Back to Journals » Vascular Health and Risk Management » Volume 16
Sex-Related Differences in the Outcomes of Endovascular Interventions for Chronic Limb-Threatening Ischemia: Results from the LIBERTY 360 Study
Authors Giannopoulos S , Shammas NW , Cawich I, Staniloae CS, Adams GL , Armstrong EJ
Received 18 January 2020
Accepted for publication 15 May 2020
Published 8 July 2020 Volume 2020:16 Pages 271—284
DOI https://doi.org/10.2147/VHRM.S246528
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Takashi Kajiya
Stefanos Giannopoulos,1 Nicolas W Shammas,2 Ian Cawich,3 Cezar S Staniloae,4 George L Adams,5 Ehrin J Armstrong1
1Division of Cardiology, Rocky Mountain Regional VA Medical Center, University of Colorado, Denver, CO, USA; 2Midwest Cardiovascular Research Foundation, Davenport, IA, USA; 3Arkansas Heart Hospital, University of Arkansas for Medical Sciences, Little Rock, AR, USA; 4Leon H. Charney Division of Cardiology, New York University Langone Medical Center, New York, NY, USA; 5Department of Cardiology, North Carolina Heart and Vascular, Rex Hospital, UNC School of Medicine, Raleigh, NC, USA
Correspondence: Ehrin J Armstrong Email [email protected]
Introduction: Previous studies have suggested that women with chroniclimb-threatening ischemia (CLTI) may have worse outcomes than men. The aim of this study was to determine whether there are sex-related differences in outcomes of patients with CLTI undergoing endovascular treatment with current endovascular technologies.
Patients and Methods: Data were derived from the LIBERTY 360 study (NCT01855412). Hazard ratios and the respective 95% confidence intervals were synthesized to examine the association between sex and all-cause mortality, target vessel revascularization (TVR), major amputation, major adverse event (MAE) and major amputation/death up to 3 years of follow-up.
Results: A total of 689 patients with CLTI (female: N=252 vs male: N=437) treated with any FDA approved or cleared device were included. The mean lesion length was 126.9± 117.3mm and 127.4± 113.3mm for the female and male patients, respectively. Although a slightly higher incidence of in-hospital mortality was observed in the female group (1.2% vs 0.0%, p=0.049), there was no difference in female vs male survival rates during follow-up. However, the risk of major amputation at 18 months was higher for the male group (male vs female: HR: 2.36; 95% CI: 1.09– 5.12; p=0.030). No difference between the two groups was detected in terms of TVR or MAE during follow-up.
Discussion: Data regarding sex-related disparity in outcomes after endovascular therapy of patients with CLTI are conflicting. Gender-related characteristics rather than biological sex characteristics might be the cause of these conflicting findings. Further studies are needed to evaluate the role of sex in revascularization outcomes among this high-risk population.
Keywords: endovascular repair, sex-specific, peripheral vascular disease, critical limb ischemia, chronic limb-threatening ischemia; revascularization
Introduction
Peripheral artery disease (PAD) affects more than 8 million patients in the United States1,2 and has been associated with morbidity and mortality rates similar to or greater than coronary artery disease (CAD).3–5 Up to 10% of patients with PAD suffer from chronic limb-threatening ischemia (CLTI).6,7 CLTI is a multilevel disease and is mainly caused by atherosclerosis.8 It has been associated with poor limb salvage (amputation rate up to 50% if left untreated), high mortality9,10 and increased utilization of health-care resources,11–13 costing more than $4 billion per year in the United States.2,14,15 The American College of Cardiology/American Heart Association (AHA/ACC) guidelines recommend that revascularization is a reasonable treatment option for CLTI,16 however data regarding best revascularization strategies for CLTI are sparse.17–19
 |
Figure 1 Kaplan–Meier estimates of freedom from major amputation during follow-up. |
 |
Figure 2 Kaplan–Meier estimates of freedom from major amputation/death during follow-up. |
Endovascular intervention is a viable treatment approach for CLTI with acceptable hemodynamic improvement and safety profile.15,20 Although endovascular therapy has been increasingly utilized,15,21 the rates of restenosis22–26 and cardiovascular events are still considerable in CLTI patients.25,26 Variable factors, including heart failure (HF), coronary artery disease (CAD), end-stage renal disease (ESRD) and diabetes, have been associated with an independent risk for higher mortality and worse outcome in patients with CLTI.27–31 Moreover, several studies have suggested that female patients with symptomatic PAD commonly present at an older age, with more advanced atherosclerosis32,33 and therefore they may have a worse prognosis compared to males.34–36
However, it is not yet clear to what extent sex affects the clinical outcomes among CLTI patients who undergo endovascular revascularization.34,37 Identification of such risk factors for worse prognosis could optimize the management of this highly morbid population.31,33,38-40 Thus, the aim of this study was to determine whether sex is associated with short- and long-term outcomes of endovascular therapy in patients with CLTI. We utilized data from the LIBERTY 360 study, which is a modern, real-world cohort of patients with PAD treated with endovascular approaches.20
Patients and Methods
Study Design and Patient Enrollment
LIBERTY 360 is a prospective, real-world, multicenter study (ClinicalTrials.gov; identifier: NCT01855412) that examined predictors of clinical and economic outcomes in patients undergoing lower extremity endovascular interventions for symptomatic PAD, with any FDA approved or cleared devices, between 2013 and 2016. Lesions above and below the knee were revascularized, while the target area at the infrapopliteal segment was any lesion in a native vessel located within or extending into 10 cm above the medial epicondyle to the digital arteries. A steering committee, including principal investigators, representatives from the study core laboratories, and the sponsor (Cardiovascular Systems, Inc) developed the study’s protocol, while Cardiovascular Systems, Inc was also responsible for oversight of the research process. The protocol for the LIBERTY 360 study was approved by the institutional review board of all the participating sites. The 53 sites which participated in the LIBERTY 360 study are demonstrated in Supplementary Table 1. All patients provided written informed consent, and that this trial was conducted in accordance with the Declaration of Helsinki. Details regarding inclusion and exclusion criteria of the LIBERTY 360 study were previously published41 and can also be found at: https://clinicaltrials.gov/ct2/show/NCT01855412?cond=NCT01855412&rank=1.
