Back to Journals » Clinical Ophthalmology » Volume 17
Short-Term Treatment Outcomes of Brolucizumab in Patients with Neovascular Age-Related Macular Degeneration: A Multicentre Indian Real-World Evidence Study
Authors Chakraborty D , Thakkar M, Venkatesh R , Roy S, Bhavsar M, Karcher H
Received 26 April 2023
Accepted for publication 25 July 2023
Published 10 August 2023 Volume 2023:17 Pages 2295—2307
DOI https://doi.org/10.2147/OPTH.S415044
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Scott Fraser
Debdulal Chakraborty,1 Milan Thakkar,2 Ramesh Venkatesh,3 Sangeeta Roy,4 Maulik Bhavsar,5 Helene Karcher6
1Disha Eye Hospital, Kolkata, West Bengal, India; 2Dr. Milan’s Retina Care Centre, Rajkot, Gujarat, India; 3Narayana Nethralaya, Bangalore, Karnataka, India; 4Susrut Eye Foundation and Research Centre, Kolkata, West Bengal, India; 5Novartis Healthcare Pvt. Ltd., Mumbai, Maharashtra, India; 6Novartis Pharma AG, Basel, Switzerland
Correspondence: Debdulal Chakraborty, Disha Eye Hospital, Kolkata, West Bengal, India, Email [email protected]
Objective: To evaluate the short-term effectiveness and safety outcomes following brolucizumab treatment in patients with neovascular age-related macular degeneration (nAMD) as a part of real-world clinical practice in India.
Methods: This was a retrospective, observational, multicentre study including patients (≥ 50 years old) diagnosed with nAMD. Anonymized data of the patients receiving the first dose of brolucizumab intravitreal injection (IVI) who were either treatment-naïve or previously treated with a single or a combination of other anti-VEGF IVIs were included. The present study reported the change in retinal fluid levels from baseline to month 3, best-corrected visual acuity (BCVA), central retinal thickness (CRT), and the number of injections received. The adverse events in the three months after brolucizumab treatment initiation were also monitored.
Results: The study included 63 patients (65 eyes) from four study centres across India (mean age: 69.1 ± 9.7 years). A total of 82 brolucizumab injections were administered during the 3 months of study duration, with 52/65 (80.0%) eyes receiving only 1 injection. Resolution of IRF, SRF, and PED was observed in 76.9%, 64.6%, and 67.7% of eyes, respectively. Further, a significant reduction in CRT was observed (baseline: 403.5 ± 118.7 μm; month 3: 308.3 ± 73.8 μm; p < 0.001), and BCVA also improved notably from 0.7 ± 0.5 logMAR at baseline to 0.5 ± 0.4 logMAR at month 3 (p < 0.001). Adverse events (AEs) were reported in 3 eyes from 3 patients; retinal pigment epithelial rip (1) and subretinal hemorrhage (2) after the first injection of brolucizumab, however, none discontinued the treatment.
Conclusion: The study reports on the short-term effectiveness and tolerability of brolucizumab therapy in the management of nAMD in both treatment-naïve and switch eyes. Brolucizumab was observed to have a favourable benefit-risk profile, and study results were within the known safety profile, with no instances of intraocular inflammation.
Keywords: anti-VEGF, brolucizumab, nAMD, real-world evidence, India
Introduction
Age-related macular degeneration (AMD) is one of the major causes of irreversible central visual impairment and is responsible for 8.7% of all cases of blindness worldwide.1–4 Globally, the number is estimated to rise to nearly 300 million by the year 2040.5 In India, early AMD and the resulting vision loss are a growing concern, with reports of a prevalence ranging from 1.4% to 3.1%, and age is found to be affecting the prevalence.6 The wet or neovascular AMD (nAMD), though less frequent, has been associated with 90% of cases of acute blindness.7–9
The main objectives of nAMD treatment are to prevent the formation of new blood vessels (macular neovascularization) and to improve and stabilize vision through fluid reduction.10–13 The anti-vascular endothelial growth factor (anti-VEGF) agents are gold standards for the management of nAMD.11,12 The commonly used anti-VEGFs, bevacizumab (off-label), ranibizumab, and aflibercept have resulted in significant improvement in visual function and thus ameliorate the quality of life of the affected patients. However, some patients may require repeated intravitreal injection (IVI) administration of monthly anti-VEGF injections for a longer duration. This reduces patients’ compliance with the treatment, leading to decreased monitoring and poor visual outcomes, which further imposes a significant burden on patients, caregivers, and treating physicians.14,15 As per the American Society of Retina Specialists (ASRS) preference and trends (PAT) survey, the physicians have identified a significant unmet need in the management of nAMD due to the frequent injections required for treatment and further expressed a need for more effective treatment with longer intervals between injections.16
