Back to Journals » Neuropsychiatric Disease and Treatment » Volume 17
Short- and Long-Term Effects of Vitamin D Treatment on Bacillus Calmette-Guerin-Induced Depressive-Like Behavior in Mice
Authors Saleh LA, Almutairi FM, Alorabi WK, Alkuhayli BA, Alzaidi SS, Alzahrani SB, Aljumayi FA, Abduljabbar MH, Alharthi AS, Alsufyani MA, Alhazmi MH, Althobaiti AA, Almutairi FN, Alshehri FS , Altowairqi E, Althobaiti YS
Received 7 December 2020
Accepted for publication 6 February 2021
Published 2 March 2021 Volume 2021:17 Pages 711—720
DOI https://doi.org/10.2147/NDT.S291793
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Lobna A Saleh,1– 3 Farooq M Almutairi,2,4 Wejdan K Alorabi,1 Bashayr A Alkuhayli,1 Shaden S Alzaidi,1 Shahad B Alzahrani,1 Futun A Aljumayi,1 Maram H Abduljabbar,1 Ayidh S Alharthi,1 Mashhour A Alsufyani,1 Mohammed H Alhazmi,1 Abdulbari A Althobaiti,1 Fahad N Almutairi,1 Fahad S Alshehri,5 Ebtehal Altowairqi,1 Yusuf S Althobaiti1,2,6
1Department of Pharmacology and Toxicology, College of Pharmacy, Taif University, Taif, 21944, Saudi Arabia; 2Addiction and Neuroscience Research Unit, College of Pharmacy, Taif University, Taif, Saudi Arabia; 3Department of Clinical Pharmacology, Faculty of Medicine, Ain Shams University, Cairo, Egypt; 4Department of Clinical Laboratories Sciences, College of Applied Medical Sciences, University of Hafar Al-Batin, Hafar Al-Batin, Saudi Arabia; 5Department of Pharmacology and Toxicology, College of Pharmacy, Umm Al-Qura University, Makkah, Saudi Arabia; 6General Administration for Precursors and Laboratories, General Directorate of Narcotics Control, Ministry of Interior, Riyadh, Saudi Arabia
Correspondence: Yusuf S Althobaiti
Department of Pharmacology and Toxicology, College of Pharmacy, Taif University, Health Science Campus, P.O. Box 11099, Airport Road, Al Haweiah, Taif, 21944, Saudi Arabia
Tel +966 545736200
Email [email protected]
Purpose: Depression is one of the most common psychological disorders. The nutritional etiology of the depression proposes that vitamin D may play a significant role in the pathogenesis of depression. Further, vitamin D deficiency has been found to aggravate depression in animals. Therefore, vitamin D treatment might be a potential therapeutic aid in depression management. This study aimed to explore the antidepressant effects of vitamin D in a Bacillus Calmette-Guerin (BCG)-induced depression model.
Methods: Thirty-six mice were randomly assigned to short-term and long-term experimental groups. In each group, mice were randomly subcategorized into three subgroups: 1. control (received vehicle), 2. BCG (received BCG [107 CFU/mouse]), and 3. BCG + vitamin D (received vitamin D [60.000 IU/kg] before BCG [107 CFU/mouse] inoculation). After completion of the two experimental periods (3 days for the short-term group and 2 weeks for the long-term group), the mice underwent three behavioral tests: locomotor activity, the forced swimming test (FST), and the tail suspension test (TST).
Results: Locomotor activity did not significantly differ among the subgroups in either the long-term or short-term groups. In the short-term group, the total immobility time on the FST was decreased in the vitamin D-treated group compared to the BCG group. However, in the TST, no significant difference was found between the vitamin D-treated group and the BCG group. In the long-term group, the immobility time on the FST was decreased in the vitamin D-treated group compared to the BCG group. Similarly, the total immobility time on the TST was also significantly lower in the vitamin D-treated mice than in the BCG-treated mice.
Conclusion: Vitamin D is useful in the management of depressive behavior. The potential role of vitamin D in the etiology of depression should be investigated in future work.
