Back to Journals » Clinical Ophthalmology » Volume 16
Sex-Specific Changes in Choroid Vasculature Among Patients with Schizophrenia and Bipolar Disorder
Authors Li CY, Garg I , Bannai D, Kasetty M, Katz R , Adhan I, Douglas KAA , Wang JC, Kim LA , Keshavan M, Lizano P, Miller JB
Received 29 December 2021
Accepted for publication 17 March 2022
Published 28 July 2022 Volume 2022:16 Pages 2363—2371
DOI https://doi.org/10.2147/OPTH.S352731
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Chloe Y Li,1,* Itika Garg,1,2,* Deepthi Bannai,3 Megan Kasetty,1 Raviv Katz,1,2 Iniya Adhan,3 Konstantinos AA Douglas,1 Jay C Wang,1 Leo A Kim,2 Matcheri Keshavan,3 Paulo Lizano,3,* John B Miller1,2,*
1Harvard Retinal Imaging Laboratory, Massachusetts Eye and Ear, Boston, MA, USA; 2Retina Service, Massachusetts Eye and Ear, Boston, MA, USA; 3Department of Psychiatry, Beth Israel Deaconess Medical Center, Boston, MA, USA
*These authors contributed equally to this work
Correspondence: John B Miller, Harvard Medical School, Department of Ophthalmology, Massachusetts Eye and Ear, 243 Charles St, Boston, MA, 02114, USA, Tel +1 617-573-3529, Email [email protected] Paulo Lizano, Harvard Medical School, Department of Psychiatry, Beth Israel Deaconess Medical Center, 75 Fenwood Road, Room 612, Boston, MA, 02115, USA, Tel +1 617-754-1227, Email [email protected]
Purpose: While structural changes within the retina of psychosis patients have been established, no detailed studies of choroidal microvasculature in these patients have been performed. Given evidence of microvascular disruption in psychosis patients, this study sought to determine whether there exists evidence of microvascular disruption in the choroids in these patients.
Methods: Fifty-six subjects (20 controls and 36 psychosis patients) were recruited from April 2018 to February 2020. Five were excluded due to imaging artifact or missing demographic information. Swept-source optical coherence tomography angiography (SS-OCTA) images were obtained. Choroid vascular enface images (12 mm × 9mm) were exported every 2.6 μm from Bruch’s membrane to the choroid–scleral interface from Topcon to ImageJ. The images were binarized using Otsu’s method, signal from the optic disk and retinal vasculature was removed, and average choroid vascular density (CVD) was calculated as the average of percent area occupied by choroidal vasculature across images in the stack. Choroid vascular volume (CVV) was calculated as the CVD multiplied by maximum CT and image area. During image analysis, study staff were blinded to the phenotype of the study subjects.
Results: Compared with same-sex controls, male psychiatric patients had significantly lower CVD. Compared with same-sex controls, female psychiatric patients had significantly lower maximum CT with correspondingly decreased CVV, after adjusting for age. When all psychiatric patients were compared with all healthy controls, no significant differences in CT, CVD, or CVV were noted.
Conclusion: These results suggest that the pathogenesis of psychotic illness affects choroidal microvasculature in a sex-specific manner.
