Back to Journals » Clinical Ophthalmology » Volume 14
Short-Term Efficacy of Selective Laser Trabeculoplasty in Omani Eyes with Glaucoma: A Single Institutional Study
Authors Al Busaidi A, Shenoy K, Panchatcharam S , Al-Mujaini A
Received 28 June 2020
Accepted for publication 6 August 2020
Published 14 September 2020 Volume 2020:14 Pages 2631—2638
DOI https://doi.org/10.2147/OPTH.S269508
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Aisha Al Busaidi,1 Kashinatha Shenoy,1 Sathiya Murthi Panchatcharam,2 Abdullah Al-Mujaini1,3
1Department of Ophthalmology, Sultan Qaboos University Hospital, Muscat, Oman; 2Research Section, Oman Medical Specialty Board, Muscat, Oman; 3College of Medicine and Health Sciences, Sultan Qaboos University Hospital, Muscat, Oman
Correspondence: Aisha Al Busaidi Department of Ophthalmology
Sultan Qaboos University Hospital, PO Box 38, PC 123, Muscat, Oman
Tel +968 24144547
Email [email protected]
Purpose: There is some evidence suggesting a different nature of response to selective laser trabeculoplasty (SLT) among different races. Therefore, we aimed to assess the short-term efficacy, safety and nature of outcome of SLT in Omani eyes.
Patients and Methods: A retrospective review was performed of patients with open-angle glaucoma (OAG) or ocular hypertension (OHTN) who underwent a single session of 360-degree SLT between January 1, 2017 and December 31, 2018. The main outcome was mean IOP reduction and attainment of treatment success at 5 weeks and 12 weeks post treatment defined as at least 20% IOP reduction from baseline without further medications or interventions. Secondary outcomes were frequency of adverse events and factors predicting success.
Results: A total of 33 eyes of 33 Omani patients who underwent treatment with SLT were analyzed. The nature of response to laser followed a gradual pattern as the mean IOP reduction from baseline was 20.2% (5.21 mm Hg, P < 0.001) at 5 weeks and further enhanced to 27.2% (6.95 mm Hg, P < 0.001) at 12 weeks. Short-term success was achieved in 51.5% and 72.2% of eyes at 5 and 12 weeks, respectively. SLT was most effective in OHTN subgroup and those with higher baseline IOP (both P < 0.001). Side effects were an infrequent occurrence, minor and transient.
Conclusion: The short-term success of SLT in Omani eyes was clinically relevant and comparable to the gradual pattern seen in patients of Indian ancestry. It is a safe therapeutic option in selective Omani eyes.
Keywords: SLT, race, outcome, open-angle glaucoma, ocular hypertension
Introduction
Recent information regarding glaucoma in Oman is limited. The Oman Eye Study 2005 found the prevalence of glaucoma to be 4.75% and according to the National Center for Statistics and Information, the Sultanate registered 5076 cases of people suffering from glaucoma in the year 2018 alone.1,2 Since the burden of disease is as high as worldwide figures, challenges exist in the approach to glaucoma and its care delivery. Khandekar et al had reported a high rate of non-compliance to treatment (75.2%) among Omani glaucoma patients.3 In addition, many patients on topical IOP-lowering therapy report symptoms suggestive of ocular surface disease. This is not surprising as a study abroad had reported that 50% of patients on glaucoma medical therapy report some degree of ocular surface discomfort.4 This is exacerbated by the fact that in Oman, preservative-free formulations of topical antiglaucoma medication are not readily available. With free healthcare services delivered in the country, the burden of eye care cost both direct and indirect are tangible. In view of these challenges surrounding glaucoma medical therapy, on one hand, and the high-risk profile of glaucoma surgery, laser trabeculoplasty (LT) should be more readily offered either as first line or adjunct treatment.