Keywords: depression, natural supplements, tail suspension test, forced swimming test
Introduction
Major depression (MD) is one of the most common psychiatric disorders affecting public health globally, and is reported to affect more than 264 million individuals worldwide.1 Anhedonia and a depressed mood are among the main characteristics of MD and negatively affect the quality of life of individuals with depression.2 Despite the significant social burden of this disease, the pathophysiological mechanisms underlying depression are not fully understood. Furthermore, the existing diagnostic techniques do not sufficiently reveal the significant alterations in neurobiology that conclude the behavioral changes in patients. Different hypotheses have been postulated to explain the etiology of depression. One of the most common hypotheses, the neurotransmitter hypothesis, suggests that low levels of brain neurotransmitters, including serotonin, norepinephrine, and dopamine, can cause depression.3–6 Accordingly, several drugs have been developed to modulate the levels of these neurotransmitters.6–8 However, one in three patients with depression do not respond to conventional treatments.9 Another hypothesis suggests that the hypothalamic-pituitary-adrenal axis can be involved via the stress-induced release of corticotropin-releasing hormone and the subsequent release of adrenal cortisol.10–14 Additionally, several brain-derived neurotrophic factors have been reported to be reduced in various brain regions in patients with depression.15–17 Since the early 1990s, accumulating data on the pathophysiology of depression have shown that inflammation is an important factor in the development of depression. Patients with MD were found to have increased inflammatory markers in the blood; such increases were associated with poorer outcomes of antidepressant treatment.18
Recent studies have suggested that low levels of vitamins such as vitamin D, vitamin B6, vitamin B12, and other essential elements may play a role in the pathogenesis of depression.19,20 Indeed, vitamin D deficiency is reported to negatively affect overall body health, muscle function, bone strength, and mental state.21 Moreover, meta-analyses and reviews have indicated that MD may be associated with decreased vitamin D levels.22–24 Moreover taking a vitamin D supplement was shown to be beneficial and reduced some biomarkers of inflammation and oxidative stress.25
Vitamin D is a steroid that has many important functions in the central nerve system (CNS), such as in brain development, neuroimmunomodulation, neuroprotection, synaptic plasticity and neurotransmission.26 The metabolites of vitamin D have the ability to protect neural integrity in brain areas (such as prefrontal cortex, amygdala and hippocampus) involved in mood regulation and the stress response.26,27 Nevertheless, some studies have shown that vitamin D is involved in neuroinflammatory pathway modulation, and a malfunction in these pathways is linked to depression and an altered stress response.27,28 It is important to note that vitamin D has been shown to modulate the hypothalamic-pituitary-adrenal axis and regulate several neurotransmitters via its activity on vitamin D receptors (VDRs) in the adrenal cortex.29 This vitamin has been reported to be neuroprotective and prevent dopamine and serotonin depletion.30 Recent studies have also found that vitamin D might be beneficial in patients with depression.31,32
Depressive-like behaviors can be induced in laboratory animals through different approaches. One of these methods is Bacillus Calmette-Guerin (BCG) inoculation in mice.33,34 This inoculation has been found to induce long-term depressive-like behavior for up to 3 weeks after inoculation, that is, ahead of the period during which signs of acute phase sickness-like behavior appear.33 This depression model has several advantages: ease of application, low cost, and requiring little effort to train animals. BCG-induced changes occur at numerous levels, including through neurochemical alterations in the brain, neuroimmune function, and neuroendocrine and behavioral changes, which resemble the MD pathophysiology in humans.20 However, the effects of vitamin D were not investigated in BCG-induced depression in mice.Therefore, this study aimed to explore the short- and long-term antidepressant effects of vitamin D in a BCG-induced depression model. We hypothesized that vitamin D would prevent BCG-induced depressive behavior in both short- and long-term experiments.
Methods
Animals
A total of 36 male albino mice (25–30 g) were housed in collective cages (six animals per cage). All animals were kept under a standard 12-h light/dark cycle in a temperature-controlled (22±2°C) environment with ad libitum access to rodent chow. All efforts were made to minimize unnecessary stress to the animals. Before starting the experiment, all of the mice were handled daily for at least 1 week for acclimatization purposes and to minimize stress reactions related to manipulation. All experiments were conducted following the procedures and guidelines set by the Animal Unit Committee and Biomedical Ethics at Taif University and in accordance with the Institutional Animal Care and Use Committee of the National Institutes of Health. The study was approved by the Animal Unit Committee and Biomedical Ethics at Taif University.
Induction of Depressive-Like Behavior
BCG was obtained from the Veterinary Serum and Vaccine Research Institute, Bacterial Diagnostic Product Research Department (Cairo, Egypt). The dose of BCG (107 CFU/mouse) was selected based on its ability to induce depression-like symptoms. BCG was administered intraperitoneally (IP) at a volume of 0.2 mL/mouse.33 BCG was diluted in Sauton’s synthetic liquid medium used to grow Mycobacterium bovis (BCG) on the day of inoculation. Mice in the control group received the same amount of vehicle.
Drug and Administration
Vitamin D3 (cholecalciferol) was purchased in a commercially available injectable form (Drevarol®) from Memphis Pharmaceuticals and Chemical Industries (Cairo, Egypt) dissolved in propylene (glycol: ethanol 90:10) and subsequently administered to mice as a single dose of 60.000 IU/kg by intraperitoneal injection.35
Experimental Protocol
Mice were randomly divided into short- and long-term treatment groups. In each treatment group, the mice were subdivided into three subgroups (each n = 6): A) control group, which received Sauton’s synthetic liquid medium (IP) once; B) BCG group, which received the BCG vaccine (0.2 mL/mouse, IP) once; and C) BCG + vitamin D group, which received vitamin D (60.000 IU/kg) via a single intraperitoneal injection immediately before BCG inoculation.
Group 1
Short-term study (behavioral testing was performed on two consecutive days, 3 days after BCG inoculation). The test for locomotor activity and the forced swimming test (FST) was conducted on the first day, and the tail suspension test (TST) was conducted on the second day, as shown in Figure 1.
 |
Figure 1 Flow diagram of the study schedule. |
Group 2
A long-term study (behavioral testing was performed on two consecutive days, 2 weeks after BCG inoculation). Tests for locomotor activity and the FST were conducted on the first day (14 days following BCG inoculation), and the TST was conducted on the second day (15 days following BCG inoculation), as shown in Figure 1.