Keywords: choroid vascular density, swept source optical coherence tomography angiography, psychosis, schizophrenia, bipolar disorder
Introduction
Schizophrenia (SZ), schizoaffective disorder (SZA), and bipolar I disorder with psychosis (BD) are severe psychiatric illnesses with the shared characteristic symptom of episodic psychosis.1 SZ and SZA impact an estimated 1% of the global population,2 whereas BD affects about 0.7% of the global population.3 Previous work by the Bipolar and Schizophrenia Network for Intermediate Phenotypes (B-SNIP2) consortium has established that the clinical phenotype of psychosis is an especially important marker of SZ, SZA, and BD and that these three disorders share significant clinical, cognitive, structural and functional disturbances when compared to healthy controls.1 While medications such as antipsychotics and mood stabilizers have allowed for the treatment of psychotic symptoms, a clear understanding of their pathogenesis remains elusive. Additionally, there are no reliable biomarkers of psychosis that can be used to diagnose or monitor response to treatment, but efforts have been made to categorize psychosis spectrum disorders based on neurobiological phenotypes.4
Vision and bottom-up visual processing deficits have been described in patients with psychotic disorders.5 Previous studies have described lower macular full retinal thickness across retinal layers in SZ6,7 and BD,7,8 as well as smaller peripapillary ganglion cell layer and retinal nerve fiber layer thickness in both conditions.8,9 Outer retinal layer alterations have also been found in SZ and BD, as evidenced by smaller outer nuclear layer and larger outer plexiform layer thicknesses.10 Abnormalities in the primary visual cortex of psychosis patients, such as structural thinning,11 have also been reported. Functional impairments of vision, including abnormal face recognition and eye movements, among psychosis patients have likewise been noted.12,13
Neuroinflammation, with consequent compromise of the nervous system microvasculature and the blood–brain barrier, is thought to contribute to the pathogenesis of psychosis.14,15 Elevated peripheral and cerebrospinal fluid markers of oxidative stress and inflammation, including reactive oxygen species and C-reactive protein, have been frequently reported among psychosis patients.15,16 Abnormal vascularization is a robustly documented response to inflammation in the choroid17,18 and central nervous system.19 The choroid lies underneath Bruch’s membrane and the retinal pigmented epithelium (RPE), considered to be the blood-retinal barrier, and provides oxygen and nutrients to the retina. Choroidal alterations occur in both structure and function in uveitis, systemic lupus erythematosus, rheumatoid arthritis, and other inflammatory disorders.20 The choroid plexus of the brain ventricles, which shares several structural and functional features with the choroid, including fenestrated vasculature21 and immune surveillance,22 is increased in volume in patients with psychosis compared to controls and is associated with higher levels of interleukin-6 (IL-6), a pro-inflammatory cytokine.23
Given the known increase in inflammatory mediators in SZ and BD patients and their effects on the central nervous system, we hypothesize that there are also disruptions within the choroidal vasculature in individuals with SZ and BD. No previous studies have focused on characterizing the choroidal vasculature in either disease, though several have reported no changes in the choroidal thickness of SZ and BD patients.6,8–10
Materials and Methods
Study Design
This was a cross-sectional pilot study performed at the Harvard Retinal Imaging Laboratory, Massachusetts Eye and Ear Infirmary (MEEI), Boston, United States and the Department of Psychiatry, Beth Israel Deaconess Medical Center (BIDMC), Boston, United States. This research adhered to the tenets of the Declaration of Helsinki. The Institutional Review Boards of MEEI and of BIDMC approved the research protocol.
Subjects
Participants from the Bipolar and Schizophrenia Network on Intermediate Phenotype-2 (B-SNIP2) were considered for inclusion. Proband diagnoses were based on the Diagnostic and Statistical Manual of Mental disorders IV24,25 and consensus diagnosis. Healthy controls were excluded if they had a personal history of major mood or psychotic disorder (SCID-Nonpatient edition), a family history of psychosis or SZ-spectrum diagnoses, and treatment with medications affecting cognition. Fifty-six subjects were recruited, including 20 healthy controls (HCs) and 36 probands (schizophrenia (SZ), n = 18, schizoaffective disorder (SZA), n = 9, psychotic bipolar I disorder (BD), n = 5). All recruited subjects had both eyes imaged. Demographic and clinical information was collected as part of the interview. Five subjects, 1 control and 4 psychosis subjects, were excluded due to lack of demographic information or significant motion artifact during imaging. All included participants provided written informed consent. Subjects were recruited with the following exclusion criteria: (1) substance dependence within the past 6 months, (2) glaucoma, macular degeneration, retinal vascular occlusions, ocular trauma, or myopia greater than 4.0 diopters, (3) currently pregnant or breastfeeding, (4) head injury with neurological sequelae, (5) intellectual disability, and (6) history of neurologic disorders. No female probands were on any form of hormonal contraception. Three healthy control females were on hormonal contraception. Healthy controls were excluded if they had a personal history of psychosis or mood disorder, a family history of psychosis, or a family history of SZ-spectrum diagnosis. Duration of illness was determined based on the subject's first psychotic disorder diagnosis. The duration of untreated psychotic illness was not available.