Selective laser trabeculoplasty (SLT), a type of LT which works by the principle of selective photo-thermolysis, is utilized worldwide as a modality to lower IOP by increasing trabecular meshwork outflow. Since its commercialization in 2001, it has achieved a proven track record of efficacy and safety. SLT is effective as both adjunctive and initial therapy for glaucoma. One prospective study showed that SLT reduced IOP 30% when used as a first treatment.5 A randomized clinical trial known as SLT/MED trial compared initial SLT versus prostaglandin analog. It showed a comparable reduction in IOP between patients receiving SLT or medications (26.4% vs 27%, respectively) as initial treatment.4 However, there was a trend toward more adjunctive treatment required for adequate IOP control in the medication arm compared to the SLT group.4 Cost studies have revealed significant cumulative savings of SLT over medications and filtering surgery.6,7 An analysis in the US of direct cost-comparison between initial treatment with two trabecular micro-bypass stents, SLT or medications for open angle-glaucoma; revealed that SLT had the lowest initial year-zero cost compared to the others and a lower average 5-year cumulative cost and marginal annual cost in years 1–5 compared to medications only.8
Most studies have shown no significant effect of race on the long-term success rates of SLT. Favorable results have been obtained in eyes of Asian descent.9 A more recent study found that SLT was effective in producing clinically significant IOP reduction among South African adults and good therapeutic responses but significantly less efficacious with a different pattern of response in socioeconomic comparable patients of Indian ancestry.10 It should be interesting to investigate how Omani eyes respond to this intervention and whether it resembles the pattern of response exhibited by African or Indian eyes. This study looks at the short-term efficacy of SLT in Omani eyes with OAG and ocular hypertension (OHTN). This will help to establish or reinforce its usefulness in the right clinical setting as a modality of treatment provided in Oman. To the best of our knowledge, this is the first study examining the outcome of SLT on Omani eyes with glaucoma.
Patients and Methods
This study was approved by the institutional medical research and ethical committee at Sultan Qaboos University (Medical Research Ethics Committee #1847). This study conformed to the provisions of the Declaration of Helsinki as amended in 2013. Consent was waived due to the retrospective nature of the review but the privacy of data from study eyes were maintained with confidentiality. This retrospective chart review assessed all patients with glaucoma treated with selective laser trabeculoplasty (SLT) from January 2017 till December 2018 at the Department of Ophthalmology in Sultan Qaboos University Hospital. Inclusion criteria were patients older than 18 years of age with ocular hypertension (OHTN) or a type of open angle glaucoma (OAG) that had undergone SLT treatment during the above-mentioned study period. If both eyes had undergone SLT, only one eye was chosen at random for analysis. Patients who underwent previous intraocular surgeries, previous LT, who had the SLT procedure aborted mid-way for any reason or who were lost to follow-up post SLT were excluded from the study.
Pre-treatment data were extracted from medical records at baseline visit and post-treatment data at 1-hour, 5 weeks and 12 weeks post laser. At baseline, data recorded included age, gender, phakic status, central corneal thickness (CCT), glaucoma subtype, glaucoma severity based on visual field analysis consisting of mean deviation (VF MD), number of glaucoma medications, refraction, best corrected visual acuity (BCVA) and baseline IOP. SLT parameters such as number of laser spots delivered, and laser power settings were obtained as well as the total energy of SLT delivered. Post-treatment documentation of side effects, changes in baseline medications and IOP measurements at 1-hour, 5 weeks and 12 weeks were retrieved.
The SLT platform used was a 532 nm Q-switched frequency doubled Nd:YAG laser source from the Ellex Solo™ SLT laser delivery machine. The laser platform uses a standardized spot size of 400 microns and pulse width of 3 ns. Eyes were prepared with topical pilocarpine 2% to allow proper visualization of angle structures and apraclonidine 0.5% eye drops instilled 30 minutes prior to laser treatment to prevent IOP spikes. Laser was delivered immediately after instillation of topical anesthetic oxybuprocaine 0.4% and application of Latina SLT Gonio Laser Lens with a coupling methylcellulose agent on the surface of the eye. The standard treatment protocol consisted of treating 360 degrees of the trabecular meshwork (TM) with total laser applications ranging from 65 to 121 continuous non-overlapping laser spots delivered during one session. The energy level per shot used in our study eyes ranged from 0.4 to 0.9 mJ which was adjusted at 0.1 mJ increments while titrating to individual target end responses. The end response was gauged by the first appearance of micro-cavitation bubbles observed next to the TM. That point was taken as the obtained treatment threshold. Topical apraclonidine 0.5% was instilled immediately after the procedure to blunt any potential for IOP spikes post laser. No topical corticosteroids or NSAIDs were used before or after the SLT treatment.
