Back to Journals » Clinical Interventions in Aging » Volume 9
Short-term efficacy of calcium fructoborate on subjects with knee discomfort: a comparative, double-blind, placebo-controlled clinical study
Authors Pietrzkowski Z, Phelan M, Keller R, Shu C, Argumedo R, Reyes-Izquierdo T
Received 21 March 2014
Accepted for publication 23 April 2014
Published 5 June 2014 Volume 2014:9 Pages 895—899
DOI https://doi.org/10.2147/CIA.S64590
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Zbigniew Pietrzkowski, 1 Michael J Phelan, 2 Robert Keller, 3 Cynthia Shu, 1 Ruby Argumedo, 1 Tania Reyes-Izquierdo 1
1FutureCeuticals, Inc., Applied BioClinical Laboratory; 2Department of Statistics, School of Information and Computer Science, University of California at Irvine; 3NutraClinical Inc., Irvine, CA, USA
Abstract: Calcium fructoborate (CFB) at a dose of 110 mg twice per day was previously reported to improve knee discomfort during the first 14 days of treatment. In this study, 60 participants with self-reported knee discomfort were randomized into two groups receiving CFB or placebo. Initial levels of knee discomfort were evaluated by Western Ontario and McMaster Universities Arthritis Index (WOMAC) and McGill Pain Questionnaire (MPQ) scores at the beginning of the study and also at 7 and 14 days after treatment. Results showed that supplementation with CFB significantly improved knee discomfort in the study subjects; significant reductions of mean within-subject change in WOMAC and MPQ scores were observed for the CFB group compared to the placebo group at both 7 and 14 days after treatment. Estimated treatment differences for the MPQ score were -5.8 (P=0.0009) and -8.9 (P< 0.0001) at Day 7 and 14, respectively. Estimated differences for the WOMAC score were -5.3 (P=0.06) and -13.73 (P< 0.0001) at Day 7 and 14, respectively. Negative values indicate greater reductions in reported discomfort. On both Day 7 and Day 14, the trend was toward greater improvement in the CFB group. The placebo group did not exhibit any change in the WOMAC and MPQ scores. In conclusion, supplementation with 110 mg CFB twice per day was associated with improving knee discomfort during the 2 weeks of intake.
Keywords: CFB, joint discomfort, WOMAC score, McGill pain score
Corrigendum for this paper has been published
Introduction
Calcium fructoborate (CFB) is a natural plant mineral borate complex produced by a patented process first described by Miljkovic (US Patent #5,962,049).1 The chemical structure and identity of CFB have been previously described.1 Of all the boron and borate supplements available, CFB has shown the most potential to improve several health conditions.1,3 For example, CFB has been identified as a potential anti-inflammatory agent.4–6 Also, earlier clinical studies have shown that C-reactive protein (CRP), a blood marker secreted by the liver in response to inflammation or infection,7 can be reduced with CFB supplementation in subjects with angina pectoris8,9 and cardiovascular conditions.10 Most recently, published clinical research has suggested that CFB has the ability to improve symptoms associated with inflammatory conditions, such as joint discomfort and osteoarthritis.2,6
Joint discomfort is a common complaint, typically associated with limited joint function, decreased feelings of energy, and decreased quality of life.11 There are numerous causes of joint discomfort, including osteoarthritis, trauma, rheumatoid arthritis, and gout.12 Current medical treatments aim at decreasing discomfort and increasing mobility. They generally include nonsteroidal anti-inflammatory drugs (NSAIDs) to control discomfort and inflammation. Unfortunately, chronic use of these medications can lead to significant adverse effects, including gastrointestinal bleeding and loss of kidney function.13 In a previous pilot clinical trial, we evaluated the effect of CFB in a small group of subjects with symptoms of osteoarthritis.2 Participants in that study had been previously diagnosed with osteoarthritis and reported a statistically significant reduction of Western Ontario and McMaster Universities Arthritis Index (WOMAC) score and McGill Pain Questionnaire (MPQ) index values over 14 days of supplementation. Based upon these findings, we hypothesized that we could show that CFB has a beneficial effect on knee pain in a larger group of subjects with self-reported knee discomfort, based on self-diagnosis and their own subjective perception of the pain.