Swept-Source Optical Coherence Tomography Angiography
All subjects were imaged in the morning using the Topcon DRI OCT-1 Atlantis (Capelle aan den Ijssel, The Netherlands). Choroid thickness (CT) was measured with the native device software, Topcon FastMap (Topcon, Tokyo, Japan). A conventional Early Treatment Diabetic Retinopathy Study (ETDRS) grid was used to automatically generate thickness maps. For all subjects, an experienced investigator (C.Y.L.) examined the position of the ETDRS grid, as well as the retinal and choroidal segmentations for all volume scans. If the automated map or segmentation was deemed inaccurate, manual correction was performed. Any discrepancy was adjudicated by a senior author (J.B.M).
Choroid Vascular Density and Volume
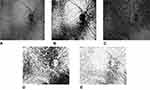
The image processing protocol (Figure 1) used here was described in detail previously by our group.26 Enface choroid vascular images (12 mm × 9 mm) were exported every 2.6 μm from Bruch’s membrane to the choroid–scleral interface from Topcon to ImageJ (National Institutes of Health, Bethesda, Maryland, USA). All the images were binarized (ImageJ command “Image > Adjust > Threshold > Apply”) using Otsu’s method. The signal from the optic disk and retinal vasculature was removed by creating an inverted mask of the image at Bruch’s membrane, then overlaying it with all remaining images in the stack (ImageJ command “Process > Image Calculator > Multiply”). The average choroid vascular density (CVD) was calculated as the average of percent area occupied by choroidal vasculature across all images in the stack. Choroid vascular volume (CVV) was calculated as the CVD multiplied by the maximum CT and the area of the image.
Statistical Analysis
Statistical analysis was performed in R version 3.4.1 (R Foundation for Statistical Computing, Vienna, Austria). The level of significance was set as p < 0.05. Categorical variables were expressed as proportion and assessed for significance by Pearson’s chi-square test with Yates’ continuity correction. The distribution of continuous variables was checked by boxplots and Shapiro–Wilk Normality test. Non-normally distributed variables were described by median with interquartile range (IQR) and assessed for statistical significance by Mann Whitney U-test and Kruskal Wallis rank sum test. Given the inclusion of both eyes of all subjects, mixed-effects multiple linear regression modeling fit by restricted maximum likelihood was used to test for group differences for various choroidal vascular indices as the dependent variable. Post-hoc multivariate analysis, with Tukey’s honestly significant difference test (Tukey’s HSD test) to adjust for multiple comparisons was performed to examine group-by-sex differences.
Results
Demographics and Clinical Characteristics of Study Subjects
A total of 112 eyes from 56 patients were examined for this study. One hundred and two eyes from 51 patients were ultimately included after 10 eyes (5 subjects) were removed due to motion artifact or incomplete demographic information. Clinical and demographic characteristics of the cohort, including race, sex, body mass index (BMI) and best corrected visual acuity (BCVA) are described in Table 1. Proband subjects did not differ significantly in demographic or clinical characteristics from controls.
 |
Table 1 Demographic and Clinical Characteristics of Study Subjects |
Choroid Thickness and Vascularity
Choroid vascular indices, including maximum CT, CVD, and CVV, among controls and probands are described in Table 2. There were no statistically significant differences among these measures by group. Mixed effects multiple linear regression analysis also revealed no significant differences in maximum CT, CVD, and CVV in healthy controls versus probands, regardless of proband diagnosis and after adjusting for age and BMI. We adjusted for age and BMI because previous authors have reported that increasing age27 and BMI28 are significantly associated with lower choroid thicknesses in otherwise healthy individuals. These results are described in Table 3.