IOP measurements were recorded at baseline prior to treatment and at follow-up post laser at 1 hour, 5 weeks and 12 weeks. If there was an IOP rise greater than 5 mm Hg from baseline, the elevation was treated with a short course of oral acetazolamide. All IOP measurements recorded were performed using a calibrated standard Goldmann Applanation Tonometry (GAT).
The main outcome in our study was % IOP reduction achieved by SLT in our study population. This was calculated as: % IOP reduction at 5 weeks or 12 weeks’ time points = absolute IOP reduction (i.e. baseline IOP – follow-up IOP at 5 weeks or 12 weeks)/baseline IOP x 100. Achieving a reduction of at least 20% of baseline IOP without requiring additional medication, laser or surgery within the follow-up period was considered a treatment success. Other outcome measures included the presence and degree of transient IOP rise at 1-hour post procedure, reporting other side effects as well as identifying predictive factors of success.
Statistical Analysis
The collected data were analysed using IBM SPSS Statistics 25.0 (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.). For descriptive purposes, continuous variables were presented with mean and standard deviation, whereas, categorical variables were presented with number and percentages. Baseline measurements of IOP were compared with specific time points post SLT using Wilcoxon-signed rank test. Other clinical parameters were looked at for any association with IOP reduction using Mann–Whitney, Kruskal–Wallis and Pearson correlation tests based on the nature of the variables. Independent predictors of clinically significant IOP reduction (treatment success) over the follow-up time period were looked for using multivariate analysis by an adjusted Generalized Estimated Equations (GEE) model. The P-value of <0.05 was considered statistical significance.
Results
A total of 36 patients underwent SLT during the study period. However, 33 eyes of 33 patients met the study eligibility criteria. Baseline demographic and clinical characteristics are presented below (Table 1). The age of the population ranged from 29 to 88 years with a mean of 59.61 years (SD 12.80 years), of which 45.5% were female. Majority of the study eyes were phakic (87.9%) and of the primary open angle glaucoma (POAG) subtype (42.4%). Other subtypes of glaucoma in decreasing occurrence were pseudo-exfoliative glaucoma (PXG), mixed-mechanism glaucoma (MMG) and ocular hypertension (OHTN). Three eyes had undergone previous LASIK and one had a remotely failed trabeculectomy. The BCVA ranged from counting fingers (CF) to 6/6 with a baseline MD on Humphrey visual field (HVF) ranging from advanced defects to visual fields within normal limits (mean −5.55 dB SD 8). The proportion of eyes that was not on any concurrent anti-glaucoma medication at the time of SLT was 45.5% (n=15/33). These eyes were either treatment naïve eyes or had a washout of an anti-glaucoma medication. The rest of the eyes were on anti-glaucoma medications ranging from 1–4 eye drops with a median of one drop (mean of 1.21 drops, 1 med= 8/33, 24.3%, 2 meds= 2/33, 6.1%, 3 meds or 4 meds=12.1% each). Out of the eyes on anti-glaucoma eye drops, 66.7% (n=12/18) were being treated with a prostaglandin analogue (PGA) either alone or in combination with other eye drops. All patients had no prior treatment of laser trabeculoplasty.