Renal disease was defined as calculated eGFR < 60 or kidney damage of at least 3 months; hyperlipidemia was defined as cholesterol levels > 200mg/dl or LDL > 100mg/dl or dyslipidemia requiring medication; hypertension was defined as systolic blood pressure > 140 mmHg or diastolic blood pressure > 90 mmHg or requiring medication for blood pressure control. For the current study, only patients with CLTI were included and sex-related comparisons were performed (female vs male). A total of 689 patients treated with endovascular procedures for CLTI were ultimately identified. Angiographic data were adjudicated by SynvaCor/Prairie Educational and Research Cooperative (PERC; Springfield, IL, USA). In the analyses of this LIBERTY 360 sub-study core lab data were preferred in order to minimize any potential bias. However, in cases where the core laboratory was not able to assess significant angiographic complications, site reported data were used. Patient demographics and lesion characteristics stratified by sex are summarized in Tables 1 and 2 respectively.
 |
Table 1 Baseline Characteristics of Participants |
 |
Table 2 Lesion Characteristics |
Study Endpoints and Statistical Analysis
Descriptive statistics were used for baseline demographics and lesion characteristics. Categorical variables are presented as absolute and relative frequencies (ie, percentages) and were compared with Monte Carlo approximation of the Fisher's exact test. Numeric data are presented as mean ± standard deviation (SD) and compared using ANOVA or a paired t test, while discrete data were compared with the Kruskal–Wallis test or Wilcoxon signed-rank test for paired data. Site reported data regarding significant angiographic complications (ie, flow-limiting dissection, perforation, distal embolization, acute vessel closure), procedural and lesion success of core lab identified lesions were used, when core lab was unable to perform angiographic assessment. Primary endpoints were: i) procedural success assessed by the angiographic core laboratory as less than 50% residual stenosis without significant angiographic complications (ie, flow-limiting dissection, perforation, distal embolization, abrupt closure) and ii) incidence of major adverse events (MAE) defined as death within 30 days of the primary procedure, unplanned major amputation of the target limb, and clinically driven target vessel revascularization (CD-TVR) as assessed by the angiographic core laboratory when angiographic images were available. Secondary endpoints were lesion success (<50% residual stenosis, without significant angiographic complications) target vessel revascularization (TVR), death, major amputation of the target limb, wound healing and the combined outcome of death or major amputation during follow-up. Secondary outcomes also included ankle brachial Index (ABI) and Rutherford class (RC). The ABI and RC were also assessed during follow-up, however as the 3-year follow visit was a phone visit, ABI and RC could be assessed only up to 2 years of follow-up. In addition, Cox regression among males vs females was synthesized for MAE, death, major amputation, and major amputation or death in up to 36 months of follow-up. As no clinically significant differences were identified between male and female baseline demographic, lesion and procedural characteristics, no sensitivity analyses were synthesized. Kaplan-Meier curves for female vs male patients were estimated for primary and secondary outcomes and compared with the Log-rank test. All statistical analyses were conducted by NAMSA (Northwood, OH, USA), and for all tests, p-values <0.05 were considered statistically significant.
Results
Patients and Lesion Characteristics
A total of 689 patients with CLTI (Female: N=252 vs Male: N=437), with 923 treated lesions (Female: N=327 vs Male: N=596), were included. Detailed patient characteristics are presented in Table 1 and Supplementary Table 2. Based on the baseline case report forms, women had lower rates of previous smoking history (Females: 52.8% vs Males: 70.0%; p <0.001), lower rates of coronary artery disease (CAD) (Females: 56.3% vs Males: 65.7%; p= 0.018), while there were significantly fewer Caucasians in the female group (Females: 77.0% vs Males: 84.0%; p= 0.025). Moreover, women had higher rates of dual-anti-platelet therapy (DAPT) prescription at discharge (Females: 73.8% vs Males: 65.0%; p= 0.018). Women had 126.9±117.3mm mean target lesion length vs men: 127.4±: 113.3mm, without any statistical difference between the two groups (p= 0.950). The women compared to men had more lesions located at the superficial femoral artery (SFA) extending to the popliteal artery (Females: 14.7% vs Males: 8.6%; p= 0.005), while lesions isolated below the knee (BTK) were more commonly observed in men (Females: 54.4% vs Males: 62.9%; p= 0.014). Detailed lesion characteristics are summarized in Table 2.