 |
Table 2 Summary of Choroid Vascular Characteristics in Controls and Psychosis Patients |
 |
Table 3 Mixed-Effects Multiple Linear Regression Analysis of Choroid Vascular Parameters in Healthy Controls versus Psychosis Patients, Adjusted for Age and BMI |
Sex-Specific Changes in Choroid Structure and Vascularity
In post-hoc regression analysis, we report that there are sex-specific differences in choroid vasculature alterations among probands, summarized in Table 4 and Figure 2. We chose to perform a post-hoc analysis of the effect of sex-by-phenotype interaction based on previous work by our group that reported sex-specific reduction in visual cortical volume in patients with SZ and BD.29 The average CVD was lower among male probands compared to male controls (β = −0.51, p = 0.006, Tukey’s HSD test), whereas CVD was not different between female probands and controls. Female probands’ maximum CT (β = −86.9, p = 0.03) and CVV (β = −1.80, p = 0.02) were lower compared to female controls (Tukey’s HSD test). When male controls were compared to female controls, the average CVD was not different, and females tended to have greater maximum CT (β = 75.2, p = 0.04) and CVV (β = 1.48, p = 0.047) as compared to males.
Discussion
Our results represent the first report of sex-specific changes in the vasculature of the eye among patients with psychosis. This is of particular interest as it may correlate with previous sex-specific changes in the central nervous system, as Turkozer et al29 previously reported sex-specific alterations in area and volume of primary (V1) and secondary (V2) visual cortices among psychosis patients, with only female probands demonstrating significant reduction in V1 and V2 area and volume. Also, as reported by Turkozer et al,29 our results suggest that psychosis augments the physiologic difference between males and females, with female controls having a lower CT than male controls, and female probands in turn having a significantly lower CT and CVV than female controls.
Given the functional homology between the choroidal microvasculature and choroid plexus, this report on choroid vasculature disruption in psychosis builds upon previous work on the choroid plexus.23 Our group previously demonstrated significantly increased choroid plexus volume in patients with psychosis spectrum illness.23 While these results are orthogonal, it is important to examine how each measure is generated. For example, choroid plexus volume is a composite measure of microvasculature, stroma, and choroid plexus epithelium, while CVV takes into account the choroidal microvascular but not stromal volume. Thus, it could be suggested that the larger choroid plexus volume observed in our large study of individuals with psychosis spectrum illness is not necessarily due to microvascular enhancement but due to increases in stroma volume. This observation coincides with our hypothesis that there may be an inflammation/vascular mediated effect on the choroid plexus, as well as other brain structures, resulting in what we call pseudothickening.15,30 However, further investigations are needed to determine if there are sex-specific changes in the choroid plexus of psychosis patients and to dissect whether greater choroid plexus volume is due to microvascular or stromal changes.
Normal brain development is sexually dimorphic, with evidence of differential white matter tract development, cerebral perfusion, cortical thickness and total cortical volume in healthy adolescent males and females.31 In patients with psychosis, there are marked sex differences in when and how symptoms appear; males tend to present with psychotic symptoms earlier in adolescence, and males tend to present with more negative symptoms, while females present with more mood-related symptoms.32 Generally, female probands tend to have a better prognosis. These disparities are thought to arise due to differential exposure to estrogen, testosterone, and other sex hormones in males versus females throughout childhood and adolescence,33 as well as differing psychosocial experiences and exposures to adverse childhood events such as childhood physical or sexual abuse.34
Although there have been no previous studies demonstrating sex-specific differences in the retinas of psychosis patients, there are many demonstrating sex-specific changes in visual, frontal, and temporal cortical volumes of psychosis patients.32 The differences we describe may be due to unequal time since diagnosis in males versus females, as by the median age of 33–38 years, males are likely to have been symptomatic for several more years. Another possibility is differing amount and length of antipsychotic use among male versus female probands, given that increasing dose and duration of antipsychotic use is associated with worse cortical volume loss in psychosis patients.35 Of note, we did not find that adjusting for the length of illness or duration of treatment with antipsychotics correlated significantly with max CT, CVD, or CVV (Supplemental Table 1). Further investigation of the timing and degree of difference in choroid vasculature between male and female psychosis probands is necessary.