Brolucizumab, a humanized, single-chain variable fragment antibody that inhibits VEGF-A,17 is the latest addition to the armamentarium of anti-VEGF agents, approved (in October 2019) by the United States Food and Drug Administration (US FDA) and by the Drugs Controller General of India (DCGI) in July 2020.18 With a molecular mass of approximately 26 kDa, brolucizumab readily penetrates the retina resulting in greater efficacy and longer durability of the effect than other anti-VEGF agents. Additionally, it exhibits rapid systemic clearance and minimal systemic exposure.19,20 The results of two large Phase III, multicentre, randomized clinical trials, HAWK and HARRIER, have established the efficacy and safety of brolucizumab in the treatment of nAMD.21,22 The once-every-12 weeks (q12w) regimen of the drug provides adequate treatment outcomes while reducing the number of IVIs required for patients with nAMD.17 In a post hoc analysis of the HAWK and HARRIER studies performed by the safety review committee (SRC), an overall incidence of intraocular inflammation (IOI) was 4.6% with brolucizumab, 3.3% for IOI with retinal vasculitis (RV) and 2.1% for IOI with RV and retinal vascular occlusion. The overall incidence of moderate visual acuity loss associated with IOI was <1%.23,24 Also, in a network meta-analysis conducted by Finger et al,25 it was observed that among all anti-VEGF treatments, brolucizumab showed better reduction in retinal thickness, comparable BCVA gains and discontinuation rates, with lesser number of injections compared to other anti-VEGF agents.
Currently, there are very limited data available on the effectiveness and safety of brolucizumab in the real-world patient population in India. The available literature evidence is restricted to either other countries or a specific institute in India with limited patient follow-up data.26–30 These findings are, therefore, difficult to extrapolate to the Indian population due to differences in the demographics, ethnicity, and clinical presentations.
In order to address the gap in the literature, the present study was designed to understand the effectiveness and safety of brolucizumab in real-world clinical practice in a broader Indian nAMD population, and how it differs in practice from other countries or prescription as per label.
Methods
Study Design
This retrospective, observational, multicentre study included all patient eyes treated with brolucizumab in the 6 months period from 4 sites across India viz. Dr. Milan’s Retina Care Centre, Rajkot (Gujarat), Disha Eye Hospital, Kolkata (West Bengal), Susrut Eye Foundation and Research Centre, Kolkata (West Bengal), and Narayana Nethralaya, Bangalore (Karnataka). Data were collected from the Electronic Medical Records (EMRs) database of the study sites, and patients’ confidentiality was maintained using anonymized and de-identified data at the source level. A grader specific to each individual centre performed the OCT evaluation. The grader was masked for the details of the study and diagnosis.
This study complied with the tenets of the Declaration of Helsinki and was approved by an Independent Ethics Committee (IEC), Royal Pune Independent Ethics Committee, India (Regn Number: ECR/45/Indt/MH/2013/RR-19) for Dr. Milan’s Retina Care Centre, Rajkot, India, along with Institutional Review Boards (IRBs) at Susrut Eye Foundation and Research Centre, Kolkata, India (Regn Number: ECR/408/Inst/WB/2013/RR-20); Disha Eye Hospitals, Kolkata, India (Regn Number: ECR/846/Inst/WB/2016/RR-19); and Narayana Nethralaya, Bangalore, India (Regn Number: ECR/187/Inst/Kar/2013/RR-19). Informed consent waiver was obtained from IEC and IRBs considering the risk of this research was not greater than minimal risk and the waiver would not adversely affect the rights and welfare of study participants. This study was registered in the Clinical Trial Registry of India (CTRI/2021/11/037815).
Study Population
Patients (male or female) aged ≥50 years treated with brolucizumab IVI [first dose during the index period (01 October 2020 to 31 March 2021)] for nAMD were included in the study. They were either treatment-naïve or previously treated with a single or a combination of other anti-VEGF IVIs. Recalcitrant cases were defined as eyes with fluid on the spectral-domain OCT, which was either worsening or persistent (<100μm reduction) despite repeated consecutive doses of aflibercept, ranibizumab or bevacizumab injections. Patients were required to have records of VA and optical coherence tomography (OCT) assessments at baseline and month 3 after initiating treatment with brolucizumab. The decisions whether to administer loading doses, the frequency of monitoring visits and of injections were solely at the discretion of the treating clinician. The retreatment criteria included drop of one or more lines in the Snellen’s visual acuity chart; an increase in OCT CRT of at least 100 μm; worsening of existing fluid (intraretinal fluid [IRF] or subretinal fluid [SRF]); or appearance of new fluid (PED/SRF/IRF); or new location of CNVM as compared to the previous visit; or evidence of persistent fluid on OCT 1 month after the previous injection.31 For polypoidal choroidal vasculopathy (PCV) cases, diagnosis was confirmed based on standard SD OCT criteria, fundus fluorescein angiography (FFA) and indocyanine green angiography (ICGA).32
Patients with dry AMD, geographic atrophy, and other retinal diseases in the eyes were excluded besides those who were undergoing additional ocular treatment along with anti-VEGF agents for nAMD.