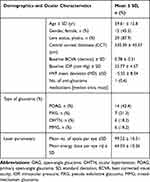 |
Table 1 Baseline Characteristics of Omani Eyes with OAG and OHTN Treated with SLT |
At baseline, before SLT, the mean IOP (treated or untreated with medications) was 25.77 mm Hg (SD 4.57). Study eyes underwent 360 degrees SLT treatment with mean applications of 99.5 shots (SD 10.31) on the trabecular meshwork resulting in a mean total energy of 64.03 mJ ± 10.26. The mean IOP reduction from baseline at 5 and 12 weeks were 20.2% (5.21 mm Hg, P <0.001) and 27.0% (6.95 mm Hg, P <0.001), respectively (Table 2, Figure 1). The proportion of eyes which achieved success of 20% or more of IOP reduction without additional medications or interventions was 51.5% at 5 weeks and 72.7% at 12 weeks (i.e. 3 months) post laser (Figure 2).
 |
Table 2 Evolution of Mean IOP (mm Hg) and Percentage IOP Reduction (%) in Omani Eyes at Different Time Points Post-SLT |
 |
Figure 1 The course of mean IOP in Omani eyes post-SLT. Over time, the difference in mean was statistically significant: F (1.6, 45.8) = 6.306, p= 0.007. |
Only 3 out of 33 eyes (9.1%) experienced an IOP spike 1-hour post SLT defined as IOP above 5 mm Hg. The IOP was normalized by the next day after a few doses of oral acetazolamide. Other post laser complications included complaints of redness and ocular pain/discomfort experienced by 2 eyes (6.1%) a day after laser and resolved spontaneously shortly after without the need for any topical cycloplegics or NSAIDs. One patient had an unusual presentation of a corneal epitheliopathy a day after laser which resolved with viscous lubricating eye drops within a week. This eye had not undergone prior laser-assisted in situ keratomileusis.
Eight patients failed to achieve treatment success at the end of the study period. Half of them had moderate-advanced disease which could explain the ineffectiveness of the procedure in these eyes, but the rest were mild cases. Four out of the 8 (50%) required additional anti-glaucoma medication and one (12.5%) failed treatment and needed a glaucoma surgical intervention (trabeculectomy with mitomycin-C) because of refractory high IOP despite maximum tolerated medical treatment. Another patient, who despite qualifying success as per the study’s criteria still required an Ahmed glaucoma tube shunt insertion at 3 months because target IOP was not reached. Majority of eyes that achieved treatment success were kept on the same pharmaceutical treatment by the end of the study period with only 3 eyes weaned off one anti-glaucoma eye drop each.
On bivariate analysis, IOP at baseline and glaucoma subtype were found to be strongly associated with IOP reduction (P <0.01), whereas, CCT showed significant but mildly positive correlation (r=0.289, P=0.004) (Table 3). After adjusting for other factors on multivariate analysis, only higher baseline IOP and OHTN subtype were shown to be independent predictors of SLT success in this study. Those with higher baseline IOP (>21 mm Hg) had IOP lowered 1.3 times more than those with lower baseline IOP (i.e. 20 mm Hg or less) (OR=1.31; 95% C.I. 1.16–1.48, P=0.001). OHTN showed 1.26 times more IOP reduction compared to POAG group (OR = 1.26; 95% C.I.1.05–1.52, P= 0.012).
 |
Table 3 Association of Clinical Parameters with IOP Reduction in Omani Eyes |
Discussion
SLT is known to provide a clinically significant IOP reduction in patients with OAG and OHTN. A recent metanalysis identified that among OAG patients who range from newly diagnosed to those on maximally tolerated medical therapy, SLT results in a 6.9–35% IOP reduction at ≥12 months post-SLT.11 Our study has shown the short-term outcome of a single session of 360° SLT treatment on Omani eyes with OAG and OHTN who were either on concurrent anti-glaucoma medications or not. The mean reduction in IOP following SLT was 20.2% (P <0.001) at 5 weeks and 27.0% (P <0.001) at 12 weeks (3 months). These levels of IOP reduction are comparable to another study which looked at the short-term efficacy of SLT in a Romanian glaucoma population and found IOP decreased by 22.47% at 1 month and 26.58% at 3 months (p=0.001).12 These findings also fall within the range reported in numerous studies for average IOP reductions even at 6 months post-SLT of 21.8–29.4%.13–16