Behavioral Testing
All behavioral experiments were performed between 8 am and 2 pm, under dim light conditions and low noise, as described in the report by Moreau et al in 2005.20 Behavior was monitored via a video camera and videotaped to be scored later by a trained observer. Each mouse underwent locomotor activity testing, the FST, and the TST. Each test was performed only once for each animal, as previously reported.36
Assessment of Locomotor Activity
The motor effects of BCG and vitamin D were assessed as previously described by O’Connor et al in 2009.37 Briefly, the motor activity of each mouse was assessed in a clean cage over a 5-min period. The numbers of line crossings (horizontal movement) and rearing (vertical movement) by all four limbs were counted.
FST
The FST was performed as previously described.36,37 Each mouse was placed in a transparent cylindrical glass beaker filled with water for 6 min. A mouse was judged to be immobile when it floated in an upright position and made only small movements to keep its head above water. The swimming time criterion was strong movements of all four limbs, jumping, struggling, thrashing, and climbing on the glass cylinder wall. The duration of immobility was evaluated during the final 5 min of the test. Each mouse was tested once.36 After the test, mice were dried with a towel and placed in cages.
TST
The TST was conducted as described by Steru et al in 1985.38 This method is based on observation of the mouse’s agitation and immobility when it is suspended by the tail for 6 min. To avoid observer bias, two trained, blinded observers manually recorded the immobility duration during the final 5 min interval of the test. The mouse was considered immobile only when it hung passively and completely motionless.38
Statistical Analysis
A one-way analysis of variance (ANOVA), followed by Tukey’s multiple comparison test, was used to analyze the behavioral data. GraphPad Prism-9 was used to perform all statistical analyses in this study, with P<0.05 indicating statistical significance. All data are expressed as the mean ± standard error of the mean.
Results
Locomotor Activity
In the short-term group, locomotor activity did not significantly differ among the subgroups in terms of the number of lines crossed (F (2, 15) = 0.2704, P=0.7667) (Figure 2A), or in the number of rearing episodes (F (2, 15) = 0.3992, P=0.6778) (Figure 2B).
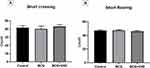 |
Figure 2 Crossing and rearing results in the short-term group. Number of (A) limb crossings and (B) rearing episodes in the short-term group. Data are expressed as the mean ± SEM. |
Similarly, in the long-term group, locomotor activity did not significantly differ among the subgroups in terms of the number of lines crossed (F (2, 15) = 0.8055, P=0.4653) (Figure 3A) or in the number of rearing episodes (F (2, 15) = 1.812, P=0.1973) (Figure 3B).
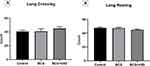 |
Figure 3 Crossing and rearing results in the long-term group. Number of (A) limb crossings and (B) rearing episodes in the long-term group. Data are expressed as the mean ± SEM. |
Short-Term Effect of Vitamin D on Depressive-Like Behavior in BCG-Inoculated Mice
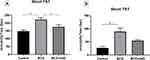
The short-term effects of vitamin D and BCG on the immobility time on the FST are shown in Figure 4A. The one-way ANOVA revealed a significant effect of vitamin D and BCG on the total immobility time on the FST (F (2, 13) = 9.787, P=0.0026). A significant increase in the total immobility time was found in the BCG group relative to the control group (P=0.0019). Further, the immobility time was significantly lower in the BCG + vitamin D group than in the BCG group (P=0.0474). No significant difference in immobility time was found between the control and vitamin D-treated groups.
The short-term effects of vitamin D and BCG on the immobility time on the TST are presented in Figure 4B. The one-way ANOVA revealed a significant effect of vitamin D and BCG on the total immobility time on the TST (F (2, 6) = 8.811, P=0.0164). Tukey’s multiple comparisons test indicated a significant increase in the total immobility time in the BCG group compared to the control group (P=0.0139). However, no significant difference was noted between the BCG + vitamin D group and the BCG group (P=0.1873). In addition, the BCG + vitamin D group and the control group did not significantly differ in terms of immobility time (P=0.3278).
Long-Term Effect of Vitamin D on Depressive-Like Behavior in BCG-Inoculated Mice
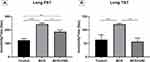
The long-term effects of vitamin D and BCG on the immobility time on the FST are shown in Figure 5A. The one-way ANOVA revealed a significant effect of vitamin D and BCG on the total immobility time on the FST (F (2, 16) = 23.50, P<0.0001). A significant increase was observed in the total immobility time in the BCG group compared to the control group (P<0.0001). The immobility time was significantly lower in the BCG + vitamin D group than in the BCG group (P=0.0123). Further, the immobility time was increased in the control group compared to the BCG + vitamin D group.
The long-term effects of vitamin D and BCG on the immobility time on the TST are shown in Figure 5B. The one-way ANOVA revealed a significant effect of vitamin D and BCG on the total immobility time on the TST (F (2, 15) = 10.20, P=0.0016). Tukey’s multiple comparisons test showed a significant increase in the total immobility time in the BCG group compared to the control group (P=0.0091). A significant reduction in the immobility time was observed in the BCG + vitamin D group compared to the BCG group (P=0.0025). However, the immobility time did not significantly differ between the BCG + vitamin D group and the control group (P=0.9032).
