Back to Journals » Vascular Health and Risk Management » Volume 18
Severity and Extent of Lead-Related Venous Obstruction in More Than 3000 Patients Undergoing Transvenous Lead Extraction
Authors Czajkowski M, Jacheć W , Polewczyk A , Kosior J, Nowosielecka D , Tułecki, Stefańczyk P, Kutarski A
Received 4 April 2022
Accepted for publication 7 July 2022
Published 17 August 2022 Volume 2022:18 Pages 629—642
DOI https://doi.org/10.2147/VHRM.S369342
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Pietro Scicchitano
Marek Czajkowski,1 Wojciech Jacheć,2 Anna Polewczyk,3,4 Jarosław Kosior,5 Dorota Nowosielecka,6 Łukasz Tułecki,7 Paweł Stefańczyk,6 Andrzej Kutarski8
1Department of Cardiac Surgery, Medical University of Lublin, Lublin, Poland; 2Department of Cardiology, Zabrze, Faculty of Medical Science in Zabrze, Medical University of Silesia in Katowice, Zabrze, Poland; 3Department of Physiology, Pathophysiology and Clinical Immunology, Collegium Medicum of Jan Kochanowski University, Kielce, Poland; 4Department of Cardiac Surgery, Świętokrzyskie Center of Cardiology, Kielce, Poland; 5Department of Cardiology, Masovian Specialistic Hospital of Radom, Radom, Poland; 6Department of Cardiology, The Pope John Paul II Province Hospital of Zamość, Zamość, Poland; 7Department of Cardiac Surgery, The Pope John Paul II Province Hospital of Zamość, Zamość, Poland; 8Department of Cardiology, Medical University of Lublin, Lublin, Poland
Correspondence: Anna Polewczyk, Department of Physiology, Pathophysiology and Clinical Immunology, Collegium Medicum of Jan Kochanowski University, Aleja IX Wieków Kielc 19A, Kielce, 25-317, Poland, Tel +48600024074, Email [email protected]
Background: Lead-related venous stenosis/obstruction (LRVSO) may be a major challenge in patients with cardiac implantable electronic devices (CIED) when device upgrade, insertion of central lines, or creation of an arteriovenous fistula for hemodialysis is indicated. The aim of this study was to evaluate the extent and severity of LRVSO.
Methods: We performed a retrospective analysis of 3002 venograms from patients awaiting transvenous lead extraction (TLE) to assess the occurrence, severity, and extent of LRVSO.
Results: Mild LRVSO occurred in 19.9%, moderate in 20.7%, severe in 19.9% and total venous occlusion in 22.5% of the patients. Moderate/severe stenosis or total occlusion of the subclavian and brachiocephalic veins was found in 38.2% and 22.5% of the patients, respectively. LRSVO was not detected in 16.9% of the patients. Moderate and severe superior vena cava (SVC) obstruction and total SVC occlusion were rare (0.4%, 0.3%, and 0.3%, respectively). Lead insertion on the left side of the chest contributed to an increased risk of LRVSO compared to right-sided implantation. Major thoracic veins on the opposite side may be narrowed in varying degrees.
Conclusion: A total of 60% of the patients with pacemaker or high-voltage leads have an advanced form of LRVSO. Any attempt to insert new pacing leads, central lines, venous ports, or catheters for hemodialysis, or to create dialysis fistula on the same side as the existing lead should be preceded by venography. Furthermore, venography may provide useful information, if it is planned to implant the lead or the catheter on the opposite side of the chest.
Keywords: lead-related venous obstruction, risk factors, abandoned leads
Introduction
Varying degrees of venous obstruction in the thorax in patients with cardiac implantable electronic devices (CIED) are relatively common, but usually asymptomatic.1–23 Lead-related venous obstruction (LRVSO) can make it difficult or even impossible to insert a new lead, venous port, central line, or to create an arteriovenous (AV) fistula for hemodialysis.24–26 Most of the studies address the occurrence and severity of venous obstruction and risk factors for LRVSO,1–7,9–11,13–20,23 but nobody has performed a precise analysis of the location and extent of the obstructed venous segment in relation to the side of the implanted device. This study, as a continuation of risk factor analysis, attempts to assess the extent of the phenomenon in patients referred to high-volume center for transvenous lead extraction (TLE).21,22 Transvenous lead extraction plays a key role in managing patients with CIED-related complications.21,27–32 In patients undergoing TLE a venogram is needed before the procedure to check the possibility of preserving vessels for potential future use.21,22,28,29 Scarce information on the location of the obstructed vein segment, its extent and relation to the side of the implanted device prompted us to perform an in-depth analysis of LRVSO in 3002 patients referred for TLE at three high-volume centers, coordinated by the same cardiologist.
This study attempted to establish the occurrence, location, extent (number of venous segments affected) of vein obstruction and the relationship between LRVSO and the chest side of CIED implantation. An additional goal was to evaluate patency of major veins of the thorax on the opposite side of device placement.
Materials and Methods
Study Population
This analysis used clinical data of 3546 patients who underwent transvenous lead extraction (TLE) between June 2008 and July 2021. Patients with any contraindication to venography were excluded from the study. Finally, the data of 3002 patients who underwent venography were analyzed.