Our study has found that SLT was effective in lowering IOP 20% or more below baseline pressure on average in 72.2% of Omani eyes with OAG and OHTN at 3 months. This was defined as a success since there was at least 20% IOP lowering with no additional medical, laser or surgical interventions as per a similar criterion set in previous large studies.17 Such successful IOP lowering effects were seen in a similar proportion of eyes up to 3 years post laser. This effect, however, was seen to wane over time. Preceding data have shown that success was achieved post SLT in 66.7–75% of eyes at 6 months,16,18,19 58–94% at 12 months,15,16,20–22 40–85% at 2 years,15,20,24 38–74% at 3 years,15,17,20,23 38–68% at 4 years15,17,20 and 11.1–31% at 5 years.14,17,23 The wide range of success rates in various studies may be explained by the differences in study design and factors which may affect the outcome of SLT including: glaucoma type, angle status, extent of angle treatment (180 vs 360°), pre-treatment IOP, number and type of medications and duration of medical treatment before SLT was performed.24
The initial IOP response in our study eyes was evident as early as 5 weeks post laser but the IOP reduction continued beyond that (over 3 months) but to a lesser extent than the initial period (Figure 1). This general reported pattern of gradual nature of response and decrease rate of decline with time was documented in several other studies and in majority of eyes even in different races such as in Romanian and Indian eyes.10,12,25 A distinct difference in pattern of response to treatment with SLT in Blacks versus those of Indian heritage in a South African study was noted. Although both races exhibited a good IOP response, Indian eyes showed more of a gradual response pattern whereas Blacks showed a more uniform response pattern fully manifest within 1 month of treatment and sustained throughout a 1-year interval.10 Another previous study found that the only significant predictor of IOP lowering at 12 months across all eyes was time, with the maximum IOP reduction seen at 3 months followed by a slow decline in effect subsequently.25 Long-term data showed the mean survival time (time for 50% of eyes to fail) is around 2 years.17,20
The side effects observed following SLT in our eyes were an infrequent occurrence, transient and minor in nature. This is in concordance with the low complication rate published in numerous case reports. The definition of various side effects post SLT varied widely among different studies in the literature, especially on the aspect of severity. The proportion of IOP spikes post procedure have been observed in almost all published series, whether or not the patients received perioperative antihypertensive treatment. The reported incidence of IOP spikes varied from 0% to 28.8% or 62% depending on whether prophylactic anti-glaucoma medication was used or not, respectively.11 In our subset of eyes, the incidence of IOP spikes (an IOP rise above 5 mm of Hg) fell within this documented range at 9.1% (n=3/33) despite all eyes receiving empirical anti-glaucoma medication. The proportion of eyes with complaints of eye pain/discomfort and redness in the literature ranges from 0% to 65.7%11 and in our study only 6.1% of eyes experienced such an occurrence which was transient and resolved spontaneously. Only one eye experienced a corneal-related side effect consistent with a picture of corneal punctate epitheliopathy which resolved with conservative treatment. Prior LASIK, although documented in previous reports to be one possible predisposing condition to corneal-related adverse effects,26–28 in our series of eyes there were none who developed post SLT corneal haze, edema or lamellar keratitis. Other side effects such as anterior chamber reaction, retinal side effects (e.g. cystoid macular edema, macular burns/scars, choroidal effusions) or other corneal side effects (e.g. corneal endothelial abnormality) were not seen in our study population. None of these adverse events required any surgical intervention or threatened sight.