Procedure Characteristics and Short-Term Outcomes
For almost all lesions, balloon angioplasty was the preferred treatment approach (Females: 319/324; 98.5% vs Males: 562/584; 96.2%; p= 0.067), with bailout stenting occurring in 2.8% (N=9/324) of females and in 4.3% (25/584) of males (p=0.280). Important procedural characteristics are provided in Table 3. Overall, significant angiographic complications occurred in 10.5% of all lesions treated (Females: 38/324; 11.7% vs Males: 58/591; 9.8%; p= 0.485). In total, target lesion success, was 78.9% (N=243/307) in the female group vs 78.3% (N=443/566) in male group, without any significant difference between the two groups (p=0.864). In-hospital TVR, MAE and major amputation rates were not statistically different between the two groups, however more in-hospital death occurred among females (Females: 1.2%; N=3/247 vs Males: 0.0%; N=0/422; p= 0.049). The causes of in-hospital death are presented in Supplementary Table 3. Detailed information regarding periprocedural complications and short-term outcomes is presented in Table 4 and Supplementary Table 4.
 |
Table 3 Procedure Characteristics and Target Lesion Device Use |
 |
Table 4 Periprocedural Complications and Short-Term Outcomes (in-Hospital) |
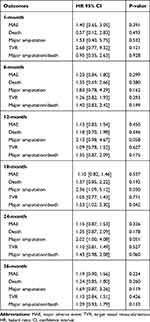 |
Table 5 Hazard Ratios (HR) and 95% Confidence Intervals of Outcomes During Follow-Up (Male Vs Female) |
Outcomes in Follow-up
Periprocedural (within 30 days) ABI was improved compared to preprocedural values of each group, with males having a better 30-day ABI overall. ABI values remained higher for males until two years of follow up, however, at 2 years of follow up the change in median ABI from baseline was not statistically different between the two groups. The periprocedural (within 30 days) median Rutherford classification of both groups was similar. No differences in median Rutherford classification between female and male patients were observed during 2 years of follow up. Details about ABI and Rutherford classification (categorical and continuous variables) during follow up are presented in Supplementary Table 2.
Female patients treated for CLTI had a lower risk for major amputation or death during 18-month follow up, compared to males (HR: 1.53; 95% CI: 1.02 - 2.30; p= 0.042). The risk of major amputation at 18-months was lower for the female group (HR: 2.36; 95% CI: 1.09 -5.12; p= 0.030), whereas the 18-month death rates were similar between the groups. At 24 months after the primary procedure although female sex was strongly correlated with less risk for major amputation (HR: 2.02; 95% CI: 1.00 - 4.08; p= 0.051) or the combined outcome of major amputation or death (HR: 1.43; 95% CI: 0.98 - 2.08; p= 0.060), no statistical difference was reached. The risk for major amputation remained similar for females vs males at 36-month follow up as well (HR: 1.69; 95% CI: 0.87-3.26; p= 0.119). The MAE, TVR and mortality risk rates were similar between the two groups and did not change during 36-month follow up.The KM survival curves for major amputation and major amputation or death combined are illustrated in Figures 1 and 2 respectively. The HRs of the outcomes and the corresponding KM estimates at several follow up time intervals are reported in Table 5 and Supplementary Table 5 respectively.
Wound Healing Rates
At baseline all patients presented with wound(s) on the target limb. At 6 months of follow-up 20.3% (38/187) female and 29.4% (86/293) male patients were seeing a wound-care specialist for wounds on target limb. Among these patients, the change in seeing a wound care specialist for wound(s) was similar between the two groups (worsened: Females: N=15/187; 8.0% vs Males: N=13/293; 4.4%; p= 0.113; improved: Females: 21.9% vs Males: 16.4%; p= 0.148). The change in seeing a wound care specialist for wound(s) on the target limb remained similar among females and males during 1 year (worsened: Females: N=11/159; 6.9% vs Males: N=10/266; 3.8%; p= 0.168; improved: Females: N=33/159; 20.8% vs Males: N=64/266; 24.1%; p= 0.475) and 2 years (worsened: Females: N=3/127; 2.4% vs Males: N=4/201; 2.0%; p= 1.000; improved: Females: N=32/127 25.2% vs Males: N=54/201; 26.9%; p= 0.797) of follow-up.
Discussion
This study utilized data from the multicenter LIBERTY 360 trial20 to investigate the role of sex in outcomes of CLTI patients undergoing endovascular therapy. In general, there were only a few differences among the groups in terms of baseline characteristics, with fewer female patients being previous smokers, Caucasians or having diagnosed CAD. Our study was based on real-world data and indicated that both female and male patients presented, most commonly, with isolated infrapopliteal disease. This study also demonstrated that females had higher in-hospital all-cause mortality rates compared to men. However, none of the deaths were attributed to the procedure. Separate analyses at several time intervals after the primary procedure provided evidence that although female sex was associated with lower rates of major amputation and major amputation/death combined at 18-month follow-up, the 36-month MAE, TVR and mortality risk rates were similar between the two groups.