There are several strengths to this study. It is among the first to focus on changes to the choroid vasculature in psychosis patients, and uses en-face SS-OCT angiography, which may be superior to more widely available SD-OCT angiography in analyzing choroidal vascularity.26,36 Limitations of this study include a small sample size, in particular the limited number of BD patients, and the inclusion of multiple diagnostic groups. While we investigated the impact of important possible confounders, including age, race, BMI, visual acuity, length of illness, and antipsychotic dosage, there were some we could not account for, including eye axial length, which is known to correlate with choroid thickness,37 among patients with psychosis. Among females, CT has also been shown to vary with phases of the menstrual cycle,38 which represents another variable we did not take into account here.
Psychosis is an important clinical entity warranting further investigation with quick, reliable, objective, and high-resolution imaging biomarkers. Recent advances in OCT with both swept source scan acquisition and OCT-angiography offer new non-invasive methods to carefully investigate the posterior segment of this psychiatric patient population, especially given that the eye is considered to be a “window into the brain”. In the present work, we used SS-OCTA to identify sex-specific changes in the choroid that may support analogous changes in the choroid plexus of the ventricles. Additional study is warranted to investigate this potentially important link between the eye and these psychotic disorders.
Meeting Presentation
The work in this manuscript was presented at the Association of Research in Vision and Ophthalmology Annual Meeting May 1–7, 2021, and The Retina Society Annual Meeting September 29–October 2, 2021.
Ethical Approval
This study was conducted in accordance with the Declaration of Helsinki. The collection and evaluation of all protected patient health information was performed in a Health Insurance Portability and Accountability Act (HIPAA)-compliant manner.
Statement of Informed Consent
Informed consent was obtained prior to performing the procedure, including permission for publication of all photographs and images included herein.
Disclosure
- P.L., D.B., and M. Keshavan. were supported in part by the Harvard Medical School Dupont Warren Fellowship and Livingston Grant; Sydney R. Baer Jr Foundation; National Institute of Health Harvard Catalyst [grant number 1KL2TR002542]; National Institute of Mental Health [grant number R01 MH078113]; and National Institute of Mental Health [grant number R01 MH077945]. We would like to thank the following B-SNIP2 principal investigators (Carol Tamminga, Brett Clementz, Elliot Gershon, and Godfrey Pearlson) for their input and support of this study.
- L.A.K. has received financial support from the National Eye Institute and CureVac AG and has financial arrangement with Pykus Therapeutics.
- J.B.M. is a consultant for Alcon, Allergan, Carl Zeiss, Sunovion, Topcon and Genentech.
- C.Y.L., I.G., M. Kasetty, R.K., I.A., K.D., and J.C.W. have no conflicts of interest to report.
References
1. Tamminga CA, Pearlson G, Keshavan M, Sweeney J, Clementz B, Thaker G. Bipolar and schizophrenia network for intermediate phenotypes: outcomes across the psychosis continuum. Schizophr Bull. 2014;40(Suppl 2):S131–S137. doi:10.1093/schbul/sbt179
2. Haller CS, Padmanabhan JL, Lizano P, Torous J, Keshavan M. Recent advances in understanding schizophrenia. F1000Prime Rep. 2014;6. doi:10.12703/P6-57