Materials and methods
Materials
CFB was provided and standardized by VDF FutureCeuticals, Inc., Momence, IL, USA. Placebo consisted of silica oxide (Sigma-Aldrich, St Louis, MO, USA) and fructose (Sigma-Aldrich).
Inclusion criteria
Subjects included in the study were >35 to <65 years of age; average, 50 years (standard deviation [SD] ±7.95). The average age for subjects in the placebo group was 51 years (SD ±6.67); subjects assigned CFB averaged 49 years of age (SD ±8.94). A Student’s t-test was performed; the age difference between groups was not significant (P=0.16). Subject body mass index (BMI) was >21 to <30 kg/m2 (average, 26.7; SD ±2.4; P=0.55). Subjects’ initial MPQ scores were >35 and <60 (average, 50; SD ±5.9, P=0.13); included subjects reported knee discomfort for more than 4 weeks prior to enrollment in the study. Aside from reported knee discomfort, subjects were generally healthy, with no visible evidence of having respiratory or other infections, nondiabetic, and free of allergies to dietary products.
Exclusion criteria
Subjects <35 or >65 years were excluded from the study, as were subjects with a BMI <21 or >30 and those who were pregnant, nursing, or planning to get pregnant. Also excluded from the study were subjects currently enrolled in another study, subjects with cardiovascular diseases or any knee injury, and subjects taking NSAIDs or other medications for pain, supplements, or vitamin D 2 weeks prior to the start of this trial.
Consent
This study was conducted according to the guidelines put forth in the Declaration of Helsinki. All procedures involving human subjects were approved by the Institutional Review Board at Vita Clinical S.A. Avenida Circunvalacion Norte #135, Guadalajara, JAL, Mexico 44270 (IRB Number: ABC-NCI-13-08-FRXB). All study subjects were generally healthy and affirmed that they had not used any type of medication or supplement for a period of 15 days prior to the start of the study. This study was performed by NutraClinical Inc. (Irvine, CA, USA), according to a protocol designed by VDF FutureCeuticals, Inc./Applied BioClinical Lab (Irvine, CA, USA). All participants signed an informed consent.
Study population
Subjects with self-reported joint discomfort were recruited for this study. Subjects were recruited based on the MPQ score. Participants were generally healthy, as self-reported and observed. No medications or supplements of any kind were permitted within 2 weeks prior to or during the study period. Participants were advised to abstain from taking vitamin D, testosterone supplements, and steroid-containing over-the-counter drugs or prescribed medications for 30 days prior to the study period.
Sixty male and female participants aged 35 to 65 years were recruited for the study. Participants were instructed to fast overnight prior to baseline collection of blood sera. On Day 1, baseline medical history and physical examinations were performed, blood was collected, and the MPQ and WOMAC questionnaires were administered. Blood samples and WOMAC and MPQ scores were also obtained at Day 7 and Day 14 after treatment. Participants were assigned into two groups of 30 subjects by simple randomization: subjects were separated into two groups by gender, then given a piece of paper marked with either a number 1 or 2. These numbers corresponded to either the placebo or the supplemented group. Each group contained an equal number of males and females, and they received either the test product CFB or a placebo formulation according to treatment assignment. All 60 subjects received test material (placebo or CFB) immediately after blood collection on Day 1. This was considered time point 0, or baseline.
CFB and placebo preparations were provided in identical bottles and in identical capsules. Placebo formulation consisted of 80 mg of fructose and 15 mg of silica oxide per capsule. Active formulation consisted of 110 mg of CFB per capsule. Subjects were instructed to take one capsule with a cup of water, twice per day. All subjects were instructed to take the capsules before breakfast and lunch, at a minimum of 15 minutes prior to eating.
Western Ontario and McMaster Universities Arthritis Index
The WOMAC is a widely used questionnaire used to assess the physical function of joints.14 The WOMAC consists of 24 items divided into three subscales, including pain (five items; scores range from 0 to 20), stiffness (two items; scores range from 0 to 8), and functional limitations (17 items; scores range from 0 to 68). Total scores range from 0 (best) to 96 (worst). The WOMAC index was administered at baseline and at 7 and 14 days after treatment.
McGill Pain Questionnaire
The MPQ is a multidimensional pain questionnaire used to quantify the quality and intensity of pain.15,16 The scale contains four subscales consisting of 78 words that participants use to indicate feelings of pain. Seven words are chosen from categories of pain description, pain components, evaluation of pain, and a miscellaneous descriptor category. Each chosen word has an associated numerical value, and total scores range from 0 (no pain) to 78 (severe pain). The MPQ was administered at baseline and at 7 and 14 days after treatment.