Discussion
This study investigated the effects of vitamin D on depressive behavior after inoculation with BCG. BCG inoculation was used to induce chronic activation of the immune system to mimic depression-like behavior in animals,33,37 since neuroinflammation has shown to be a risk factor in the development of depression. Chronic depressive-like behavior in this model was preceded by acute sickness-like behavior that persisted for approximately 2 days. This was evidenced by reduced motor activity and the significantly lower number of crossings and rearings in the activity cage. This effect was followed by a long-term depressive-like effect, during which signs of depression appeared. Male rather than female mice were selected in this study because estrogen has been shown to have antidepressant-like effects in animal paradigms of depression, resulting in an advantageous action in female rodents.39–41 Besides, estradiol replacement in ovariectomized females decreases depression-like behavior.39,40 Variations in endogenous levels of estrogen can change depressive behavior in rodents.42 Furthermore, rodents display reduced depressive behavior during the late proestrus phase of the estrous cycle, when estrogen levels are elevated. Fluctuations in estrogen levels also appear to be concomitant with changes in progestin and androgen.42 Collectively, these variations in hormone levels might influence the antidepressant activity of vitamin D.
Two behavioral tests, the FST and the TST, were used to assess despair aspect of depressive-like behavior. Although these behavioral tests are similar in terms of the construct, they differ according to the biological substrates that underly the behavior.36–38,43 A single vitamin D dose (60.000 IU/kg/day) ameliorated the depressant effects of BCG, as demonstrated in both the FST and TST. The immobility times on the FST and TST were reduced in the BCG + vitamin D group compared to the BCG group (Figures 4 and 5). However, there was no significant difference in the short-term TST results between the BCG + vitamin D group and the BCG group (Figure 4B), although there was a trend towards a decrease in immobility time in the BCG + vitamin D group. This could be due to differences in sensitivity between the FST and TST. Several studies have reported that many factors could cause such variations, including water temperature, genetic differences, and level of aggression in mice.44–46 When comparing the TST and FST, implementation of the experimental procedure significantly differed, thus affecting the results; the results of the TST may not be reproducible in the FST. Therefore, we chose to perform both the FST as well as TST, since they have been validated and widely used. This dose was selected in accordance with the work of Aygun et al, who demonstrated the anxiolytic-antidepressive effect of a bolus dose of vitamin D (60.000 IU/kg) in WAG/Rij rats (a model of absence epilepsy with comorbidity of depression), which is not significantly different from the effects induced by chronic daily doses.35 Our findings regarding depressive-like behavior are in line with those of a previous study by Camargo et al, which revealed that a 100 IU/kg/day dose of vitamin D3 in the week after chronic corticosterone administration reduced depressive-like behavior in mice.47 However, while vitamin D effectively reduced the immobility time in the long-term FST (Figure 5B), it did not restore the immobility time to normal level in the control group. In this study, we used a single pretreatment injection of vitamin D before BCG inoculation, which may not fully protect against the depressant effects of BCG. The use of a higher dose of vitamin D or multiple doses may achieve this, and further research is warranted.
Interestingly, vitamin D signaling in the hippocampus of depressed rats was found to protect from the deteriorating effects of stress as a compensatory mechanism during uncontrolled chronic mild stress.48 Moreover, vitamin D has been shown to improve ‘anhedonia-like symptoms’, which was possibly mediated by restoring the expression of dopamine transporters in the nucleus accumbens in rats subjected to depression-like symptoms by chronic mild stress.49
In the current study, locomotor activity did not significantly differ among the subgroups. We also found no significant difference in locomotor activity between the short- and long-term groups (Figures 2 and 3), thus excluding any influence of vitamin D or BCG on the locomotor system.
Several reports have found that vitamin D plays an essential role in brain development and neuritogenesis.50–53 Vitamin D is also involved in cognitive function and mental health.54,55 Previous reports have found that decreased plasma levels of vitamin D are linked to mood disorders56,57 and depression.58–62 Accordingly, the involvement of vitamin D in depression has been explored in several studies. Indeed, calcitriol, the bioactive form of vitamin D, plays an essential role in the brain by activating VDRs and hydroxylases in many brain areas50,63 such as the amygdala, where behavior and emotions are controlled.64 Other studies have reported that vitamin D has a neuroprotective function through different mechanisms, such as regulating the neuronal calcium concentration, which reduces the neuronal toxicity caused by excess calcium.52,65,66 Vitamin D has also been reported to enhance glutathione antioxidant activity in neurons, protecting them from oxidation processes.58,66 Furthermore, vitamin D is known to play a role in the synthesis of neurotransmitters involved in mood regulation, such as dopamine, serotonin, and norepinephrine, by regulating the gene expression of tyrosine hydroxylase in the biosynthesis of these neurotransmitters.67
Several studies have found that vitamin D possesses protective properties against reactive oxygen species (ROS) and glutamate neurotoxicity.68 Similarly, fluoxetine, an antidepressant compound, has shown protective properties against ROS and nitrate levels in a corticosterone depression model in mice.47,69,70 Other reports have shown that vitamin D could potentially produce an antidepressant activity by modulating inducible nitric oxide synthase expression in the brain.71
Amassing data related to mood and behavioral disorders have shown that there is a critical interplay between the immune system and alterations in brain circuits. The immune responses to many stressful stimuli start with the prompt activation of astrocytes and microglial cells and the release of inflammatory cytokines, such as prostaglandin E2, tumor necrosis factor and interleukin-1β in the CNS. These inflammatory markers are elevated in patients with MD and related to the duration and severity of the mood disorder, as shown in some clinical studies.72 Observations from such studies suggested that inhibiting the release of inflammatory cytokines can be a therapeutic approach to treat depressive symptoms. This was supported by Wang and colleagues,73 who found that vitamin D could suppress the Akt/NF-κB/COX-2 pathway by inhibiting macrophage-mediated inflammatory processes. These observations indicate that depression can be treated by targeting the neuroinflammatory system, and can aid in developing new therapeutic strategies in future research in this filed.