3. Ferrari AJ, Stockings E, Khoo JP, et al. The prevalence and burden of bipolar disorder: findings from the global burden of disease study 2013. Bipolar Disord. 2016;18(5):440–450. doi:10.1111/bdi.12423
4. Clementz BA, Sweeney JA, Hamm JP, et al. Identification of distinct psychosis biotypes using brain-based biomarkers. AJP. 2016;173(4):373–384. doi:10.1176/appi.ajp.2015.14091200
5. Keane BP, Cruz LN, Paterno D, Silverstein SM. Self-reported visual perceptual abnormalities are strongly associated with core clinical features in psychotic disorders. Front Psychiatry. 2018;9:69. doi:10.3389/fpsyt.2018.00069
6. Topcu-Yilmaz P, Aydin M, Cetin Ilhan B. Evaluation of retinal nerve fiber layer, macular, and choroidal thickness in schizophrenia: spectral optic coherence tomography findings. Psychiatr Clin Psychopharmacol. 2019;29(1):28–33. doi:10.1080/24750573.2018.1426693
7. Joe P, Ahmad M, Riley G, Weissman J, Smith RT, Malaspina D. A pilot study assessing retinal pathology in psychosis using optical coherence tomography: choroidal and macular thickness. Psychiatry Res. 2018;263:158–161. doi:10.1016/j.psychres.2018.03.011
8. Polo V, Satue M, Gavin A, et al. Ability of swept source OCT to detect retinal changes in patients with bipolar disorder. Eye. 2019;33(4):549–556. doi:10.1038/s41433-018-0261-6
9. Lizano P, Bannai D, Lutz O, Kim LA, Miller J, Keshavan MA. Meta-analysis of retinal cytoarchitectural abnormalities in schizophrenia and bipolar disorder. Schizophr Bull. 2020;46(1):43–53. doi:10.1093/schbul/sbz029
10. Bannai D, Lizano P, Kasetty M, et al. Retinal layer abnormalities and their association with clinical and brain measures in psychotic disorders: a preliminary study. Psychiatry Res Neuroimaging. 2020;299:111061. doi:10.1016/j.pscychresns.2020.111061
11. Reavis EA, Lee J, Wynn JK, Engel SA, Jimenez AM, Green MF. Cortical thickness of functionally defined visual areas in schizophrenia and bipolar disorder. Cereb Cortex. 2016;bhw151. doi:10.1093/cercor/bhw151
12. Reilly JL, Frankovich K, Hill S, et al. Elevated antisaccade error rate as an intermediate phenotype for psychosis across diagnostic categories. Schizophr Bull. 2014;40(5):1011–1021. doi:10.1093/schbul/sbt132
13. McBain R, Norton D, Chen Y. Differential roles of low and high spatial frequency content in abnormal facial emotion perception in schizophrenia. Schizophr Res. 2010;122(1–3):151–155. doi:10.1016/j.schres.2010.03.034
14. Watkins CC, Andrews SR. Clinical studies of neuroinflammatory mechanisms in schizophrenia. Schizophr Res. 2016;176(1):14–22. doi:10.1016/j.schres.2015.07.018
15. Lizano P, Lutz O, Xu Y, et al. Multivariate relationships between peripheral inflammatory marker subtypes and cognitive and brain structural measures in psychosis. Mol Psychiatry. 2020;26:3430–3443. doi:10.1038/s41380-020-00914-0
16. Ök T, Sarısoy G, Bilgici B, et al. Oxidative stress in bipolar and schizophrenia patients. Psychiatry Res. 2015;228(3):688–694. doi:10.1016/j.psychres.2015.04.046
17. Campa C, Costagliola C, Incorvaia C, et al. inflammatory mediators and angiogenic factors in choroidal neovascularization: pathogenetic interactions and therapeutic implications. Mediators Inflamm. 2010;2010:1–14. doi:10.1155/2010/546826
18. Weber ML, Heier JS. Choroidal neovascularization secondary to myopia, infection and inflammation. In: Nguyen QD, Rodrigues EB, Farah ME, Mieler WF, Do DV, editors. Developments in Ophthalmology. Vol. 55. S. Karger AG; 2015:167–175.