The WOMAC score and the MPQ are both reliable assessment tools for measuring function and feelings of discomfort, respectively. There is evidence that both instruments together give better quantification of pain experiences than did the WOMAC alone. Even though there is correlation between these instruments, they cannot be used interchangeably; rather, they complement each other.17
Statistical methods
To address the a priori hypothesis that treatment would improve mean reported discomfort in study subjects with self-reported knee joint discomfort, the primary analysis tested the effect of treatment on the mean 7-day and 14-day change from baseline in WOMAC score and MPQ. A repeated measures analysis of variance18 was used to estimate treatment effects on within-subject changes in mean WOMAC and MPQ scores over the 7- and 14-day period. Specifically, each score was regressed on an indicator of treatment group, post-treatment day, and the treatment-day interaction. In this case, a test of the coefficients for the treatment-day interaction equaling zero is equivalent to a test of the treatment effect at Day 7 and Day 14. Both the WOMAC and MPQ score were analyzed using this approach.
Results
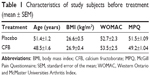
Demographic characteristics of the study population are presented in Table 1. The average age of the 60 study participants was 49.2 years (SEM ±1.02). The groups were comparable with respect to ethnicity and BMI. All participants completed the study. All recruited subjects experienced knee discomfort at starting point. Baseline WOMAC values ranged from 41.6 to 53.5 and MPQ values ranged from 49.2 to 51.5. There were no significant differences at baseline between placebo and supplemented group in either WOMAC (P=0.79) or MPQ (P=0.13) scores.
The average WOMAC and MPQ scores by treatment condition at baseline and at 7 and 14 days after treatment are described in Table 2. The average scores of WOMAC and MPQ decreased after 7 and 14 days of supplementation with CFB. Treatment had a significant effect on the WOMAC score over time (F=9.05; P<0.0001). Similarly, CFB had a significant effect on the MPQ score over time (F=7.38; P<0.0001). The effect of CFB was significantly more efficacious than that of placebo. The analysis of effects was based on mean within-subject change from baseline.
Table 3 shows the estimated effects of CFB versus placebo at 7 and 14 days. In each case the mean within-subject change from baseline for CFB is compared to the mean within-subject change for the placebo. A negative estimate indicates that treatment was involved in greater reductions in reported knee discomfort. The within-subject change in mean WOMAC score over 14 days was estimated to be 13.73 points lower on CFB when compared to control (estimated effect =−13.73, P<0.0001, 95% confidence interval [CI] −19.28 to −8.19). The within-subject change in mean MPQ score over 14 days was estimated to be 8.9 points greater on CFB when compared to control (estimated effect =−8.9, P<0.0001, 95% CI −12.32 to −5.48). CFB had a significant effect on mean within-subject change for MPQ scores at Day 7 (estimated effect =−5.8, P=0.009); the estimated effect on WOMAC scores was not significant at Day 7, but there is a positive indication in the trend. Overall the results supported significant activity for CFB in reducing reported discomfort due to joint pain during the 2-week course of the study.
Discussion
CFB is a patented,1 nature-identical analog of a plant mineral complex commonly found in plants.19 Previous studies have demonstrated the potential effects of CFB.20–24 CFB has been identified as a potential anti-inflammatory agent with the ability to modulate key markers associated with inflammation-related conditions such as osteoarthritis.2,5 CFB performed better than placebo in reducing subject-reported feelings of knee distress and joint discomfort. Participants reported subjectively an improvement in feelings of flexibility, comfort, and quality of life.2,5 This prospective randomized trial of CFB was performed to further validate these previous results. However, in the previous pilot study, subjects had been diagnosed with a joint condition.2 This current study is in agreement with, and extends, the results of our previous report: a larger group of participants was recruited, and the knee discomfort was only self-diagnosed. As in the previous study, subjects were either given a placebo or were supplemented with 108 mg/day CFB for 14 days. The amount of CFB supplemented to the participants was within the daily dietary boron requirements, which should not exceed 10 mg per day.1,25–29 The results from the current study confirm our previous observations. Both studies showed improvement in discomfort and function over the 2-week study period. None of the subjects reported any adverse effects associated with treatment.