SLT is not uniformly effective in all treated eyes. The most consistently reported factor which is associated with higher SLT success and/or greater IOP reduction is higher baseline (pre-SLT) IOP.29 Our study findings are in keeping with this; as one of the factors associated with SLT success was baseline (pre-SLT) IOP. This association was apparent when pre-SLT IOP was considered as dichotomized at a threshold of 21 mm Hg. This predictor of success was noted up to a certain level as extremely high pressures may not be effectively managed by SLT; requiring repeat SLT or surgery as the magnitude of IOP reduction to control disease progression is larger and unachievable by single SLT treatment alone.30 This was evident in one of the eyes which had a baseline IOP of 40 mm Hg and despite achieving success with 30% IOP reduction resulting in post SLT IOP of 28 mm Hg at 3 months, required an Ahmed glaucoma tube shunt insertion. Although the literature does not support that glaucoma subtype is a predictor of SLT success (even the once presumed association of pseudo-exfoliative glaucoma), our study found OHTN to be an independent predictor of success compared to POAG. Since the nature of this association was found after adjusting for other factors associated with IOP reduction, this could not be reflected or explained by the fact that OHTN have higher baseline IOPs and thus greater IOP reduction. A possible reason is that majority of OHTN eyes in our study were treatment naïve eyes on no anti-glaucoma medications which could impact success of laser treatment since SLT initially performed result in better IOP reduction than in those performed as an adjuvant treatment. Another contradicting finding in this study was that the total energy level employed in SLT therapy was not found to be an important prognostic factor, unlike some previous reports.31 Habib et al32 divided patients receiving 360 degree SLT treatment into those who received low (<85 mJ), medium (85–105 mJ) and high (>105 mJ) energy SLT and so a possible explanation for the lack of association of this as a prognostic factor was that in reference to these categories the mean total energy dose delivered in our study eyes was low (64.03 mJ SD 10.26). This could have undermined the impact of total energy on SLT success in our study population. Also, this might explain the lack of AC inflammation and low frequency of IOP spikes in our population. Other patient factors including age, gender, phakic status, CCT, baseline visual acuity, visual field MD and concurrent antiglaucoma eye drops (including prostaglandin analogs) were not found to be significant predictors of success neither in our study nor in previous reports.18,33,34
This study had several strengths, as to our knowledge, it was the first of its kind examining the effects of SLT in Omani eyes. It analyzed SLT’s response on a spectrum of open-angle ocular hypertension and glaucoma cases ranging from primary to secondary forms including PXG and MMG (which included steroid-induced glaucoma). This helped to confirm the efficacy and broad utility of SLT in various types of glaucoma among Omani eyes. All lasers were performed by the same experienced ophthalmologist, minimizing the bias of differing laser technique and target treatment end-points. The obvious limitations are its retrospective design, small sample size and a success criterion that does not necessarily reflect success in the real-world setting. Although the short-term outcome of SLT was the primary focus of this study, we intend to continue following up our study patients long term; over 5 years, to elucidate the full nature and longevity of response in Omani eyes.
Conclusions
This study confirms that SLT is efficacious in lowering IOP, as an initial treatment or when medical therapy is insufficient in various types of OAG, OHTN and in various races including Omani eyes which was the focus of the study. It emphasized the short-term usefulness, safety and scope of clinical application of SLT thus should be strongly considered in every ophthalmologist’s armamentarium for reducing IOP in selective eyes. However, its wide acceptance as a treatment in daily practice by ophthalmologists and patients in Oman will be influenced by their preference, surgeon’s experience and access to laser facilities among others.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. All authors have read and approved the manuscript.
Disclosure
We, the authors, declare that: 1) There was no financial support in terms of grants or funds for this study from any source. 2) We have no proprietary interests or any potential conflicts of interest. 3) This study was presented at Muscat International Ophthalmology Conference, March 28th, 2019.
References
1. Khandekar R, Shama ME, Mohammed AJ. Noncompliance with medical treatment among glaucoma patients in Oman – a cross-sectional descriptive study. Ophthalmic Epidemiol. 2005;12(5):303–309. doi:10.1080/09286580500224602
2. Times of Oman. North Al Sharqiyah sees highest number of glaucoma cases in Oman. [online]. 2019. Available from: https://timesofoman.com/article/2293393/oman/health/north-al-sharqiyah-sees-highest-number-of-glaucoma-cases-in-oman.
