Back to Journals » International Journal of Nanomedicine » Volume 9 » Issue 1
Short communication: selective cytotoxicity of curcumin on osteosarcoma cells compared to healthy osteoblasts
Authors Chang R, Sun L, Webster T
Received 6 October 2013
Accepted for publication 5 November 2013
Published 10 January 2014 Volume 2014:9(1) Pages 461—465
DOI https://doi.org/10.2147/IJN.S55505
Review by Single anonymous peer review
Peer reviewer comments 4
Run Chang,1 Linlin Sun,1 Thomas J Webster1,2
1Department of Chemical Engineering, Northeastern University, Boston, MA, USA; 2Center of Excellence for Advanced Materials Research, King Abdulaziz University, Jeddah, Saudi Arabia
Abstract: Curcumin is a natural phenolic compound extracted from the plant Curcuma longa L. In previous studies, curcumin has been shown to have anticancer, antioxidant, and anti-inflammatory effects. In this study, the cytotoxicity of different concentrations (5, 10, 25, 50, 75, and 100 µM) of curcumin dissolved in dimethyl sulfoxide was compared between MG-63 osteosarcoma and healthy human osteoblast cells. Consequently, the viability of osteosarcoma cells was less than 50% at a concentration of 10 µM compared to the control sample without curcumin, but healthy osteoblast cells had at least 80% viability throughout all the concentrations tested. The results demonstrated that MG-63 osteosarcoma cells were much more sensitive in terms of cytotoxicity to curcumin, while the healthy human osteoblasts exhibited a higher healthy viability after 24 hours of curcumin treatment. Therefore, this study showed that at the right concentrations (5 µM to 25 µM), curcumin, along with a proper nanoparticle drug delivery carrier, may selectively kill bone cancer cells over healthy bone cells.
Keywords: curcumin, osteosarcoma, human osteoblast, viability, bone cancer
A Letter to the Editor has been received and published for this article.
Introduction
Osteosarcoma is the most frequent primary bone cancer today, and exhibits cancerous osteoblastic differentiation and malignant osteoid.1 It originally arises from primitive transformed mesenchymal cells, and mostly occurs at the end of long bones, such as the knee, hip, and shoulder. There are about 800 cases of osteosarcoma each year in the United States. The survival rate of patients highly affected by osteosarcoma has been 60% to 75% for over a decade.2 At present, the most commonly used therapy for osteosarcoma is a treatment cycle that consists of preoperative chemotherapy, a tumor amputation or limb salvage procedure, and postoperative chemotherapy. However, the drugs used for chemotherapy (such as doxorubicin and cisplatin) induce high toxicity on both tumorous and normal tissues, causing significant side effects such as anemia, neutropenia, thrombocytopenia, and heart damage, which may decrease the survival rate of osteosarcoma patients.3 It is clear that we need a new approach for the treatment of bone cancer, one in which osteosarcoma cells are killed selectively without affecting healthy bone cell viability.