19. Mastorakos P, McGavern D. The anatomy and immunology of vasculature in the central nervous system. Sci Immunol. 2019;4(37):eaav0492. doi:10.1126/sciimmunol.aav0492
20. Cunningham ET, Ferrara D, Mrejen S, Freund KB, Zierhut M. Imaging the choroid and choroidal neovascularization in eyes with inflammation. Ocul Immunol Inflamm. 2016;24(3):243–245. doi:10.1080/09273948.2016.1180040
21. Lutty GA, McLeod DS. Development of the hyaloid, choroidal and retinal vasculatures in the fetal human eye. Prog Retin Eye Res. 2018;62:58–76. doi:10.1016/j.preteyeres.2017.10.001
22. McMenamin PG, Saban DR, Dando SJ. Immune cells in the retina and choroid: two different tissue environments that require different defenses and surveillance. Prog Retin Eye Res. 2019;70:85–98. doi:10.1016/j.preteyeres.2018.12.002
23. Lizano P, Lutz O, Ling G, et al. Association of choroid plexus enlargement with cognitive, inflammatory, and structural phenotypes across the psychosis spectrum. AJP. 2019;176(7):564–572. doi:10.1176/appi.ajp.2019.18070825
24. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV; Includes ICD-9-CM Codes Effective 1..
25. American Psychiatric Association, American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR.
26. Wang JC, Laíns I, Providência J, et al. Diabetic choroidopathy: choroidal vascular density and volume in diabetic retinopathy with swept-source optical coherence tomography. Am J Ophthalmol. 2017;184:75–83. doi:10.1016/j.ajo.2017.09.030
27. Hirata M, Tsujikawa A, Matsumoto A, et al. Macular choroidal thickness and volume in normal subjects measured by swept-source optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52(8):4971. doi:10.1167/iovs.11-7729
28. Yilmaz I, Ozkaya A, Kocamaz M, et al. Correlation of choroidal thickness and body mass index. Retina. 2015;35(10):2085–2090. doi:10.1097/IAE.0000000000000582
29. Turkozer HB, Lizano P, Adhan I, et al. Regional and Sex-Specific Alterations in the Visual Cortex of Individuals With Psychosis Spectrum Disorders. Biological Psychiatry. 2022;S0006-3223(22)01159-3. doi:10.1016/j.biopsych.2022.03.023
30. Pong S, Karmacharya R, Sofman M, Bishop JR, Lizano P. The role of brain microvascular endothelial cell and blood-brain barrier dysfunction in schizophrenia. Complex Psychiatry. 2020;6(1–2):30–46. doi:10.1159/000511552
31. Kaczkurkin AN, Raznahan A, Satterthwaite TD. Sex differences in the developing brain: insights from multimodal neuroimaging. Neuropsychopharmacol. 2019;44(1):71–85. doi:10.1038/s41386-018-0111-z
32. Abel KM, Drake R, Goldstein JM. Sex differences in schizophrenia. Int Rev Psychiatry. 2010;22(5):417–428. doi:10.3109/09540261.2010.515205
33. Gogos A, Ney LJ, Seymour N, Van Rheenen TE, Felmingham KL. Sex differences in schizophrenia, bipolar disorder, and post‐traumatic stress disorder: are gonadal hormones the link? Br J Pharmacol. 2019;176(21):4119–4135. doi:10.1111/bph.14584
34. Prokopez CR, Vallejos M, Farinola R, et al. The history of multiple adverse childhood experiences in patients with schizophrenia is associated with more severe symptomatology and suicidal behavior with gender-specific characteristics. Psychiatry Res. 2020;293:113411. doi:10.1016/j.psychres.2020.113411
35. Andreasen NC, Liu D, Ziebell S, Vora A, Ho BC. Relapse duration, treatment intensity, and brain tissue loss in schizophrenia: a prospective longitudinal MRI study. AJP. 2013;170(6):609–615. doi:10.1176/appi.ajp.2013.12050674
36. Spaide RF, Fujimoto JG, Waheed NK. Image artifacts in optical coherence tomography angiography. Retina. 2015;35(11):2163–2180. doi:10.1097/IAE.0000000000000765
37. Barteselli G, Chhablani J, El-Emam S, et al. Choroidal volume variations with age, axial length, and sex in healthy subjects: a three-dimensional analysis. Ophthalmology. 2012;119(12):2572–2578. doi:10.1016/j.ophtha.2012.06.065
38. Ulaş F, Doğan Ü, Duran B, Keleş A, Ağca S, Çelebi S. Choroidal thickness changes during the menstrual cycle. Curr Eye Res. 2013;38(11):1172–1181. doi:10.3109/02713683.2013.811258
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.