Outcome Assessments
The primary endpoint of the study was to evaluate the short-term effectiveness of brolucizumab on fluid levels [absence/reduction of IRF, SRF, and pigment epithelial detachment (PED)] in treatment eyes (n = 65) at month 3 compared to the baseline. The secondary endpoint measures included changes in visual outcome in terms of mean change in the best-corrected visual acuity (BCVA) from baseline to month 3 as measured by logarithm of the minimum angle of resolution (logMAR; measurement by Snellen chart converted to logMAR for analysis), the evaluation of the effectiveness of brolucizumab on fluid levels (IRF, SRF, PED) from baseline to months 1 and 2, and change in the CRT as assessed by OCT from baseline to months 1, 2, and 3. Further, the number of anti-VEGF injections, non-injection visits (monitoring visits), and the total number of patient visits during the first 3 months of treatment with brolucizumab were recorded. The ocular and non-ocular safety of brolucizumab was determined by capturing the incidence and characteristics of treatment-emergent adverse events (AEs) at each visit. The exploratory endpoint was to assess whether the improvements observed at month 3 were sustained at month 6.
Statistical Analysis
Assuming that up to 81% of patients’ eyes would be fluid-free at month 3 (as observed in HAWK and HARRIER clinical trials), with a power of 80%, a sample size of approximately 44 patients’ eyes was considered enough for this real-world study.
As this was a descriptive study, no comparative analysis was conducted. Descriptive statistics were tabulated for the demographic and clinical characteristics as well as outcome variables. Continuous variables were summarized as the number of observations, means, and standard deviations (SD). On the other hand, categorical variables were summarized as counts and proportions (overall number of injections, the total number of visits, the number of non-injection visits, etc.). For continuous data, the changes from baseline to the follow-up period were assessed using the Wilcoxon-signed rank test. Likewise, for categorical data, the changes from the baseline to the follow-up period were assessed using the Chi-square test. A p-value of <0.05 was considered statistically significant. No missing value imputation was performed. The analyses were conducted separately for the study eyes (patients) with 1, 2, and 3 months of follow-up from the index date (first injection of brolucizumab). A window period of ±15 days was considered during the entire study period. All calculations were performed using R language (version 4.2.2) with R studio software (version 1.4.1106) and Microsoft Excel version 2021.
Results
A total of 76 patients were screened, of which 63 patients met the selection criteria [65 eyes {oculus dexter (OD) = 27, oculus sinister (OS) = 34 and oculus uterque (OU) = 4}] and were included in the study (Figure 1). Of these, 25 (38.5%) eyes were treatment-naïve, and 40 (61.5%) eyes had received prior anti-VEGF therapy for nAMD. The mean age of patients was 69.16 ± 9.72 years. Ranibizumab was the most prescribed prior anti-VEGF (55.0% of eyes), followed by aflibercept (25.0% of eyes). No patients had a prior history of intraocular inflammation (IOI), retinal vascular occlusion, or retinal vasculitis (Table 1).