Previous studies have investigated several risk factors for the prognosis of endovascular treatment in patients with CLTI.5,42–45 Although the role of sex has been investigated in CAD and cerebrovascular disease,34,46-48 sex-related differences in CLTI patients requiring endovascular treatment remains understudied.33,34 Thus, the American Heart Association has called to action studies of women and PAD outcomes.34 CLTI has been associated with high morbidity and mortality, significantly increasing health-care costs.11–13,49 Moreover, CLTI causes severe physical function restriction with devastating consequences for the patients.50 Thus, as there are no specific guidelines to determine the prognosis of endovascular therapy in CLTI patients, identifying several risk factors related to poor outcomes, could improve management and delay major amputations.49
In general, it has been observed that female patients often present with more advanced PAD, at an older age compared to men and as such are at higher risk of adverse outcomes.32 A previous retrospective analysis demonstrated that these differences in presentation persisted among patients treated with PTA alone, primary stenting, or atherectomy with/without PTA for symptomatic PAD.51 Additional to differences in presentation, it has been considered that biological sex characteristics might influence the long-term outcomes of revascularization procedures in patients with CLTI.35,36,52–54 A previous analysis of representative state administrative databases indicated that female sex was associated with higher risk of mortality, especially when women had a history of CAD or cerebrovascular disease.35 Similarly, Ramkumar et al, utilizing data from the Vascular Quality Initiative (VQI) database, suggested that women undergoing endovascular therapy for symptomatic PAD, were at higher risk for reintervention and/or re-occlusion during a median follow-up of 1-year.51 Nonetheless, these studies did not exclusively include patients with CLTI.35,51
A retrospective study by McCoach et al, investigating the outcome of angioplasty in 97 women and 122 men with CTLI exclusively, demonstrated that women were at higher risk for major adverse cardiovascular events during a median follow-up of 2.2 years, although women had lower prevalence of CAD at baseline.33 In our study (N= 689) no difference was observed in terms of all-cause death during a 3-year follow-up, although the female population of our study had higher prevalence of several comorbidities (eg, CAD, hypertension, diabetes, etc.). Considering that the most common cause of mortality among this high-risk population is cardiovascular death,6 the results of our study were different from the McCoach study. Interestingly, a large multicenter observational study (N=2523) comparing clinical outcomes of endovascular therapy for PAD between women and men showed that female sex was a risk factor for death, MI and major amputation among caludicants.55 However, a sensitivity analysis of this study, including only CLTI cases, failed to show any difference between the female and male group over a median follow-up of 701 days, which was similar to our results.55 Thereby it could be hypothesized that lesion and procedural characteristics rather than biological sex differences affect the prognosis of CLTI patients undergoing endovascular revascularization procedures. However, as data are sparse, more research is warranted in order to identify whether female sex is a risk factor for clinical outcomes of endovascular interventions for CLTI.
Although the late survival rate does not seem to be affected by sex, our study demonstrated a higher incidence of in-hospital death among female patients indicating that sex might play a role in short- rather than long-term outcomes. However, all causes of in-hospital death were not related to the procedure (ie, two death events were attributed to end-stage renal disease and one to amputation due to necrotic toes). ESRD, a high-risk co-morbidity that is a patient-specific variable for early mortality, is independent of sex, as such we believe that female sex is not a risk factor for undergoing endovascular therapy for the treatment of CLTI. In accordance with that, a recent prospective study utilizing data from the Nationwide Inpatient Sample (NIS) database failed to show any association between sex and in-hospital mortality among CLTI patients.56 Miller et al, studying patients with lifestyle limiting claudication, who received endovascular or open surgical repair, demonstrated that women had higher inpatient mortality compared to men, however they suggested that sex might be a predictor for patients with claudication rather CLTI.57 Although several etiologies, including hormonal differences between men and women, have been considered to affect outcomes of revascularization strategies in patients with PAD, the results are conflicting. Therefore, sex-related disparity in CLTI patients undergoing endovascular treatment warrants further research in order to identify whether female sex is a risk factor for late mortality.
Interestingly, our study based on real-world data indicated that female sex might be a protective factor for major amputation at 18-month follow-up. The KM survival estimates for the outcome of major amputation at 18 months (Females: 96.5% vs Males: 91.8%; Log-rank test: p= 0.025) and 24 months (Females: 95.3% vs Males: 91.1%; Log-rank test: p= 0.046) of follow-up were better in females compared to males undergoing endovascular revascularization. Moreover, the KM estimates of freedom from major amputation or death at 18 months of follow-up were also favorable for the female patients, although these results might be driven by the significantly lower amputation rates among females. However, previous studies including either only CLTI patients33 or mixed groups of patients with CLTI/claudication reported similar rates of major amputation between the two groups.33,58 As in our study significantly more men presented with isolated BTK disease, we believe that different lesion characteristics might have affected our results.