3. Fechtner RD, Godfrey DG, Budenz D, et al. Prevalence of ocular surface complaints in patients with glaucoma using topical intraocular pressure-lowering medications. Cornea. 2010;29(6):618–621. doi:10.1097/ICO.0b013e3181c325b2
4. Katz LJ, Steinmann WC, Kabir A, Molineaux J, Wizov SS, Marcellino G. Selective laser trabeculoplasty versus medical therapy as initial treatment of glaucoma: a prospective, randomized trial. J Glaucoma. 2012;21(7):460–468. doi:10.1097/IJG.0b013e318218287f
5. Melamed S, Ben Simon GJ, Levkovitch-Verbin H. Selective laser trabeculoplasty as primary treatment for open-angle glaucoma: a prospective, nonrandomized pilot study. Arch Opthalmol. 2003;121(7):957–960. doi:10.1001/archopht.121.7.957
6. Lee R, Hutnik CM. Projected cost comparison of selective laser trabeculoplasty versus glaucoma medication in the Ontario Health Insurance Plan. Can J Ophthalmol. 2006;41(4):449–456. doi:10.1016/S0008-4182(06)80006-2
7. Cantor LB, Katz LJ, Cheng JW, Chen E, Tong KB, Peabody JW. Economic evaluation of medication, laser trabeculoplasty and filtering surgeries in treating patients with glaucoma in the US. Curr Med Res Opin. 2008;24(10):2905–2918. doi:10.1185/03007990802379996
8. Berdahl JP, Khatana AK, Katz LJ, et al. Cost-comparison of two trabecular micro-bypass stents versus selective laser trabeculoplasty or medications only for intraocular pressure control for patients with open-angle glaucoma. J Med Econ. 2017;20(7):760–766. doi:10.1080/13696998.2017.1327439
9. Lai JS, Chua JK, Tham CC, Lam DS. Five-year follow-up of selective laser trabeculoplasty in Chinese eyes. Clin Exp Ophthalmol. 2004;32(4):368–372. doi:10.1111/j.1442-9071.2004.00839.x
10. Goosen E, Coleman K, Visser L, Sponsel WE. Racial differences in selective laser trabeculoplasty efficacy. J Curr Glaucoma Pract. 2017;11(1):22–27. doi:10.5005/jp-journals-10008-1216
11. Wong MO, Lee JW, Choy BN, Chan JC, Lai JS. Systematic review and meta-analysis on the efficacy of selective laser trabeculoplasty in open-angle glaucoma. Surv Ophthalmol. 2015;60(1):36–50. doi:10.1016/j.survophthal.2014.06.006
12. Chiselita D, Cantemir A, Pantalon AD. Selective laser trabeculoplasty – short term efficacy and safety profile in open angle glaucoma or ocular hypertension treatment. Rom J Ophthalmol. 2015;59(3):148–153.
13. Martinez-de-la-Casa JM, Garcia-Feijoo J, Castillo A, et al. Selective vs argon laser trabeculoplasty: hypotensive efficacy, anterior chamber inflammation and postoperative pain. Eye. 2004;18(5):498–502. doi:10.1038/sj.eye.6700695
14. Woo DM, Healey PR, Graham SL, Goldberg I. Intraocular pressure-lowering medications and long-term outcomes of selective laser trabeculoplasty. Clin Exp Ophthalmol. 2015;43(4):320–327. doi:10.1111/ceo.12452
15. Gracner T, Falez M, Gracner B, Pahor D. Long-term follow up of selective laser trabeculoplasty in primary open-angle glaucoma. Klin Monbl Augenheilkd. 2006;223(9):743–747. (). doi:10.1055/s-2006-926725
16. Kent SS, Hutnik CM, Birt CM, et al. A randomized clinical trial of selective laser trabeculoplasty versus argon laser trabeculoplasty in patients with pseudoexfoliation. J Glaucoma. 2015;24(5):344–347. doi:10.1097/IJG.0b013e31829e55e4
17. Bovell AM, Damji KF, Hodge WG, Rock WJ, Buhrmann RR, Pan YI. Long term effects on the lowering of intraocular pressure, selective laser or argon trabeculoplasty? Can J Ophthalmol. 2011;46(5):408–413. doi:10.1016/j.jcjo.2011.07.016