 |
Table 1 Baseline Demographics and Disease Characteristics |
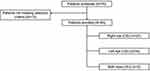 |
Figure 1 Study population. |
Changes in IRF, SRF, and PED at Month 3
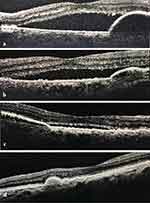
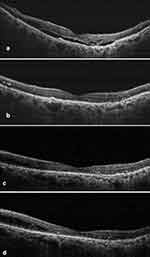
Among all the 65 eyes, notable changes in IRF, SRF, and PED were observed as early as month 1 following the first brolucizumab injection. Around 27 (41.5%) and 36 (55.4%) eyes had decreased and complete IRF resolution, respectively, at the end of month 1. By the end of month 3, IRF decreased in 13 (20.0%) eyes and completely resolved in 50 (76.9%) eyes (naïve eyes: 76.0% and switch eyes: 77.5%). Similarly, at the end of month 1, SRF decreased in 39 (60.0%) eyes and resolved in 19 (29.2%) eyes. By the end of month 3, SRF decreased in 17 (26.1%) eyes and resolved in 42 (64.6%) eyes (naïve eyes: 64.0% and switch eyes: 65.0%). Further, PED decreased and resolved in 20 (30.7%) and 41 (63.1%) eyes, respectively, at the end of month 1 and decreased in 16 (24.6%) eyes and resolved in 44 (67.7%) eyes (naïve eyes: 60.0% and switch eyes: 72.5%) at the end of month 3. The changes in IRF, SRF, and PED were statistically significant at month 3 compared to baseline (p < 0.05) (Figure 2). The percentage of eyes achieving dryness (absence of both IRF and SRF) increased from 9 (13.8%) at baseline to 34 (52.3%) eyes at the end of month 3 (p < 0.001). The details are provided in Supplementary Table 1A–C. Amongst 65 eyes, only one PCV case was identified. Figures 3 and 4 are representative case examples of treatment-naïve and previously treated cases of nAMD who received brolucizumab, respectively.
 |
Figure 2 Changes in intraretinal fluid (IRF), subretinal fluid (SRF), and pigment epithelial detachment (PED) from baseline till month 3. |
Changes in BCVA and CRT from Baseline
A significant improvement in mean BCVA was observed at the end of month 3 compared to baseline (baseline: 0.7 ± 0.5 logMAR; month 3: 0.5 ± 0.4 logMAR, mean change: −0.2; p < 0.001) (Figure 5). Further, a significant reduction in mean CRT was also noted at the end of month 3 (308.3 ± 73.8 μm) compared to baseline (403.5 ± 118.7 μm) (p < 0.001) (Figure 6).
 |
Figure 5 Mean best-corrected visual acuity (BCVA) from baseline till month 3. |
 |
Figure 6 Mean change in central retinal thickness (CRT) as assessed by optical coherence tomography (OCT) from baseline till month 3 (N=65). |
Number of Injections and Treatment Interval
At the end of month 3, a total of 82 injections were administered in 65 eyes. Overall, 52 (80.0%) eyes received a single injection, 9 (13.8%) eyes received two injections, and 4 (6.2%) eyes received three injections. The details are enlisted in Table 2. In the switch group (n = 40 eyes), prior to brolucizumab, a total of 253 anti-VEGF injections were administered (Table 1). The mean number of previous injections in the switch group was 6.66 (range: 1–15).
 |
Table 2 Brolucizumab Injection Details from Baseline to Month 3 (N = 65) |
Monitoring Visits
In addition to the patient visits for brolucizumab injection, visits for monitoring or visits due to AEs were also captured during the first 3 months of treatment. Of the total 53 visits without anti-VEGF injections, 50 visits were for monitoring purposes by the end of month 3, and the remaining 3 visits were due to AEs during the 1st month.
Safety
Overall, 3 AEs were reported (retinal pigment epithelial rip, n = 1, and subretinal hemorrhage, n = 2). All the events occurred after the first injection of brolucizumab and were suspected to be related to the study drug. However, the drug was not discontinued in any of the cases. The patient with subretinal hemorrhage underwent a vitrectomy. Of note, no IOI was observed in any of the eyes.
Exploratory Analysis (Extended Follow-Up Up to Month 6)
The short-term effectiveness of brolucizumab injections on fluid levels, number of injections received, change in BCVA and CRT from baseline, and incidence of AEs were further evaluated for months 4, 5, and 6 in all 65 eyes (naïve eyes: 38.5% and switch eyes: 61.5%). During the month 4 to 6, 36 (55.4%) eyes received the second injection, and 2 (3.1%) eyes received the third injection (Supplementary Table 2). None of the eyes had a reappearance of the fluids from months 4 to 6. Further, at the end of month 6, 38 (58.5%), 37 (56.9%), and 37 (56.9%) eyes sustained the resolution of IRF, SRF, and PED, respectively, as it was observed at month 3 [Supplementary Table 3A–C]. BCVA was observed to be reduced at the end of month 6 (0.5 logMAR) compared to baseline (0.7 logMAR) (p < 0.001). Further, a significant reduction in CRT was noted at the end of month 6 (288.51 μm) compared to baseline (403.5 μm) (p < 0.001) (Supplementary Table 4). No additional adverse events were reported between month 3 to month 6.