One limitation of the present study is that the findings are not supported by biochemical testing (eg plasma levels of vitamin D, confirmation of the stimulation of the vitamin D and VDR system in brain tissue, and inflammatory and oxidative markers). Therefore, future studies should investigate potential fluctuations in these parameters in BCG-induced mouse models of depression. Another limitation of this study is the lack of a positive control group (vitamin D alone), which could demonstrate the effect of using the same vitamin D dose in non-BCG-induced mice. In addition, standard antidepressant drugs, such as fluoxetine, a selective serotonin reuptake inhibitor or Imipramine, a tricyclic antidepressant were not used as a positive control in our study. Future studies should utilize vitamin D along with positive controls to confirm the role of vitamin D in depression. Furthermore, the toxicity of vitamin D treatment on morphological and biochemical parameters in the kidneys, liver, and plasma of mice treated with BCG was not evaluated.
Conclusion
The results of this study demonstrate the important role of vitamin D in the management of depressive behavior. Short- and long-term effects of vitamin D and BCG on the immobility time on the FST and TST were observed in this study. We observed significant BCG-induced depressive effects, represented by increases in the immobility time on the FST and TST. Vitamin D was able to reverse the depression-like effects of BCG and considerably reduce immobility time on the FST and TST. Vitamin D might be a good therapeutic option in patients with depression, and further studies are needed to explore its therapeutic potential on this disease.
Abbreviations
MD, major depression; VDRs, vitamin D receptors; BCG, Bacillus Calmette-Guerin; IP, intraperitoneally; FST, forced swimming test; TST, tail suspension test; ANOVA, analysis of variance; ROS, reactive oxygen species; CNS, central nervous system.
Acknowledgments
YSA was supported by Taif University Researchers Supporting Project number (TURSP-2020/78), Taif University, Taif, Saudi Arabia.
Disclosure
The authors declare no conflicts of interest for this work.
References
1. da Silva Souza SV, da Rosa PB, Neis VB, Moreira JD, Rodrigues ALS, Moretti M. Effects of cholecalciferol on behavior and production of reactive oxygen species in female mice subjected to corticosterone-induced model of depression. Naunyn Schmiedebergs Arch Pharmacol. 2020;393(1):111–120. doi:10.1007/s00210-019-01714-2
2. Jiang C, Salton SR. Chapter 7 - Neuroprotective roles of neurotrophic growth factors in mood disorders. In: Gozes I, Levine J, editors. Neuroprotection in Autism, Schizophrenia and Alzheimer’s Disease. Academic Press; 2019:145–172.
3. Meltzer HY. Role of serotonin in depression. Ann N Y Acad Sci. 1990;600:
4. Delgado PL, Moreno FA. Role of norepinephrine in depression. J Clin Psychiatry. 2000;61(Suppl 1):5–12.
5. Nutt DJ. Relationship of neurotransmitters to the symptoms of major depressive disorder. J Clin Psychiatry. 2008;69(Suppl E1):4–7.
6. D’Aquila PS, Collu M, Gessa GL, Serra G. The role of dopamine in the mechanism of action of antidepressant drugs. Eur J Pharmacol. 2000;405(1–3):365–373. doi:10.1016/S0014-2999(00)00566-5
7. Andersen J, Kristensen AS, Bang-Andersen B, Stromgaard K. Recent advances in the understanding of the interaction of antidepressant drugs with serotonin and norepinephrine transporters. Chem Commun (Camb). 2009;(25):3677–3692. doi:10.1039/b903035m
8. Lucki I, O’Leary OF. Distinguishing roles for norepinephrine and serotonin in the behavioral effects of antidepressant drugs. J Clin Psychiatry. 2004;65(Suppl 4):11–24.
9. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905–1917. doi:10.1176/ajp.2006.163.11.1905
10. Heuser I, Yassouridis A, Holsboer F. The combined dexamethasone/CRH test: a refined laboratory test for psychiatric disorders. J Psychiatr Res. 1994;28(4):341–356. doi:10.1016/0022-3956(94)90017-5
11. Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23(5):477–501. doi:10.1016/S0893-133X(00)00159-7
12. McQuade R, Young AH. Future therapeutic targets in mood disorders: the glucocorticoid receptor. Br J Psychiatry. 2000;177(5):390–395. doi:10.1192/bjp.177.5.390
13. Nemeroff CB. The corticotropin-releasing factor (CRF) hypothesis of depression: new findings and new directions. Mol Psychiatry. 1996;1(4):336–342.