As reported here, 7 days’ supplementation with CFB resulted in statistically significant improvement of knee comfort as measured by MPQ. In parallel, reduction of WOMAC index values indicated a strong trend (P=0.06) at 7 days. Both results indicate that CFB could improve knee distress as a result of short-term treatment. The exact mechanism of action behind these observed and reported effects is not known at this time and requires further investigation. It has been previously reported that CFB may reduce blood levels of CRP.5 CRP, fibrinogen, and erythrocyte sedimentation rate are well-documented markers of inflammation.10 Elevations in blood CRP levels have been reported in patients with symptoms of osteoarthritis, including pain.30,31 Consequently, reduction of CRP could be associated to some extent to improvement of knee conditions. In order to evaluate this possibility, we measured blood levels of CRP at Days 1, 7, and 14 in both experimental groups. Collected results indicate that majority of our study subjects did not initially showed blood CRP levels greater than 3 mg/dL, which are considered elevated and indicative of an inflammatory disease. However, a subgroup of the study subjects (30%) had Day 1 baseline levels of CRP >3. An analysis of blood samples from this subgroup showed a statistically significant reduction of CRP at Day 7 and 14 over Day 1 in the CFB group, but not in the placebo group. These results are not presented here due to the small size of this subgroup population.
The WOMAC score and MPQ index are validated subjective assessment tools for measuring knee discomfort, joint function, and overall well-being of the evaluated subjects. Both tests used together provide more accurate evaluation.17 The two tests have some similar measure that validate each other, but also have different measures that add to the overall assessment.32 In this study, the mean WOMAC score and MPQ index demonstrated continued improvement over the short duration of the study when compared to placebo.
Conclusion
This comparative double-blind study has confirmed the effect of CFB, showing significant improvements in knee discomfort and function over a period of 2 weeks. However, the inflammatory markers that are targeted and the mechanism of action are still unknown. A future clinical study would help clarify the above, as well as the effect of the CFB supplementation on a long-term basis.
Acknowledgments
We would like to thank John M Hunter, Brad Evers (FutureCeuticals, Inc.), and Michael Sapko for their comments and suggestions.
Disclosure
The authors report no conflicts of interest in this work.
References
Miljkovic D, Scorei RI, Cimpoiaşu VM, Scorei ID. Calcium fructoborate: plant-based dietary boron for human nutrition. J Diet Suppl. 2009;6(3):211–226. | ||
Reyes-Izquierdo T, Nemzer B, Gonzalez AE, et al. Short-term intake of calcium fructoborate improves WOMAC and McGill scores and beneficially modulates biomarkers associated with knee osteoarthritis: a pilot clinical double-blinded placebo-controlled study. Am J Biomed Sci. 2012;4(2):111–122. | ||
Wagner CC, Ferraresi Curotto V, Pis Diez R, Baran EJ. Experimental and theoretical studies of calcium fructoborate. Biol Trace Elem Res. 2008;122(1):64–72. | ||
Scorei RI, Ciofrangeanu C, Ion R, et al. In vitro effects of calcium fructoborate upon production of inflammatory mediators by LPS-stimulated RAW 264.7 macrophages. Biol Trace Elem Res. 2010; 135(1–3):334–344. | ||
Scorei ID, Scorei RI. Calcium fructoborate helps control inflammation associated with diminished bone health. Biol Trace Elem Res. 2013; 155(3):315–321. | ||
Scorei R, Mitrut P, Petrisor I, Scorei I. A double-blind, placebo-controlled pilot study to evaluate the effect of calcium fructoborate on systemic inflammation and dyslipidemia markers for middle-aged people with primary osteoarthritis. Biol Trace Elem Res. 2011;144(1–3): 253–263. | ||