Discussion
The present real-world study provides insights on the short-term effectiveness of brolucizumab for the treatment of nAMD in 82 patient eyes from 4 centres in India and reports statistically significant improvement in anatomical and visual outcomes at the end of month 3 compared to baseline. Additionally, the study found that 3/82 eyes experienced AEs, which did not require discontinuation of brolucizumab. Both treatment-naïve and switch eyes responded positively to the brolucizumab treatment. At the end of month 3, ≥65% of eyes had shown complete resolution of IRF, SRF, and PED with brolucizumab therapy, including switch eyes. These observed improvements in fluid levels were found to be sustained up to month 6. Of the total 65 eyes, 80.0% (n = 52) required only one injection and did not require a second injection during the 3 months of study duration.
This retrospective real-world study in Indian nAMD patients measured the short-term effectiveness of brolucizumab with fluid status as the primary endpoint over a period of three months. Though the study population differed from other real-world studies such as BRAILLE,15 PROBE33 and REBA34 [current study; naïve eyes 38.5% and switch eyes 61.5%; BRAILLE: naïve eyes: 21.3% and switch eyes: 78.7% and REBA: naïve eyes: 23.8% and switch eyes: 76.2%, PROBE: 27 eyes] the outcomes were comparable to that of BRAILLE, REBA, and PROBE.15,33,34
IRF
In our study, IRF was resolved in half of the total 65 eyes at the end of month 1 [36 (55.4%) eyes] and was completely resolved in three-fourth of the eyes [50 (76.9%) eyes] at the end of month 3, which was a statistically significant compared to the baseline (p < 0.05). When compared with the BRAILLE study, complete IRF resolution was observed in 39.3% of eyes at the final follow-up visit.15 Similarly, in a study conducted by Montesel et al, IRF was present in 12 out of 19 (63.2%) eyes at baseline and got resolved in 9 (47.4%) eyes at the last follow-up.30
SRF
In the current study, of the 65 eyes with SRF at baseline, brolucizumab led to a reduction in 17 (26.1%) eyes and complete resolution in 42 (64.6%) eyes, whereas there was minimal/no change in 5 (7.7%) eyes at the end month 3. The BRAILLE study reported similar outcomes with reduction observed in 78.9% of eyes, complete SRF resolution in 15.5% of eyes, and no/minimal change in 5.6% of eyes.15
PED
In the present study, PED was observed to decrease in 16 (24.6%) eyes and resolved in 44 (67.7%) eyes at the end of month 3, which was significantly different compared to baseline (p < 0.05). Similarly, in the BRAILLE study, PED was reported to be reduced in 61.9% of eyes and resolved in 23.8% of eyes.15
BCVA
A significant improvement in BCVA from baseline to month 3 was noted in the present study (baseline: 0.7 ± 0.5 logMAR; month 3: 0.5 ± 0.4 logMAR; p < 0.001) which was similar to that observed in the BRAILLE study (baseline: 0.8 ± 0.5 logMAR; final: 0.7 ± 0.51 logMAR; p < 0.00001). Likewise, in another retrospective, real-life clinical study, the mean BCVA was reported to be significantly improved from 0.24 ± 0.27 logMAR at baseline to 0.12 ± 0.23 logMAR at month 3 post-brolucizumab injection (p < 0.001).29 In a retrospective, multicentric PROBE study, a significant gain in the mean BCVA was seen in treatment naïve patients undergoing brolucizumab therapy (p = 0.014).33 Additionally, in REBA study, there was a significant gain in mean BCVA in the treatment naïve and switch therapy groups following brolucizumab treatment.34 However, there was no evidence of visual benefits after brolucizumab administration in RWE studies conducted by Walter et al,35 Bulirsch et al,27 Enriquez et al,28 and Sharma et al.26
CRT
A significant reduction was noted in CRT at month 3 (308.3 ± 73.8 μm) compared to baseline (403.5 ± 118.7 μm) (p < 0.001) in the present study. Similar observations were reported in another real-world clinical study, wherein CRT decreased significantly from 461.7 ± 82.9 μm to 343.6 ± 74.3 μm (p = 0.004) 3 months after the treatment.36
Further, the extended analysis carried out in the present study demonstrated that none of the study eyes had reappearance of the fluids till month 6. Moreover, the improvements in outcomes such as BCVA and CRT observed at the end of month 3 were maintained till month 6. These findings were in concurrence with the observations of a study carried out by Abdin et al, wherein, all outcomes mentioned above were found to improve till 1 year post the first administration of brolucizumab among patients with nAMD.37 The current study findings were also consistent with the BRAILLE study conducted in Indian patients over 52 weeks wherein brolucizumab demonstrated significant improvement in BCVA with significant reductions in CST, SRF, and IRF.38
Safety Outcomes