18. Martow E, Hutnik CM, Mao A. SLT and adjunctive medical therapy: a prediction rule analysis. J Glaucoma. 2011;20(4):266–270. doi:10.1097/IJG.0b013e3181e3d2c1
19. Nagar M, Luhishi E, Shah N. Intraocular pressure control and fluctuation: the effect of treatment with selective laser trabeculoplasty. Br J Ophthalmol. 2009;93(4):497–501. doi:10.1136/bjo.2008.148510
20. Weinand FS, Althen F. Long-term clinical results of selective laser trabeculoplasty in the treatment of primary open angle glaucoma. Eur J Ophthalmol. 2006;16(1):100–104. doi:10.1177/112067210601600116
21. McIIraith I, Strasfeld M, Colev G, Hutnik C. Selective laser trabeculoplasty as initial and adjunctive treatment for open-angle glaucoma. J Glaucoma. 2006;15(2):124–130. doi:10.1097/00061198-200604000-00009
22. Nagar M, Ogunyomade A, O’Brart DPS, Howes F, Marshall J. A randomised, prospective study comparing selective laser trabeculoplasty with latanoprost for the control of intraocular pressure in ocular hypertension and open angle glaucoma. Br J Ophthalmol. 2005;89(11):1413–1417. doi:10.1136/bjo.2004.052795
23. Juzych MS, Chopra V, Banitt MR, et al. Comparison of long-term outcomes of selective laser trabeculoplasty versus argon laser trabeculoplasty in open-angle glaucoma. Ophthalmology. 2004;111(10):1853–1859. doi:10.1016/j.ophtha.2004.04.030
24. Koucheki B, Hashemi H. Selective laser trabeculoplasty in the treatment of open-angle glaucoma. J Glaucoma. 2012;21(1):65–70. doi:10.1097/IJG.0b013e3182027596
25. Realini T, Shillingford-Ricketts H, Burt D, Balasubramani GK. West Indies Glaucoma Laser Study (WIGLS):2. predictors of selective laser trabeculoplasty efficacy in Afro-Caribbeans with glaucoma. J Glaucoma. 2018;27(10):845–848.
26. Regina M, Bunya VY, Orlin SE, Ansari H. Corneal edema and haze after selective laser trabeculoplasty. J Glaucoma. 2011;20(5):327–329. doi:10.1097/IJG.0b013e3181e6668d
27. Moubayed SP, Hamid M, Choremis J, Li G. An unsual finding of corneal edema complicating selective laser trabeculoplasty. Can J Ophthalmol. 2009;44(3):337–378. doi:10.3129/i09-025
28. Holz H, Pirouzian A. Bilateral diffuse lamellar keratitis following consecutive selective laser trabeculoplasty in a LASIK patient. J Cataract Refract Surg. 2010;36(5):847–849. doi:10.1016/j.jcrs.2009.11.024
29. Kennedy JB, SooHoo JR, Kahook MY, Seibold LK. Selective laser trabeculoplasty: an update. Asia Pac J Ophthalmol. 2016;5(1):63–69. doi:10.1097/APO.0000000000000175
30. Lee JW, Lai JS. A review of selective laser trabeculoplasty in the Hong Kong Chinese population. Hong Kong Med J. 2016;22(2):165–170. doi:10.12809/hkmj154641
31. Lee JW, Wong MO, Liu CC, Lai JSM. Optimal selective laser trabeculoplasty energy for maximal intraocular pressure reduction in open-angle glaucoma. J Glaucoma. 2015;24(5):128–131. doi:10.1097/IJG.0000000000000215
32. Habib L, Lin J, Berezina T, Holland B, Fechtner RD, Khouri AS. Selective laser trabeculoplasty: does energy dosage predict response? Oman J Ophthalmol. 2013;6(2):92–95. doi:10.4103/0974-620X.116635
33. Hodge WG, Damji KF, Rock W, Buhrmann R, Bovell AM, Pan Y. Baseline IOP predicts selective laser trabeculoplasty success at 1 year post-treatment: results from a randomised clinical trial. Br J Ophthalmol. 2005;89(9):1157–1160. doi:10.1136/bjo.2004.062414
34. Tzimis V, Tze L, Ganesh J, et al. Laser trabeculoplasty: an investigation into factors that might influence outcomes. Can J Ophthalmol. 2011;46(4):305–309. doi:10.1016/j.jcjo.2011.06.005
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