Isolated infrapopliteal lesions, being commonly observed among diabetics or patients with ESRD and elderly,59 have been associated with higher incidence of limb loss due to poor initial runoff and severe comorbidities.59 Several studies have indicated that women more commonly present with diffuse femoropopliteal lesions,60 while other investigators have suggested that women with CLTI might have a higher incidence of BTK only disease.61,62 Thus, the data regarding the impact of sex on lesion location and lesion characteristics exhibit high heterogeneity. In our study, most patients had isolated infrapopliteal disease, with men having more isolated lesions located at the infrapopliteal segment than women. The effect of sex on revascularization outcomes in patients with CLTI is yet not clear and most available data are conflicting. We believe that unmeasured patient factors, rather than biological sex characteristics are the cause for the differences observed among women and men with CLTI, undergoing endovascular revascularization. Furthermore, a multivariate assessment of several risk factors will provide a more accurate prediction of outcomes, rather than the assessment of a single characteristic. Further studies are needed to evaluate sex-related variation in prognosis among patients with CLTI.
Limitations
The LIBERTY 360 study was a multicenter, core-laboratory adjudicated study with data about patients (ie, patients with CLTI) that were typically excluded from large clinical trials. However, our results should be interpreted in the context of several limitations. First, this is a post hoc analysis of data retrieved from the LIBERTY 360 study, which was an observational nonrandomized study of endovascular therapies, sparing open surgery.20 Second, site and patient participation bias may be resulted due to the requirement of extensive testing. Third, the outcomes might have been affected by the variable devices being used and the different preferred treatment algorithms among the physicians (eg, atherectomy, drug-eluting technology utilization, etc.). Thus, although this study provides important information regarding long-term outcomes (3-year follow-up) of endovascular therapy for CLTI among males vs females, its generalizability might be limited due to the lower drug-eluting technology utilization. However, taking into account the recently raised mortality concerns for DCB technology, this study significantly adds to the literature. Moreover, this study was sponsored by a company promoting atherectomy and as such bias could be attributed to extensive use of orbital atherectomy. Last, the lesion location exhibited high heterogeneity and sensitivity analyses for lesions limited to infrapopliteal or femoropopliteal segment could not be synthesized. Further studies should separately investigate the role of sex in short- and long-term outcomes of femoropopliteal/infrapopliteal revascularizations among patients with CLTI or claudication.
Conclusions
Females exhibited higher in-hospital all-cause mortality among patients undergoing endovascular revascularization, however no death was related to the procedure. At 18-months follow-up, female patients were at lower risk for major amputation and major amputation/death compared to men. Data regarding sex disparity in outcomes of endovascular therapy of patients with CLTI are conflicting. Unmeasured patient factors rather than biological sex characteristics might be the actual cause of these variable results. Further studies should guide the development of treatment algorithms based on multivariate assessment of risk factors for a more accurate prediction of outcomes among female and male patients.
Abbreviations
PAD, peripheral arterial disease; CTLI, chronic threatening-limb ischemia; AHA/ACC, American College of Cardiology/American Heart Association; HF, heart failure; CAD, coronary artery disease; ESRD, end-stage renal disease; MAE, major adverse event; CD-TVR, clinically driven target vessel revascularization; ABI, ankle-brachial index; RC, Rutherford classification; KM, Kaplan Meier; MLD, minimal lumen diameter; DAPT, dual anti-platelet therapy; RVD, reference vessel diameter; SFA, superficial femoral artery; ATK, above the knee lesions; BTK, below the knee lesions; DCB, drug-coated balloon; DES, drug-eluting stent; TVR, target vessel revascularization; IQR, interquartile range; CI, confidence interval; HR, hazard ratio; OR, odds ratio; N, number; NIS, Nationwide Inpatient Sample database.
Data Sharing Statement
Individual-deidentified participant data and study-related documents will not be made available.
Acknowledgments
All authors had unrestricted access to the data sets and can take responsibility for the integrity of the data and the accuracy of the data analysis. The authors also thank Ann Behrens, BS and Brad J. Martinsen, Ph.D. of Cardiovascular Systems, Inc, for editing and critical review of this manuscript.
Disclosure
Dr. Shammas Receives research and educational grants from BD, Boston Scientific, Phillips, Intact Vascular and VentureMed Group. Dr Cawich is a consultant to Avinger, BD, Cardiovascular Systems Incorporated (CSI) and Mercator. George L. Adams receives consultant fees from Bard Peripheral Vascular, Terumo Interventional Systems, Medtronic, Boston Scientific, Spectranetics, and Cardiovascular Systems Incorporated (CSI). Dr. Armstrong is a consultant to Abbott Vascular, Boston Scientific, Cardiovascular Systems Incorporated (CSI), Medtronic, Philips, and PQ Bypass. All other authors have no conflicts of interest to disclose.