Brolucizumab was observed to have a favorable benefit-risk profile, with 3 AEs (retinal pigment epithelial rip, n = 1, and subretinal hemorrhage, n = 2) reported during the entire course of treatment. Study results were within the known safety profile, and no new safety signal was identified. Similar AEs were reported in HAWK and HARRIER clinical trials following brolucizumab therapy.21 A short-term study conducted by Montesel et al had reported an intra-ocular inflammation following intravitreal brolucizumab.30 In a recent real-world study of brolucizumab safety in the 12–18 months after treatment initiation in the US reported a proportion of IOI-related AEs in 22 (4.6%) eyes out of 482 eyes. Majority of the eyes [14/22 (64%)] developed IOI-related AE within 90 days of the first brolucizumab injection of which 3 (14%) AE developed in the first 30 days.24 Additionally, REBA study noted two adverse events,34 whereas in another retrospective study by Sharma et al no AE was reported.26 Baumal et al in a systematic review documented the incidence of IOI ranging from 0% to 19%.23
The difference in the incidence of adverse events between this study and other studies may be attributed to variations in patient characteristics, limited sample size, as well as lower number of injections with monthly follow-ups. Additionally, since brolucizumab was approved in India in July 2020, it is likely that following the reporting of the safety signal for brolucizumab in February 2020, Indian clinicians may have benefitted with information on potential risks and better understanding of IOI and related adverse events. Evidence-based recommendations developed by various groups of ophthalmologists, providing guidance on patient selection, thorough examination of the eye for inflammation prior to injection and patient education on self-monitoring of symptoms, and report symptoms as soon as they develop, might have contributed to lower incidence of events in the real-world settings.
This current study also demonstrated that the real-world usage pattern of brolucizumab in Indian nAMD patients departs from the per-label usage.39 If the injections were given as per label, which recommends a loading phase of 3 injections 4 weeks apart, we would have observed about 3–4 injections per eye instead of 1 for most patient eyes. The finding that effectiveness outcomes at 3 months have still showed improvement (despite the less frequent injections and ~62% switch eyes) compared to outcomes before the start of brolucizumab is noteworthy and can be helpful for clinicians and their patients in weighing the benefits and risks of starting this therapy given it is also associated with a risk of adverse event. Given the patient’s socioeconomic status, chronic nature of the disease, burden of frequent injections, and visits imposed on patients and caregivers, as well as poor treatment compliance, and out-of-pocket expenditure, exploring the early effectiveness and safety outcomes of brolucizumab in real world Indian settings is pivotal.16,40
Strengths and Limitations
The strengths of the study include having a heterogeneous sample of patient eyes from 4 centres and capturing the short-term outcomes from real-world practice in India, where injection and follow-up visits are at the discretion of the treating clinician. The major limitations of the present study are its retrospective nature, limited sample size, and brief follow-up period. Other limitations include OCT evaluation done at individual centre by a single grader from each centre.
Conclusions
This real-world short-term study reported significant improvements in fluid parameters from baseline to month 3 in both treatment-naïve eyes and eyes who had received prior anti-VEGF injections. Further, a marked improvement was also observed in BCVA along with a significant reduction in CRT at the end of month 3 compared to the baseline. Brolucizumab was observed to have a favorable benefit-risk profile, and the study results were in line with the known safety profile of the drug, with no IOI reported during the study duration. Notably, the study emphasized the significance of brolucizumab in addressing the unmet need for nAMD treatment as it has the potential to reduce the treatment burden of frequent injections and improve patient compliance. However, further prospective studies with larger sample size and with long-term follow-up and comparative arms may provide better assessment and sustainability of the effectiveness outcomes and benefits in real-world practice.
Acknowledgments
Medical writing support in the development of this paper was provided by THB c/o Sekhmet Technologies Pvt. Ltd., Gurugram, Haryana, India.
Funding
The study was funded by Novartis Healthcare Pvt. Ltd., Mumbai, Maharashtra, India.
Disclosure
Maulik Bhavsar is an employee of Novartis Healthcare Pvt Ltd. India. Helene Karcher is an employee and shareholder of Novartis Pharma AG, Switzerland and Editor-in-Chief of the journal Epidemiologic Methods, a scientific journal from the publishing company De Gruyter. Helene Karcher receive a yearly honorary for this service. The other authors report no conflicts of interest in this work.