14. Pariante CM, Miller AH. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol Psychiatry. 2001;49(5):391–404. doi:10.1016/S0006-3223(00)01088-X
15. Karege F, Vaudan G, Schwald M, Perroud N, La Harpe R. Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. Brain Res Mol Brain Res. 2005;136(1–2):29–37. doi:10.1016/j.molbrainres.2004.12.020
16. Dwivedi Y, Rao JS, Rizavi HS, et al. Abnormal expression and functional characteristics of cyclic adenosine monophosphate response element binding protein in postmortem brain of suicide subjects. Arch Gen Psychiatry. 2003;60(3):273–282. doi:10.1001/archpsyc.60.3.273
17. Chen B, Dowlatshahi D, MacQueen GM, Wang JF, Young LT. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol Psychiatry. 2001;50(4):260–265. doi:10.1016/S0006-3223(01)01083-6
18. Majd M, Saunders EF, Engeland CGJ. Inflammation and the dimensions of depression: a review. Frontiers in Neuroendocrinology. 2020;56:100800. doi:10.1016/j.yfrne.2019.100800
19. Rosa PB, Ribeiro CM, Bettio LE, et al. Folic acid prevents depressive-like behavior induced by chronic corticosterone treatment in mice. Pharmacol Biochem Behav. 2014;127:1–6. doi:10.1016/j.pbb.2014.10.003
20. Moreau M, Lestage J, Verrier D, et al. Bacille Calmette-Guerin inoculation induces chronic activation of peripheral and brain indoleamine 2,3-dioxygenase in mice. J Infect Dis. 2005;192(3):537–544. doi:10.1086/431603
21. Holick MF. The vitamin D epidemic and its health consequences. J Nutr. 2005;135(11):2739S–2748S. doi:10.1093/jn/135.11.2739S
22. Shi H, Wang B, Xu X. Antidepressant effect of vitamin D: a literature review. Neuropsychiatry. 2017;7(4):337–341.
23. Anglin RE, Samaan Z, Walter SD, McDonald SD. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry. 2013;202:100–107. doi:10.1192/bjp.bp.111.106666
24. Annweiler C, Rastmanesh R, Richard-Devantoy S, Beauchet O. The role of vitamin D in depression: from a curious idea to a therapeutic option. J Clin Psychiatry. 2013;74(11):1121–1122. doi:10.4088/JCP.13ac08783
25. Jamilian H, Amirani E, Milajerdi A, et al. The effects of vitamin D supplementation on mental health, and biomarkers of inflammation and oxidative stress in patients with psychiatric disorders: a systematic review and meta-analysis of randomized controlled trials. Prog Psychopharmacol Biol Psychiatry. 2019;94:109651. doi:10.1016/j.pnpbp.2019.109651
26. Groves NJ, McGrath JJ, Burne TH. Vitamin D as a neurosteroid affecting the developing and adult brain. Annu Rev Nutr. 2014;34:117–141. doi:10.1146/annurev-nutr-071813-105557
27. Wimalawansa SJJB. Vitamin D deficiency. N Engl J Med. 2019;8(2):30.
28. Berk M, Williams LJ, Jacka FN, et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013;11(1):200.
29. Puchacz E, Stumpf WE, Stachowiak EK, Stachowiak MK. Vitamin D increases expression of the tyrosine hydroxylase gene in adrenal medullary cells. Brain Res Mol Brain Res. 1996;36(1):193–196. doi:10.1016/0169-328X(95)00314-I
30. Cass WA, Smith MP, Peters LE. Calcitriol protects against the dopamine- and serotonin-depleting effects of neurotoxic doses of methamphetamine. Ann N Y Acad Sci. 2006;1074(1):261–271. doi:10.1196/annals.1369.023
31. Sepehrmanesh Z, Kolahdooz F, Abedi F, et al. Vitamin D supplementation affects the beck depression inventory, insulin resistance, and biomarkers of oxidative stress in patients with major depressive disorder: a randomized, controlled clinical trial. J Nutr. 2016;146(2):243–248. doi:10.3945/jn.115.218883
32. Khoraminya N, Tehrani-Doost M, Jazayeri S, Hosseini A, Djazayery A. Therapeutic effects of vitamin D as adjunctive therapy to fluoxetine in patients with major depressive disorder. Aust Nz J Psychiat. 2013;47(3):271–275. doi:10.1177/0004867412465022
33. Moreau M, Andre C, O’Connor JC, et al. Inoculation of Bacillus Calmette-Guerin to mice induces an acute episode of sickness behavior followed by chronic depressive-like behavior. Brain Behav Immun. 2008;22(7):1087–1095. doi:10.1016/j.bbi.2008.04.001
34. Kelley KW, O’Connor JC, Lawson MA, Dantzer R, Rodriguez-Zas SL, McCusker RH. Aging leads to prolonged duration of inflammation-induced depression-like behavior caused by Bacillus Calmette-Guerin. Brain Behav Immun. 2013;32:63–69. doi:10.1016/j.bbi.2013.02.003
35. Aygun H, Ayyildiz M, Agar E. Effects of vitamin D and paricalcitol on epileptogenesis and behavioral properties of WAG/Rij rats with absence epilepsy. Epilepsy Res. 2019;157:106208. doi:10.1016/j.eplepsyres.2019.106208
36. Porsolt RD. Animal models of depression: utility for transgenic research. Rev Neurosci. 2000;11(1):53–58. doi:10.1515/REVNEURO.2000.11.1.53
37. O’Connor JC, Lawson MA, Andre C, et al. Induction of IDO by bacille Calmette-Guerin is responsible for development of murine depressive-like behavior. J Immunol. 2009;182(5):3202–3212. doi:10.4049/jimmunol.0802722
38. Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl). 1985;85(3):367–370. doi:10.1007/BF00428203
39. Fedotova J. Effects of mild, moderate and severe stress on depression in female rats: modifications by estrous cycle, ovariectomy and estradiol treatment. Biol Psychiat Psychopharmacol. 2006;8:45–47.