References
1. Pande RL, Perlstein TS, Beckman JA, Creager MA. Secondary prevention and mortality in peripheral artery disease: national health and nutrition examination study, 1999 to 2004. Circulation. 2011;124(1):17–23. doi:10.1161/CIRCULATIONAHA.110.003954
2. Fowkes FG, Rudan D, Rudan I, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382(9901):1329–1340. doi:10.1016/S0140-6736(13)61249-0
3. Subherwal S, Patel MR, Kober L, et al. Peripheral artery disease is a coronary heart disease risk equivalent among both men and women: results from a nationwide study. Eur J Prev Cardiol. 2015;22(3):317–325. doi:10.1177/2047487313519344
4. McDermott MM, Hahn EA, Greenland P, et al. Atherosclerotic risk factor reduction in peripheral arterial disease: results of a national physician survey. J Gen Intern Med. 2002;17(12):895–904. doi:10.1046/j.1525-1497.2002.20307.x
5. Chen DC, Singh GD, Armstrong EJ, Waldo SW, Laird JR, Amsterdam EA. Long-term comparative outcomes of patients with peripheral artery disease with and without concomitant coronary artery disease. Am J Cardiol. 2017;119(8):1146–1152. doi:10.1016/j.amjcard.2016.12.023
6. Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg. 2007;45(Suppl S):S5–S67. doi:10.1016/j.jvs.2006.12.037
7. Levin SR, Arinze N, Siracuse JJ. Lower extremity critical limb ischemia: a review of clinical features and management. Trends Cardiovasc Med. 2019.
8. Becker F, Robert-Ebadi H, Ricco JB, et al. Chapter I: definitions, epidemiology, clinical presentation and prognosis. Eur J Vasc Endovasc Surg. 2011;42(Suppl 2):S4–S12. doi:10.1016/S1078-5884(11)60009-9
9. Jaff MR, White CJ, Hiatt WR, et al. An update on methods for revascularization and expansion of the TASC lesion classification to include below-the-knee arteries: a supplement to the inter-society consensus for the management of peripheral arterial disease (TASC II): the TASC steering committee. Ann Vasc Dis. 2015;8(4):343–357. doi:10.3400/avd.tasc.15-01000
10. Tendera M, Aboyans V, Bartelink ML, et al. ESC Guidelines on the diagnosis and treatment of peripheral artery diseases: document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2011;32(22):2851–2906. doi:10.1093/eurheartj/ehr211
11. Peacock JM, Keo HH, Duval S, et al. The incidence and health economic burden of ischemic amputation in Minnesota, 2005–2008. Prev Chronic Dis. 2011;8(6):A141.
12. Varu VN, Hogg ME, Kibbe MR. Critical limb ischemia. J Vasc Surg. 2010;51(1):230–241. doi:10.1016/j.jvs.2009.08.073
13. Allie DE, Hebert CJ, Lirtzman MD, et al. Critical limb ischemia: a global epidemic.A critical analysis of current treatment unmasks the clinical and economic costs of CLI. EuroIntervention. 2005;1(1):75–84.
14. Sachs T, Pomposelli F, Hamdan A, Wyers M, Schermerhorn M. Trends in the national outcomes and costs for claudication and limb threatening ischemia: angioplasty vs bypass graft. J Vasc Surg. 2011;54(4):1021–1031.e1021. doi:10.1016/j.jvs.2011.03.281
15. Rudofker EW, Hogan SE, Armstrong EJ. Preventing major amputations in patients with critical limb ischemia. Curr Cardiol Rep. 2018;20(9):74. doi:10.1007/s11886-018-1019-2
16. Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: executive Summary: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;69(11):1465–1508. doi:10.1016/j.jacc.2016.11.008
17. Jones WS, Dolor RJ, Hasselblad V, et al. Comparative effectiveness of endovascular and surgical revascularization for patients with peripheral artery disease and critical limb ischemia: systematic review of revascularization in critical limb ischemia. Am Heart J. 2014;167(4):489–498.e487. doi:10.1016/j.ahj.2013.12.012
18. Jones WS, Schmit KM, Vemulapalli S, et al. AHRQ Comparative Effectiveness Reviews. In: Treatment Strategies for Patients with Peripheral Artery Disease. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013 May. Report No.: 13-EHC090-EF.