References
1. Stahl A. The diagnosis and treatment of age-related macular degeneration. Dtsch Arztebl Int. 2020;117(29–30):513–520.
2. Mitchell P, Liew G, Gopinath B, Wong TY. Age-related macular degeneration. Lancet. 2018;392(10153):1147–1159. doi:10.1016/S0140-6736(18)31550-2
3. García-Layana A, Cabrera-López F, García-Arumí J, Arias-Barquet L, Ruiz-Moreno JM. Early and intermediate age-related macular degeneration: update and clinical review. Clin Interv Aging. 2017;12:1579–1587. doi:10.2147/CIA.S142685
4. Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106–e116. doi:10.1016/S2214-109X(13)70145-1
5. Kawasaki R, Yasuda M, Song SJ, et al. The prevalence of age-related macular degeneration in Asians: a systematic review and meta-analysis. Ophthalmology. 2010;117(5):921–927. doi:10.1016/j.ophtha.2009.10.007
6. Likhar N, Mothe RK, Kanukula R, et al. The prevalence of age-related macular degeneration in Indian population: a systematic review. Value Health. 2015;18(3):A180. doi:10.1016/j.jval.2015.03.1041
7. Gupta SK, Murthy GV, Morrison N, et al. Prevalence of early and late age-related macular degeneration in a rural population in northern India: the INDEYE feasibility study. Invest Ophthalmol Vis Sci. 2007;48(3):1007–1011. doi:10.1167/iovs.06-0712
8. Al-Zamil WM, Yassin SA. Recent developments in age-related macular degeneration: a review. Clin Interv Aging. 2017;12:1313–1330. doi:10.2147/CIA.S143508
9. Hernández-Zimbrón LF, Zamora-Alvarado R, Ochoa-de la paz L, et al. Age-related macular degeneration: new paradigms for treatment and management of AMD. Oxid Med Cell Longev. 2018;2018:8374647. doi:10.1155/2018/8374647
10. Phan LT, Broadhead GK, Hong TH, Chang AA. Predictors of visual acuity after treatment of neovascular age-related macular degeneration - current perspectives. Clin Ophthalmol. 2021;15:3351–3367. doi:10.2147/OPTH.S205147
11. Gil-Martínez M, Santos-Ramos P, Fernández-Rodríguez M, et al. Pharmacological advances in the treatment of age-related macular degeneration. Curr Med Chem. 2020;27(4):583–598. doi:10.2174/0929867326666190726121711
12. Parravano M, Costanzo E, Scondotto G, Trifirò G, Virgili G. Anti-VEGF and other novel therapies for neovascular age-related macular degeneration: an update. BioDrugs. 2021;35(6):673–692. doi:10.1007/s40259-021-00499-2
13. Yeung L, Hsieh YT, Yang CH, et al. Management of neovascular age-related macular degeneration: Taiwan expert consensus. J Formos Med Assoc. 2021;120(12):2061–2071. doi:10.1016/j.jfma.2021.06.012
14. Ricci F, Bandello F, Navarra P, Staurenghi G, Stumpp M, Zarbin M. Neovascular age-related macular degeneration: therapeutic management and new-upcoming approaches. Int J Mol Sci. 2020;21(21):8242. doi:10.3390/ijms21218242
15. Chakraborty D, Maiti A, Sheth JU, et al. Brolucizumab in neovascular age-related macular degeneration - Indian real-world experience: the BRAILLE Study. Clin Ophthalmol. 2021;15:3787–3795. doi:10.2147/OPTH.S328160
16. Monés J, Singh RP, Bandello F, et al. Undertreatment of neovascular age-related macular degeneration after 10 years of anti-vascular endothelial growth factor therapy in the real world: the need for a change of mindset. Ophthalmologica. 2020;243(1):1–8. doi:10.1159/000502747
17. Nguyen QD, Das A, Do DV, et al. Brolucizumab: evolution through preclinical and clinical studies and the implications for the management of neovascular age-related macular degeneration. Ophthalmology. 2020;127(7):963–976. doi:10.1016/j.ophtha.2019.12.031
18. Recommendations of the 39th SEC (Ophthalmology) Meeting, 22.06.2020 at CDSCO HQ New Delhi. Available from: https://cdsco.gov.in/opencms/resources/UploadCDSCOWeb/2018/UploadCommitteeFiles/39thSECOphthalmologyRecommendation%2022.06.2020.pdf.
19. Yokota T, Milenic DE, Whitlow M, Schlom J. Rapid tumor penetration of a single-chain Fv and comparison with other immunoglobulin forms. Cancer Res. 1992;52(12):3402–3408.
20. Borras L, Gunde T, Tietz J, et al. Generic approach for the generation of stable humanized single-chain Fv fragments from rabbit monoclonal antibodies. J Biol Chem. 2010;285(12):9054–9066. doi:10.1074/jbc.M109.072876
21. Dugel PU, Koh A, Ogura Y, et al. HAWK and HARRIER: Phase 3, multicentre, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127(1):72–84. doi:10.1016/j.ophtha.2019.04.017
22. Dugel PU, Singh RP, Koh A, et al. HAWK and HARRIER: ninety-six-week outcomes from the phase 3 trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2021;128(1):89–99. doi:10.1016/j.ophtha.2020.06.028
23. Baumal CR, Sørensen TL, Karcher H, et al. Efficacy and safety of brolucizumab in age-related macular degeneration: a systematic review of real-world studies. Acta Ophthalmol. 2022;00:1–17.