Curcumin (or diferuloylmethane) is an orange-yellow phenolic compound extracted from the natural plant Curcuma longa L. In some previous studies, it has been shown to have significant anticancer, antioxidant, and anti-inflammatory effects.4 Curcumin has been shown to have an inhibitory effect on NF-κB. This is important because the activation of NF-κB in tumor cells is relatively higher than normal cells and is responsible for the development of carcinogenesis, such as antiapoptotic genes, metastasis, tumor promotion, and malignancy. However, as a result of the treatment of curcumin, NF-κB tends to maintain bonding with IκB (inhibitor of NF-κB), since curcumin hinders the phosphorylation and degradation of IκBα. Therefore, the inactivated NF-κB/IκB complex is kept in the cytoplasm, and is not able to enter the nucleus. Consequently, the carcinogenesis-related expression of genetic products of NF-κB, including cyclin D1, COX-2, and Bcl-2, is down-regulated by curcumin in various tumor cells.5 In fact, Zheng et al reported that curcumin could induce cell cycle arrest and apoptosis of melanoma cell lines A375 and MeWo in response to down-regulation of NF-κB and increased levels of the p53 tumor suppressor protein.6
In a previous study, Jin et al demonstrated that curcumin in different concentrations (5, 10, 25, 50, 75, and 100 μM) led to apoptosis (or programmed cell death) of U2OS osteosarcoma cells for different time periods (6, 12, 24, and 36 hours), showing that curcumin induces apoptosis of U2OS cells by a time- and concentration-dependent manner in vitro; also, the curcumin-treated cancer cells had higher expression of apoptosis-related proteins, including Bax, Bak, and cytochrome C, as well as a lower expression of anti-apoptosis proteins.7 In addition, curcumin also induced higher cytotoxicity of various types of brain tumor cells, while its toxicity was relatively much lower in human normal fibroblast cells.8
Some studies have demonstrated that curcumin might induce death of healthy osteoblast cells. For example, curcumin may lead to osteoblast apoptosis at low concentrations, up to 25 μM, and necrosis at high concentrations, up to 200 μM.9 However, few studies have compared the cytotoxicity of curcumin between osteosarcoma and healthy human osteoblast cell lines, or determined an exact concentration at which curcumin was toxic to osteosarcoma cells but not toxic to healthy osteoblasts. Such a finding would provide critical information to the field of a concentration of curcumin that should be delivered to bone tumors in order to kill cancer cells, but not affect healthy osteoblast functions. Thus, the purpose of this study was to evaluate if (and at what concentration) curcumin would cause a greater apoptotic effect on osteosarcoma cells than on normal osteoblast cells. Clearly, such advances would be paramount to allow curcumin to be used as a novel bone anticancer drug with minimal side effects.
Materials
Curcumin (or diferuloylmethane) powder and sterile-filtered fetal bovine serum were purchased from Sigma-Aldrich (St Louis, MO, USA). MG-63 osteosarcoma cells (ATCC-CRL-1427), Eagle’s Minimum Essential Medium, dimethyl sulfoxide (DMSO), and phosphate buffered saline were purchased from the American Type Culture Collection (Manassas, VA, USA). Human osteoblast cells and osteoblast medium (consisting of osteoblast growth medium and osteoblast growth medium supplementmix) were purchased from PromoCell GmbH (Heidelberg, Germany). An MTT dye solution was purchased from Promega (Madison, WI, USA).
Methods
Cell culture method
The human osteosarcoma cell line, MG-63, was cultured in Eagle’s Minimum Essential Medium, with 10% fetal bovine serum. Healthy osteoblasts were cultured in medium consisting of one bottle of Basal Osteoblast Growth Medium with one vial of SupplementMix and 1% of a penicillin/streptomycin solution. Cells were cultured at 37°C in a humidified incubator in an atmosphere of 95% oxygen and 5% carbon dioxide.
Preparation of curcumin stock solution
Curcumin powder was dissolved in DMSO to obtain a concentration of 100 mM, and then was stored at −20°C protected from light. Different concentrations (1, 2, 5, 10, 15, and 20 mM) of curcumin were prepared by diluting the stock solution with DMSO.
Cytotoxicity assays
Both MG-63 osteosarcoma cells and healthy human osteoblasts were seeded onto a 96-well plate separately at a density of 2 × 104 cells/cm2. After 24 hours of cell culture, 1.0 μL of each curcumin solution at various concentrations was added to both of the cell lines to obtain 200 μL of a cell solution with curcumin. Therefore, the final concentrations of curcumin were 5, 10, 25, 50, 75, and 100 μM. Samples free of the curcumin/DMSO solution served as control samples, and the 0 μM samples only contained 1.0 μL of DMSO. After this, the cells were cultured for 24 hours.
Initially, we tried to use a 10% volume ratio of DMSO (eg, 20 μL of DMSO in 200 μL of the final cell solution) with different concentrations of curcumin. However, the cell viability of osteosarcoma cells dramatically decreased for all samples with or without curcumin. Thus, in order to eliminate the interference of DMSO, a smaller amount was selected through experimentation (ie, observing no change in cell viability through using DMSO alone; data not shown) for the cytotoxicity assays.