19. Swaminathan A, Vemulapalli S, Patel MR, Jones WS. Lower extremity amputation in peripheral artery disease: improving patient outcomes. Vasc Health Risk Manag. 2014;10:417–424. doi:10.2147/VHRM.S50588
20. Mustapha J, Gray W, Martinsen BJ, et al. One-year results of the LIBERTY 360 study: evaluation of acute and midterm clinical outcomes of peripheral endovascular device interventions. J Endovasc Ther. 2019;26(2):143–154. doi:10.1177/1526602819827295
21. Goodney PP, Beck AW, Nagle J, Welch HG, Zwolak RM. National trends in lower extremity bypass surgery, endovascular interventions, and major amputations. J Vasc Surg. 2009;50(1):54–60. doi:10.1016/j.jvs.2009.01.035
22. Gerhard-Herman M, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive summary. Vasc Med. 2017;22(3):NP1–NP43. doi:10.1177/1358863X17701592
23. Katsanos K, Kitrou P, Spiliopoulos S, Diamantopoulos A, Karnabatidis D. Comparative Effectiveness of plain balloon angioplasty, bare metal stents, drug-coated balloons, and drug-eluting stents for the treatment of infrapopliteal artery disease: systematic review and bayesian network meta-analysis of randomized controlled trials. J Endovasc Ther. 2016;23(6):851–863. doi:10.1177/1526602816671740
24. Schillinger M, Sabeti S, Loewe C, et al. Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N Engl J Med. 2006;354(18):1879–1888. doi:10.1056/NEJMoa051303
25. Bunte MC, House JA, Spertus JA, Cohen DJ, Marso SP, Safley DM. Association between health status and long-term mortality after percutaneous revascularization of peripheral artery disease. Catheter Cardiovasc Interv. 2016;87(6):1149–1155. doi:10.1002/ccd.26442
26. Jones WS, Patel MR, Tsai TT, et al. Anatomic runoff score predicts cardiovascular outcomes in patients with lower extremity peripheral artery disease undergoing revascularization. Am Heart J. 2015;170(2):400–408. doi:10.1016/j.ahj.2015.04.026
27. Chang SH, Tsai YJ, Chou HH, et al. Clinical predictors of long-term outcomes in patients with critical limb ischemia who have undergone endovascular therapy. Angiology. 2014;65(4):315–322. doi:10.1177/0003319713515544
28. O’Brien-Irr MS, Dosluoglu HH, Harris LM, Dryjski ML. Outcomes after endovascular intervention for chronic critical limb ischemia. J Vasc Surg. 2011;53(6):1575–1581. doi:10.1016/j.jvs.2011.01.068
29. Krishnamurthy V, Munir K, Rectenwald JE, et al. Contemporary outcomes with percutaneous vascular interventions for peripheral critical limb ischemia in those with and without poly-vascular disease. Vasc Med. 2014;19(6):491–499. doi:10.1177/1358863X14552013
30. Meltzer AJ, Shrikhande G, Gallagher KA, et al. Heart failure is associated with reduced patency after endovascular intervention for symptomatic peripheral arterial disease. J Vasc Surg. 2012;55(2):353–362. doi:10.1016/j.jvs.2011.08.016
31. Chen DC, Armstrong EJ, Singh GD, Amsterdam EA, Laird JR. Adherence to guideline-recommended therapies among patients with diverse manifestations of vascular disease. Vasc Health Risk Manag. 2015;11:185–192. doi:10.2147/VHRM.S76651
32. Ortmann J, Nuesch E, Cajori G, et al. Benefit of immediate revascularization in women with critical limb ischemia in an intention-to-treat analysis. J Vasc Surg. 2011;54(6):1668–1678. doi:10.1016/j.jvs.2011.06.110
33. McCoach CE, Armstrong EJ, Singh S, et al. Gender-related variation in the clinical presentation and outcomes of critical limb ischemia. Vasc Med. 2013;18(1):19–26. doi:10.1177/1358863X13475836
34. Hirsch AT, Allison MA, Gomes AS, et al. A call to action: women and peripheral artery disease: a scientific statement from the American Heart Association. Circulation. 2012;125(11):1449–1472. doi:10.1161/CIR.0b013e31824c39ba
35. Egorova N, Vouyouka AG, Quin J, et al. Analysis of gender-related differences in lower extremity peripheral arterial disease. J Vasc Surg. 2010;51(2):
36. Pulli R, Dorigo W, Pratesi G, Fargion A, Angiletta D, Pratesi C. Gender-related outcomes in the endovascular treatment of infrainguinal arterial obstructive disease. J Vasc Surg. 2012;55(1):105–112. doi:10.1016/j.jvs.2011.07.050
37. Soga Y, Iida O, Takahara M, et al. Two-year life expectancy in patients with critical limb ischemia. JACC Cardiovasc Interv. 2014;7(12):1444–1449. doi:10.1016/j.jcin.2014.06.018
38. Armstrong EJ, Wu J, Singh GD, et al. Smoking cessation is associated with decreased mortality and improved amputation-free survival among patients with symptomatic peripheral artery disease. J Vasc Surg. 2014;60(6):1565–1571. doi:10.1016/j.jvs.2014.08.064
39. Armstrong EJ, Chen DC, Singh GD, Amsterdam EA, Laird JR. Angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use is associated with reduced major adverse cardiovascular events among patients with critical limb ischemia. Vasc Med. 2015;20(3):237–244. doi:10.1177/1358863X15574321
40. Westin GG, Armstrong EJ, Bang H, et al. Association between statin medications and mortality, major adverse cardiovascular event, and amputation-free survival in patients with critical limb ischemia. J Am Coll Cardiol. 2014;63(7):682–690. doi:10.1016/j.jacc.2013.09.073