24. Zubricky R, McCoy J, Donkor R, et al. Real-world frequency and management of ocular adverse events in eyes with neovascular age-related macular degeneration treated with brolucizumab [published online ahead of print, 2023 Jun 13]. Ophthalmol Ther. 2023. doi:10.1007/s40123-023-00741-w
25. Finger RP, Dennis N, Freitas R, et al. Comparative efficacy of brolucizumab in the treatment of neovascular age-related macular degeneration: a systematic literature review and network meta-analysis. Adv Ther. 2022;39(8):3425–3448. doi:10.1007/s12325-022-02193-3
26. Sharma A, Kumar N, Parachuri N, et al. Brolucizumab-early real-world experience: BREW study. Eye. 2021;35(4):1045–1047. doi:10.1038/s41433-020-1111-x
27. Bulirsch LM, Saßmannshausen M, Nadal J, Liegl R, Thiele S, Holz FG. Short-term real-world outcomes following intravitreal brolucizumab for neovascular AMD: SHIFT study. Br J Ophthalmol. 2022;106(9):1288–1294. doi:10.1136/bjophthalmol-2020-318672
28. Enríquez AB, Baumal CR, Crane AM, et al. Early experience with brolucizumab treatment of neovascular age-related macular degeneration. JAMA Ophthalmol. 2021;139(4):441–448. doi:10.1001/jamaophthalmol.2020.7085
29. Matsumoto H, Hoshino J, Mukai R, Nakamura K, Akiyama H. Short-term outcomes of intravitreal brolucizumab for treatment-naïve neovascular age-related macular degeneration with type 1 choroidal neovascularization including polypoidal choroidal vasculopathy. Sci Rep. 2021;11(1):6759. doi:10.1038/s41598-021-86014-7
30. Montesel A, Bucolo C, Sallo FB, Eandi CM. Short-term efficacy and safety outcomes of brolucizumab in the real-life clinical practice. Front Pharmacol. 2021;12:720345. doi:10.3389/fphar.2021.720345
31. Lalwani GA, Rosenfeld PJ, Fung AE, et al. A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO Study. Am J Ophthalmol. 2009;148(1):43–58.e1. doi:10.1016/j.ajo.2009.01.024
32. Anantharaman G, Sheth J, Bhende M, et al. Polypoidal choroidal vasculopathy: pearls in diagnosis and management. Indian J Ophthalmol. 2018;66(7):896–908. doi:10.4103/ijo.IJO_1136_17
33. Bilgic A, Kodjikian L, Srivastava S, et al. Initial pro re nata brolucizumab for exudative AMD: the PROBE Study. J Clin Med. 2021;10(18):4153. doi:10.3390/jcm10184153
34. Bilgic A, Kodjikian L, March de Ribot F, et al. Real-world experience with brolucizumab in wet age-related macular degeneration: the REBA Study. J Clin Med. 2021;10(13):2758. doi:10.3390/jcm10132758
35. Walter S, Saba N Efficacy and durability of brolucizumab in patients being transitioned from prior anti-VEGF therapy; 2021.
36. Rübsam A, Rau S, Pilger D, et al. Early OCT angiography changes of macular neovascularization in patients with exudative AMD Treated with brolucizumab in a real-world setting. JOphthalmol. 2022;2022:1–9. doi:10.1155/2022/2659714
37. Abdin AA, Aljundi W, Jawhari KE, Suffo S, Weinstein I, Seitz B. First year real life experience with intravitreal brolucizumab for treatment of refractory neovascular age-related macular degeneration. Front Pharmacol. 2012;13:860784.
38. Chakraborty D, Maiti A, Sheth JU, et al. Brolucizumab in neovascular age-related macular degeneration - Indian real-world experience: the BRAILLE Study - fifty-two-week outcomes. Clin Ophthalmol. 2022;16:4303–4313. doi:10.2147/OPTH.S395577
39. Pagenax [pack insert]. Mumbai, India: Novartis Healthcare Pvt. Ltd.; 2022.
40. National health estimates (2018–2019) released. Press Information Bureau (pib.gov.in). Ministry of health and family welfare; 2022. Available from: https://pib.gov.in/PressReleasePage.aspx?PRID=1858770.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.