Sharif M, Shepstone L, Elson CJ, Dieppe PA, Kirwan JR. Increased serum C reactive protein may reflect events that precede radiographic progression in osteoarthritis of the knee. Ann Rheum Dis. 2000;59(1):71–74. | ||
Scorei RI, Rotaru P. Calcium fructoborate – potential anti-inflammatory agent. Biol Trace Elem Res. 2011;143(3):1223–1238. | ||
Scorei R. Is boron a prebiotic element? A mini-review of the essentiality of boron for the appearance of life on earth. Orig Life Evol Biosph. 2012;42(1):3–17. | ||
Militaru C, Donoiu I, Craciun A, Scorei ID, Bulearca AM, Scorei RI. Oral resveratrol and calcium fructoborate supplementation in subjects with stable angina pectoris: effects on lipid profiles, inflammation markers, and quality of life. Nutrition. 2013;29(1):178–183. | ||
Neugebauer V, Han J, Adwanikar H, Fu Y, Ji G. Techniques for assessing knee joint pain in arthritis. Mol Pain. 2007;3:8. | ||
Calmbach WL, Hutchens M. Evaluation of patients presenting with knee pain: Part II. Differential diagnosis. Am Fam Physician. 2003;68(5): 917–922. | ||
Bush TM, Shlotzhauer TL, Imai K. Nonsteroidal anti-inflammatory drugs. Proposed guidelines for monitoring toxicity. West J Med. 1991; 155(1):39–42. | ||
Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833–1840. | ||
Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975;1(3):277–299. | ||
Melzack R. The McGill pain questionnaire: from description to measurement. Anesthesiology. 2005;103(1):199–202. | ||
Gandhi R, Tsvetkov D, Dhottar H, Davey JR, Mahomed NN. Quantifying the pain experience in hip and knee osteoarthritis. Pain Res Manag. 2010;15(4):224–228. | ||
Diggle P, Heagerty P, Liang KY, Zeger SL. Analysis of Longitudinal Data. 2nd ed. New York: Oxford University Press; 2009. | ||
Rotaru P, Scorei R, Harabor A, Dumitru MD. Thermal analysis of a calcium fructoborate sample. Thermochim Acta. 2010;506:8–13. | ||
Scorei IR. Calcium fructoborate: plant-based dietary boron as potential medicine for cancer therapy. Front Biosci (Schol Ed). 2011;3:205–215. | ||
Scorei R, Ciubar R, Ciofrangeanu CM, Mitran V, Cimpean A, Iordachescu D. Comparative effects of boric acid and calcium fructoborate on breast cancer cells. Biol Trace Elem Res. 2008;122(3):197–205. | ||
Scorei R, Ciubar R, Iancu C, Mitran V, Cimpean A, Iordachescu D. In vitro effects of calcium fructoborate on fMLP-stimulated human neutrophil granulocytes. Biol Trace Elem Res. 2007;118(1):27–37. | ||
Scorei RI, Popa R. Sugar-borate esters – potential chemical agents in prostate cancer chemoprevention. Anticancer Agents Med Chem. 2013; 13(6):901–909. | ||
Scorei RI, Popa R. Boron-containing compounds as preventive and chemotherapeutic agents for cancer. Anticancer Agents Med Chem. 2010;10(4):346–351. | ||
Dinca L, Scorei R. Boron in human nutrition and its regulations use. J Nutr Ther. 2013;2(1):22–29. | ||
Hunt CD. Boron. In: Coates PM, Betz JM, Blackman MR, et al, editors. Encyclopedia of dietary supplements. 2nd ed. New York: Informa Healthcare; 2010:82–90. | ||
Meacham S, Karakas S, Wallace A, Altun F. Boron in human health: evidence for dietary recommendations and public policies. Open Miner Process J. 2010;3:36–53. | ||
Devirian TA, Volpe SL. The physiological effects of dietary boron. Crit Rev Food Sci Nutr. 2003;43(2):219–231. | ||
Rainey CJ, Nyquist LA, Coughlin JR, Downing RG. Dietary Boron Intake in the United States: CSFII 1994–1996. J Food Compos Anal. 2002;15(3):237–250. | ||
Pearle AD, Scanzello CR, George S, et al. Elevated high-sensitivity C-reactive protein levels are associated with local inflammatory findings in patients with osteoarthritis. Osteoarthritis Cartilage. 2007;15(5):516–523. | ||
Sarzi-Puttini P, Cimmino MA, Scarpa R, et al. Osteoarthritis: an overview of the disease and its treatment strategies. Semin Arthritis Rheum. 2005;35(1 Suppl 1):1–10. | ||
Creamer P, Lethbridge-Cejku M, Hochberg MC. Determinants of pain severity in knee osteoarthritis: effect of demographic and psychosocial variables using 3 pain measures. J Rheumatol. 1999;26(8):1785–1792. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.