MTT assay for cell viability measurements
Followed by a 24-hour curcumin treatment, the original medium in each well was carefully aspirated. After being washed with phosphate buffered saline two times, 100 μL of the cell culture solution and 15 μL of the MTT staining solution were added to each well. Then, 100 μL of the stop solution of the MTT dye solution was added after 4 hours. The final solution was stored in an incubator overnight. The 96-well plates were then tested using a spectrophotometer at a 570 nm wavelength to obtain the optical density.
Statistical analyses
The cell density was obtained from a standard curve which was constructed as a linear curve expressing the correlation between different cell densities and optical densities. Each experiment for each cell line was conducted three times, at eight samples for each group. Data are expressed as mean ± standard error of the mean and a two-tailed Student t-test was used to evaluate differences between means, with P<0.05 being considered statistically significant.
Results
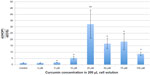
The results of the present study demonstrated that MG-63 osteosarcoma cells and healthy human osteoblasts responded differently to curcumin (Figures 1 and 2, respectively). To demonstrate this difference, the ratio between viable healthy human osteoblast to osteosarcoma cell density is shown in Figure 3. These results illustrated for the first time that curcumin induced greater cytotoxicity on MG-63 osteosarcoma cells than on healthy human osteoblasts in a dose-dependent manner. No statistical difference in healthy osteoblast densities was observed when exposed to 5 and 10 μM of curcumin, while osteosarcoma density decreased 0.6 and 5.3 times when exposed to 5 and 10 μM of curcumin compared to controls, respectively. Most significantly, the results from this study suggest that curcumin at a concentration of 25 μM decreased osteosarcoma over healthy osteoblast density the most. For the above reasons, it is suggested that curcumin concentrations between 5 and 25 μM should be further studied for various bone cancer applications.
Discussion
In this study, after 24 hours of curcumin treatment, MG-63 osteosarcoma cell densities decreased dramatically compared to control samples with cell medium only or with the same amount of DMSO only (ie, 0 μM); at curcumin concentrations from 25 μM to 100 μM, osteosarcoma cell densities demonstrated a minimum value. In contrast, healthy human osteoblast densities were much less sensitive to the curcumin treatment. For this reason, this study showed for the first time that curcumin may have greater selectivity for killing MG-63 osteosarcoma cells than healthy osteoblast cells, and thus may be a potential drug for bone cancer nanoparticle therapy with low toxicity to normal healthy bone cells.
Nevertheless, the main problem that impedes curcumin from being commonly used clinically today is its low solubility and high degradation rate in the body. Curcumin is insoluble in water in response to its polyphenol structure, but it is more soluble in ethanol, DMSO, or acetone. In addition, curcumin can exist in both an enolate and a bis-keto compound form depending on the environment. In acidic and neutral conditions and in solid phase, the keto form predominates, while the enolic form predominates in alkaline conditions.10 When pH >7.2, curcumin undergoes a degradation process, and almost completes its degradation to trans-6-(4′-hydroxy-3′-methoxyphenyl)-2,4-dioxo-5-hexanal, feruloylmethane, and ferulic acid in 30 minutes. During in vivo tests, very low amounts of curcumin were found in serum (eg, for 2 g/kg curcumin administrated orally to humans, only 0.006±0.005 μM was found in serum after 1 hour).11 As a result of metabolism by the liver, curcumin is likely to be degraded to curcumin glucuronide and curcumin sulfate, even if it is absorbed orally.12 Thus, it is necessary to find an effective drug delivery vehicle for curcumin in order to increase its water solubility, protect it from degradation, and release it onto target areas. Of course, nanoparticle curcumin drug carriers may provide an answer due to their ability to avoid immune system clearance and be functionalized to attach to cancer cell membranes; the next phase of this research is to incorporate 5 μM to 25 μM curcumin into various nanoparticle drug carriers to determine in vitro and in vivo efficacy.
