ca metabolism in breast cancer may be estrogen through a yet undetermined mechanism.
In the breast cancer group, serum Ca significantly decreased at the early phase. Early phase serum PTH and 1,25(OH)2D3 were comparably higher in the breast cancer group, potentially due to diminished Ca levels. Toulis et al13 witnessed that denosumab increased serum PTH at 7 days of treatment in primary OP patients, but not in breast cancer patients, which was inconsistent with our findings. However, their data lacked detailed information on the breast cancer treatment course;13 thus, it was possible that their study had been performed during aromatase inhibitor administration.
In this study, the percent changes of TRACP-5b re-increased every 6 month just before denosumab administration in the primary OP group, which was consistent with earlier reports,17,18 while the re-increase of the percent changes of TRACP-5b was not observed in the breast cancer group. Also, the inhibitory effects on BAP were greater in the breast cancer group than in the primary OP group, suggesting the inhibition of bone metabolism by denosumab was stronger and more prolonged in breast cancer patients. Although the mechanism of this is unclear, it may be related to a sufficiency of estrogen in bone tissues.
Cancer itself was removed by surgeries in all of the breast cancer patients and recurrence has not been observed in any case 6.8 years on average after surgeries. Thus, it is conceivable that cancer tissue might have not affected the results in this study.
During denosumab therapy, both L-BMD and H-BMD significantly increased over 24 months in both groups similarly compared with pre-treatment levels. In the ZO-FAST study,16 zoledronic acid therapy in OP patients with aromatase inhibitors showed that fracture rate was 8% for 12 months, while this study showed no fracture occurred during the 24-month observation period. In addition, L-BMD and H-BMD had increased by 5.7% and 3.6%, respectively, at 12 months in the ZO-FAST study,16 while in this study, L-BMD and H-BMD had increased by 5.2% and 4.3%, respectively, at 12 months in the breast cancer group. Thus, a previous report16 and our findings suggest that denosumab can be a good option for the treatment of OP with accompanying breast cancer.
The main limitations of our study were its small sample size and mixed population. Further investigation is warranted to ascertain if: 1) BMD increases continuously by denosumab treatment and to what extent fracture is prevented, and 2) other adverse effects occur.
Conclusion
L-BMD and H-BMD were significantly increased in both groups at 24 months of denosumab treatment. Accordingly, the drug represents a good therapeutic agent for OP with breast cancer as it is for primary OP. Since serum Ca significantly decreased in the breast cancer group during the early phase of treatment, supplemental vitamin D and Ca are advised, and estrogen may be responsible for the decrease of serum Ca in breast cancer patients.
Disclosure
The authors report no conflicts of interest in this work.
References
O’Sullivan CC, Ruddy KJ. Management of potential long-term toxicities in breast cancer patients. Curr Breast Cancer Rep. 2016;8(4):183–192. | ||
van der Burg B, de Groot RP, Isbrücker L, Kruijer W, de Laat SW. Direct stimulation by estrogen of growth factor signal transduction pathways in human breast cancer cells. J Steroid Biochem Mol Biol. 1992;43(1–3):111–115. | ||
Clarke BL, Khosla S. Androgens and bone. Steroids. 2009;74(3):296–305. | ||
Hadji P, Aapro MS, Body JJ, et al. Management of aromatase inhibitor-associated bone loss (AIBL) in postmenopausal women with hormone sensitive breast cancer: joint position statement of the IOF, CABS, ECTS, IEG, ESCEO IMS, and SIOG. J Bone Oncol. 2017;7:1–12. | ||
Stratton J, Hu X, Soulos PR, et al. Bone density screening in postmenopausal women with early-stage breast cancer treated with aromatase inhibitors. J Oncol Pract. 2017;13(5):e505–e515. | ||
Smith I, Yardley D, Burris H, et al. Comparative efficacy and safety of adjuvant letrozole versus anastrozole in postmenopausal patients with hormone receptor-positive, node-positive early breast cancer: final results of the randomized Phase III femara versus anastrozole clinical evaluation (FACE) trial. J Clin Oncol. 2017;35(10):1041–1048. | ||
Ellis GK, Bone HG, Chlebowski R, et al. Randomized trial of denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer. J Clin Oncol. 2008;26(30):4875–4882. | ||
McClung MR, Lewiecki EM, Cohen SB, et al; AMG 162 Bone Loss Study Group. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med. 2006;354(8):821–831. | ||
Silva I, Branco JC. Denosumab: recent update in postmenopausal osteoporosis. Acta Reumatol Port. 2012;37(4):302–313. | ||
Ferrari S, Adachi JD, Lippuner K, et al. Further reductions in nonvertebral fracture rate with long-term denosumab treatment in the FREEDOM open-label extension and influence of hip bone mineral density after 3 years. Osteoporos Int. 2015;26(12):2763–2771. | ||
Kamimura M, Nakamura Y, Ikegami S, et al. Significant improvement of bone mineral density and bone turnover markers by denosumab therapy in bisphosphonate-unresponsive cases. Osteoporos Int. 2017;28(2):559–566. | ||
Gnant M, Pfeiler G, Dubsky PC, et al; Austrian Breast and Colorectal Cancer Study Group. Adjuvant denosumab in breast cancer (ABCSG-18): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2015;386(9992):433–443. | ||
Toulis K, Iliadou P, Mandanas S, et al. Calcium homeostasis in women with non-metastatic breast cancer with osteoporosis after a single dose of denosumab: a pilot study. Hormones (Athens). 2016;15(4):560–562. | ||
Soen S, Fukunaga M, Sugimoto T, et al. [New diagnostic criteria and guidelines on osteoporosis. Diagnostic criteria for primary osteoporosis: year 2012 revision]. Clin Calcium. 2014;24(1):323–329. Japanese. | ||
Pacifici R, Brown C, Puscheck E, et al. Effect of surgical menopause and estrogen replacement on cytokine release from human blood mononuclear cells. Proc Natl Acad Sci U S A. 1991;88(12):5134–5138. | ||
Bundred NJ, Campbell ID, Davidson N, et al. Effective inhibition of aromatase inhibitor-associated bone loss by zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole. Cancer. 2008;112(5):1001–1010. | ||
Nakamura Y, Suzuki T, Kamimura M, et al. Vitamin D and calcium are required at the time of denosumab administration during osteoporosis treatment. Bone Res. 2017;5:17021. | ||
Nakamura T, Matsumoto T, Sugimoto T, Shiraki M. Dose-response study of denosumab on bone mineral density and bone turnover markers in Japanese postmenopausal women with osteoporosis. Osteoporos Int. 2012;23(3):1131–1140. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.