40. Rachman IM, Unnerstall JR, Pfaff DW, Cohen RS. Estrogen alters behavior and forebrain c-fos expression in ovariectomized rats subjected to the forced swim test. Proc Natl Acad Sci U S A. 1998;95(23):13941–13946. doi:10.1073/pnas.95.23.13941
41. Rocha BA, Fleischer R, Schaeffer JM, Rohrer SP, Hickey GJJP. 17β-estradiol-induced antidepressant-like effect in the forced swim test is absent in estrogen receptor-β knockout (BERKO) mice. Psychopharmacology. 2005;179(3):637–643. doi:10.1007/s00213-004-2078-1
42. Walf AA, Frye CAJN. A review and update of mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology. 2006;31(6):1097–1111. doi:10.1038/sj.npp.1301067
43. Saleh LA, Hamza M, El Gayar NH, Abd El-Samad AA, Nasr EA, Masoud SI. Ibuprofen suppresses depressive like behavior induced by BCG inoculation in mice: role of nitric oxide and prostaglandin. Pharmacol Biochem Behav. 2014;125:29–39. doi:10.1016/j.pbb.2014.07.013
44. Thierry B, Steru L, Simon P, Porsolt RJP. The tail suspension test: ethical considerations. Psychopharmacology. 1986;90(2):284–285. doi:10.1007/BF00181261
45. Can A, Dao DT, Terrillion CE, Piantadosi SC, Bhat S, Gould TDJ. The tail suspension test. Jove. 2012;(59).
46. Petit-Demouliere B, Chenu F, Bourin MJP. Forced swimming test in mice: a review of antidepressant activity. Psychopharmacology. 2005;177(3):245–255. doi:10.1007/s00213-004-2048-7
47. Camargo A, Dalmagro AP, Rikel L, da Silva EB, Simao da Silva KAB, Zeni ALB. Cholecalciferol counteracts depressive-like behavior and oxidative stress induced by repeated corticosterone treatment in mice. Eur J Pharmacol. 2018;833:451–461. doi:10.1016/j.ejphar.2018.07.002
48. Jiang P, Zhang WY, Li HD, Cai HL, Liu YP, Chen LY. Stress and vitamin D: altered vitamin D metabolism in both the hippocampus and myocardium of chronic unpredictable mild stress exposed rats. Psychoneuroendocrinology. 2013;38(10):2091–2098. doi:10.1016/j.psyneuen.2013.03.017
49. Sedaghat K, Yousefian Z, Vafaei AA, et al. Mesolimbic dopamine system and its modulation by vitamin D in a chronic mild stress model of depression in the rat. Behavioural Brain Research. 2019;356:156–169. doi:10.1016/j.bbr.2018.08.020
50. Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1α-hydroxylase in human brain. J Chem Neuroanat. 2005;29(1):21–30. doi:10.1016/j.jchemneu.2004.08.006
51. Almeras L, Eyles D, Benech P, et al. Developmental vitamin D deficiency alters brain protein expression in the adult rat: implications for neuropsychiatric disorders. Proteomics. 2007;7(5):769–780. doi:10.1002/pmic.200600392
52. Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D. New clues about vitamin D functions in the nervous system. Trends Endocrin Met. 2002;13(3):100–105. doi:10.1016/S1043-2760(01)00547-1
53. Brown J, Bianco JI, McGrath JJ, Eyles DW. 1,25-dihydroxyvitamin D3 induces nerve growth factor, promotes neurite outgrowth and inhibits mitosis in embryonic rat hippocampal neurons. Neurosci Lett. 2003;343(2):139–143. doi:10.1016/S0304-3940(03)00303-3
54. Oudshoorn C, Mattace-Raso FU, van der Velde N, Colin EM, van der Cammen TJ. Higher serum vitamin D3 levels are associated with better cognitive test performance in patients with Alzheimer’s disease. Dement Geriatr Cogn Disord. 2008;25(6):539–543. doi:10.1159/000134382
55. Kalueff A, Minasyan A, Keisala T, Kuuslahti M, Miettinen S, Tuohimaa P. The vitamin D neuroendocrine system as a target for novel neurotropic drugs. CNS Neurol Disord Drug Targets. 2006;5(3):363–371.