41. Adams GL, Mustapha J, Gray W, et al. The LIBERTY study: design of a prospective, observational, multicenter trial to evaluate the acute and long-term clinical and economic outcomes of real-world endovascular device interventions in treating peripheral artery disease. Am Heart J. 2016;174:14–21. doi:10.1016/j.ahj.2015.12.013
42. Vogel TR, Dombrovskiy VY, Carson JL, Graham AM. In-hospital and 30-day outcomes after tibioperoneal interventions in the US Medicare population with critical limb ischemia. J Vasc Surg. 2011;54(1):109–115. doi:10.1016/j.jvs.2010.12.055
43. Iida O, Soga Y, Hirano K, et al. Midterm outcomes and risk stratification after endovascular therapy for patients with critical limb ischaemia due to isolated below-the-knee lesions. Eur J Vasc Endovasc Surg. 2012;43(3):313–321. doi:10.1016/j.ejvs.2011.11.025
44. Khaira KB, Brinza E, Singh GD, et al. Long-term outcomes in patients with critical limb ischemia and heart failure with preserved or reduced ejection fraction. Vasc Med. 2017;22(4):307–315. doi:10.1177/1358863X17714153
45. Singh GD, Armstrong EJ, Waldo SW, et al. Non-compressible ABIs are associated with an increased risk of major amputation and major adverse cardiovascular events in patients with critical limb ischemia. Vasc Med. 2017;22(3):210–217. doi:10.1177/1358863X16689831
46. Lansky AJ, Ng VG, Maehara A, et al. Gender and the extent of coronary atherosclerosis, plaque composition, and clinical outcomes in acute coronary syndromes. JACC Cardiovasc Imaging. 2012;5(3 Suppl):S62–S72. doi:10.1016/j.jcmg.2012.02.003
47. Forster A, Gass A, Kern R, et al. Gender differences in acute ischemic stroke: etiology, stroke patterns and response to thrombolysis. Stroke. 2009;40(7):2428–2432. doi:10.1161/STROKEAHA.109.548750
48. Norris CM, Johnson NL, Hardwicke-Brown E, McEwan M, Pelletier R, Pilote L. The contribution of gender to apparent sex differences in health status among patients with coronary artery disease. J Womens Health (Larchmt). 2017;26(1):50–57. doi:10.1089/jwh.2016.5744
49. Shishehbor MH, White CJ, Gray BH, et al. Critical limb ischemia: an expert statement. J Am Coll Cardiol. 2016;68(18):2002–2015. doi:10.1016/j.jacc.2016.04.071
50. Suckow BD, Goodney PP, Nolan BW, et al. Domains that determine quality of life in vascular amputees. Ann Vasc Surg. 2015;29(4):722–730. doi:10.1016/j.avsg.2014.12.005
51. Ramkumar N, Suckow BD, Brown JR, et al. Role of sex in determining treatment type for patients undergoing endovascular lower extremity revascularization. J Am Heart Assoc. 2019;8(17):e013088. doi:10.1161/JAHA.119.013088
52. Eugster T, Gurke L, Obeid T, Stierli P. Infrainguinal arterial reconstruction: female gender as risk factor for outcome. Eur J Vasc Endovasc Surg. 2002;24(3):245–248. doi:10.1053/ejvs.2002.1712
53. Vouyouka AG, Egorova NN, Salloum A, et al. Lessons learned from the analysis of gender effect on risk factors and procedural outcomes of lower extremity arterial disease. J Vasc Surg. 2010;52(5):1196–1202. doi:10.1016/j.jvs.2010.05.106
54. Magnant JG, Cronenwett JL, Walsh DB, Schneider JR, Besso SR, Zwolak RM. Surgical treatment of infrainguinal arterial occlusive disease in women. J Vasc Surg. 1993;17(1):
55. Choi KH, Park TK, Kim J, et al. Sex differences in outcomes following endovascular treatment for symptomatic peripheral artery disease: an analysis from the K- VIS ELLA registry. J Am Heart Assoc. 2019;8(2):e010849. doi:10.1161/JAHA.118.010849
56. Schaumeier MJ, Hawkins AT, Hevelone ND, Sethi RKV, Nguyen LL. Association of treatment for critical limb ischemia with gender and hospital volume. Am Surg. 2018;84(6):1069–1078.
57. Miller SM, Sumpio BJ, Miller MS, Erben Y, Cordova AC, Sumpio BE. Higher inpatient mortality for women after intervention for lifestyle limiting claudication. Ann Vasc Surg. 2019;58:54–62. doi:10.1016/j.avsg.2019.01.006
58. Ferranti KM, Osler TM, Duffy RP, Stanley AC, Bertges DJ. Association between gender and outcomes of lower extremity peripheral vascular interventions. J Vasc Surg. 2015;62(4):990–997. doi:10.1016/j.jvs.2015.03.066
59. Fernandez N, McEnaney R, Marone LK, et al. Multilevel versus isolated endovascular tibial interventions for critical limb ischemia. J Vasc Surg. 2011;54(3):722–729. doi:10.1016/j.jvs.2011.03.232
60. Ortmann J, Nuesch E, Traupe T, Diehm N, Baumgartner I. Gender is an independent risk factor for distribution pattern and lesion morphology in chronic critical limb ischemia. J Vasc Surg. 2012;55(1):98–104. doi:10.1016/j.jvs.2011.07.074
61. Kumakura H, Kanai H, Araki Y, et al. Sex-related differences in Japanese patients with peripheral arterial disease. Atherosclerosis. 2011;219(2):846–850. doi:10.1016/j.atherosclerosis.2011.08.037
62. Diehm N, Shang A, Silvestro A, et al. Association of cardiovascular risk factors with pattern of lower limb atherosclerosis in 2659 patients undergoing angioplasty. Eur J Vasc Endovasc Surg. 2006;31(1):59–63. doi:10.1016/j.ejvs.2005.09.006
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