In some cases, carriers with a hydrophobic inner area and water-soluble surface successfully loaded curcumin in a noncovalently manner. For instance, a PLGA-PEG-PLGA (poly[D,L-lac-tide-co-glycolide]-b-poly[ethylene-glycol]-b-poly[D,L-lactide-co-glycolide]) copolymer,13 self-assembled peptide hydrogel MAX814 and curcumin-phospholipid complexes15 were used for improving the efficacy of curcumin. Thus, the results of this study provided promise that such drug carriers could be loaded with curcumin, delivered to bone tumors and selectively kill osteosarcoma cells without harming healthy osteoblasts; at a curcumin concentration of 25 μM, osteosarcoma cell densities were 50% less than compared to the samples without curcumin.
In conclusion, here it is reported for the first time that curcumin induced higher cytotoxicity for MG-63 osteosarcoma cells than for human osteoblasts at 5 μM to 25 μM concentrations. In the future, amphiphilic curcumin drug nanoparticle carriers with a hydrophobic core and a hydrophilic outer surface will be developed and tested as a healthy manner to decrease bone tumors.
Acknowledgment
The authors would like to acknowledge Northeastern University for funding.
Disclosure
The authors report no conflicts of interest in this work.
References
Ritter J, Bielack SS. Osteosarcoma. Ann Oncol. 2010;21(7):vii320–vii325. | |
Chow W, Haglund K, Randall RL. Bone sarcomas [webpage on the Internet]. Cancer Network; 2013 [updated May 15, 2013]. Available from: http://www.cancernetwork.com/cancer-management/bone-sarcomas. Accessed November 20, 2013. | |
McTiernan A, Jinks RC, Sydes MR, et al. Presence of chemotherapy-induced toxicity predicts improved survival in patients with localised extremity osteosarcoma treated with doxorubicin and cisplatin: a report from the European Osteosarcoma Intergroup. Eur J Cancer. 2012;48(5):703–712. | |
Tennesen HH, Greenhill JV. Studies on curcumin and curcuminoids. XXII: curcumin as a reducing agent and as a radical scavenger. Int J Pharm. 1992;87(1–3):79–87. | |
Wilken R, Veena MS, Wang MB, Srivatsan ES. Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol Cancer. 2011;10:12. | |
Zheng M, Ekmekcioglu S, Walch ET, Tang CH, Grimm EA. Inhibition of nuclear factor-jB and nitric oxide by curcumin induces G2/M cell cycle arrest and apoptosis in human melanoma cells. Melanoma Res. 2004;14(3):165–171. | |
Jin S, Xu HG, Shen JN, Chen XW, Wang H, Zhou JG. Apoptotic effects of curcumin on human osteosarcoma U2OS cells. Orthop Surg. 2009;1(2):144–152. | |
Khaw AK, Hande MP, Kalthur G, Hande MP. Curcumin inhibits telomerase and induces telomere shortening and apoptosis in brain tumour cells. J Cell Biochem. 2013;114(6):1257–1270. | |
Chan WH, Wu HY, Chang WH. Dosage effects of curcumin on cell death types in a human osteoblast cell line. Food Chem Toxicol. 2006;44(8):1362–1371. | |
Strimpakos AS, Sharma RA. Curcumin: preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid Redox Signal. 2008;10(3):511–545. | |
Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2013;4(6):807–818. | |
Kumar A, Ahuja A, Ali J, Baboota S. Conundrum and therapeutic potential of curcumin in drug delivery. Crit Rev Ther Drug Carrier Syst. 2010;27(4):279–312. | |
Song Z, Feng R, Sun M, et al. Curcumin-loaded PLGA-PEG-PLGA triblock copolymeric micelles: Preparation, pharmacokinetics and distribution in vivo. J Colloid Interface Sci. 2011;354(1):116–123. | |
Altunbas A, Lee SJ, Rajasekaran SA, Schneider JP, Pochan DJ. Encapsulation of curcumin in self-assembling peptide hydrogels as injectable drug delivery vehicles. Biomaterials. 2011;32(25):5906–5914. | |
Maiti K, Mukherjee K, Gantait A, Saha BP, Mukherjee PK. Curcumin-phospholipid complex: Preparation, therapeutic evaluation and pharmacokinetic study in rats. Int J Pharm. 2007;330(1–2):155–163. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.