56. Wilkins CH, Sheline YI, Roe CM, Birge SJ, Morris JC. Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. Am J Geriatr Psychiatr. 2006;14(12):1032–1040. doi:10.1097/01.JGP.0000240986.74642.7c
57. Przybelski RJ, Binkley NC. Is vitamin D important for preserving cognition? A positive correlation of serum 25-hydroxyvitamin D concentration with cognitive function. Arch Biochem Biophys. 2007;460(2):202–205. doi:10.1016/j.abb.2006.12.018
58. Jorde R, Waterloo K, Saleh F, Haug E, Svartberg J. Neuropsychological function in relation to serum parathyroid hormone and serum 25-hydroxyvitamin D levels. The Tromso study. J Neurol. 2006;253(4):464–470. doi:10.1007/s00415-005-0027-5
59. Schneider B, Weber B, Frensch A, Stein J, Fritz J. Vitamin D in schizophrenia, major depression and alcoholism. J Neural Transm (Vienna). 2000;107(7):839–842. doi:10.1007/s007020070063
60. Hoogendijk WJ, Lips P, Dik MG, Deeg DJ, Beekman AT, Penninx BW. Depression is associated with decreased 25-hydroxyvitamin D and increased parathyroid hormone levels in older adults. Arch Gen Psychiatry. 2008;65(5):508–512. doi:10.1001/archpsyc.65.5.508
61. Jorde R, Sneve M, Figenschau Y, Svartberg J, Waterloo K. Effects of vitamin D supplementation on symptoms of depression in overweight and obese subjects: randomized double blind trial. J Intern Med. 2008;264(6):599–609. doi:10.1111/j.1365-2796.2008.02008.x
62. Groves NJ, Zhou M, Jhaveri DJ, McGrath JJ, Burne THJP. Adult vitamin D deficiency exacerbates impairments caused by social stress in BALB/c and C57BL/6 mice. Psychoneuroendocrinology. 2017;86:53–63. doi:10.1016/j.psyneuen.2017.09.003
63. Prufer K, Veenstra TD, Jirikowski GF, Kumar R. Distribution of 1,25-dihydroxyvitamin D3 receptor immunoreactivity in the rat brain and spinal cord. J Chem Neuroanat. 1999;16(2):135–145. doi:10.1016/S0891-0618(99)00002-2
64. Walbert T, Jirikowski GF, Prufer K. Distribution of 1,25-dihydroxyvitamin D3 receptor immunoreactivity in the limbic system of the rat. Horm Metab Res. 2001;33(9):525–531. doi:10.1055/s-2001-17210
65. Kalueff AV, Eremin KO, Tuohimaa P. Mechanisms of neuroprotective action of vitamin D(3). Biochemistry (Mosc). 2004;69(7):738–741. doi:10.1023/B:BIRY.0000040196.65686.2f
66. Shinpo K, Kikuchi S, Sasaki H, Moriwaka F, Tashiro K. Effect of 1, 25‐dihydroxyvitamin D3 on cultured mesencephalic dopaminergic neurons to the combined toxicity caused by L‐buthionine sulfoximine and 1‐methyl‐4‐phenylpyridine. J Neurosci Res. 2000;62(3):374–382. doi:10.1002/1097-4547(20001101)62:3<374::AID-JNR7>3.0.CO;2-7
67. Newmark HL, Newmark J. Vitamin D and Parkinson’s disease–a hypothesis. Mov Disord. 2007;22(4):461–468. doi:10.1002/mds.21317
68. Ibi M, Sawada H, Nakanishi M, et al. Protective effects of 1α,25-(OH)2D3 against the neurotoxicity of glutamate and reactive oxygen species in mesencephalic culture. Neuropharmacology. 2001;40(6):761–771. doi:10.1016/S0028-3908(01)00009-0
69. Muraro C, Dalla Tiezza M, Pavan C, Ribaudo G, Zagotto G, Orian L. Major depressive disorder and oxidative stress: in silico investigation of fluoxetine activity against ROS. Appl Sci Basel. 2019;9(17):3631. doi:10.3390/app9173631
70. Galecki P, Szemraj J, Bienkiewicz M, Zboralski K, Galecka E. Oxidative stress parameters after combined fluoxetine and acetylsalicylic acid therapy in depressive patients. Hum Psychopharmacol. 2009;24(4):277–286. doi:10.1002/hup.1014
71. DeLuca GC, Kimball SM, Kolasinski J, Ramagopalan SV, Ebers GC. Review: the role of vitamin D in nervous system health and disease. Neuropathol Appl Neurobiol. 2013;39(5):458–484. doi:10.1111/nan.12020
72. Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65(9):732–741. doi:10.1016/j.biopsych.2008.11.029
73. Wang Q, He Y, Shen Y, et al. Vitamin D inhibits COX-2 expression and inflammatory response by targeting thioesterase superfamily member 4. J Biol Chem. 2014;289(17):11681–11694. doi:10.1074/jbc.M113.517581
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.