TLE was performed by the same experienced cardiologist at three different high-volume centers. Patients were eligible if preoperative venography allowed for an objective assessment of the venous system (axillary, subclavian, brachiocephalic veins and superior vena cava) on the side of lead insertion. All information regarding the patient and the procedure was entered into the computer database on a current basis.
This report comes from three collaborating high-volume centers performing more than 200 TLE procedures annually.
Venography Procedure
An intravenous catheter for preoperative venography was placed in the peripheral vein in the arm on the side of the existing or planned lead implantation. All patients received an injection of 20–40 mL contrast medium containing 350 mg iodine/mL. Venograms were obtained in the anteroposterior projection and the frames capturing the major veins of the thorax were stored and used for subsequent analysis of venous patency.
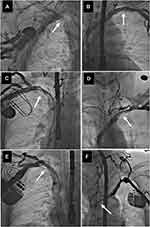
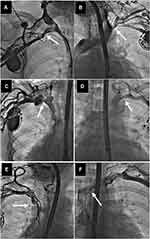
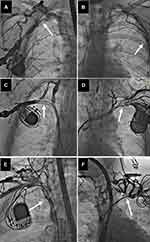
All radiographic images were evaluated by the cardiac surgeon and the cardiologist with experience in interventional radiology, and venous patency was graded on a 5-degree scale from normal flow to complete occlusion as described previously.21 Selected frames of each venogram were used to determine the degree of narrowing at each venous level and divided into five classes, ie, no stenosis (patent), mild narrowing defined as <1/3 reduction in venous diameter in the posteroanterior view (Figure 1) moderate stenosis: 1/3 to 2/3 reduction in venous diameter), severe stenosis (≥2/3 narrowing, but still patent (Figure 2) and complete occlusion of the axillary (AxV), subclavian (ScV), innominate (brachiocephalic) (AnV) veins and superior vena cava (SVC) (Figure 3).
Unilateral administration of the contrast medium to visualize the indwelling leads enabled, in some cases, evaluation of the brachiocephalic vein on the opposite side of the chest due to the development of thoracic and neck collateral vessels. Based on a subjective assessment of venous blood flow and severity of stenosis, we also made an attempt to predict long-term patency of potential ipsilateral arteriovenous fistula for hemodialysis access. This classification of vessel narrowing has, in our opinion, a practical meaning.
While mild stenosis should not affect an additional lead implantation or catheter insertion, special equipment and skills may be required in patients with moderate narrowing, as compared to simple cardiovascular, anesthesia-related, or renal interventions in subjects without any stenosis. Severe narrowing may cause even a bigger problem, when the vein, identified as patent by ultrasound, may not allow for safe catheter insertion and placement due to proximal stenosis, not detected during an ultrasound examination.
In patients with complete venous occlusion insertion of central lines may be risky, chances to effectively place the device are low and are largely dependent on the presence of collateral blood flow. There is also a very small chance that AV fistula will have adequate blood flow to support hemodialysis.
The number and total length of stenotic venous sites can affect the likelihood of replacing the existing device, upgrading or adding new devices, leads, or catheters.
Patient Groups
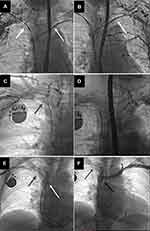
For the purposes of analysis, the study population was divided into three groups according to the side of device placement: group I – with leads implanted on the left side, group II – with leads implanted on the right side and group III – with leads implanted on both sides of the chest. The contralateral venous system was evaluated whenever possible (Figure 4).
Lead Extraction Procedure
Lead extractions were performed in compliance with the 2017 HRS and 2018 EHRA guidelines on management of lead-related complications.27–29 Indications for TLE or operation-related complications were defined as described in the 2017 HRS Expert Consensus Statement on Cardiovascular Implantable Electronic Device Lead Management and Extraction.28
Conventional mechanical sheaths were used as a first choice; powered rotational mechanical sheaths and other instruments were the second-line tools. Laser sheaths were not available at the centers.
Statistical Analysis
The Shapiro–Wilk test showed that most continuous variables were normally distributed. For uniformity, all continuous variables are presented as the mean ± standard deviation. The categorical variables are presented as number and percentage. The significance of differences between groups was determined using the nonparametric Chi2 test with Yates’ correction statistical analysis was performed using Statistica version 13.3 (TIBCO Software Inc.).
Approval of the Bioethics Committee
All patients gave their informed written consent to undergo TLE and use anonymous data from their medical records. The consent was approved by the Bioethics Committee at the Regional Chamber of Physicians in Lublin no. 288/2018/KB/VII. The study, procedures, and data collection were carried out in line with the ethical standards of the 1964 Declaration of Helsinki.
Results
The study group consisted of 3002 patients (mean age 66.87 years, 39.35% females). Ischemic heart disease (IHD) was found to be the most common medical condition (57.48%). The mean left ventricular ejection fraction (LVEF) was 49.01%. The Charlson comorbidity index33 was 4.78 points on average. The most common indication for TLE was lead failure (51.02%), lead-related infective endocarditis (LRIE) with or without pocket infection (PI) (20.72%), and local pocket infection (8.23%). Complete procedural success of TLE was achieved in 95.5% of the patients with the rate of major complications being 1.77% (Table 1).
 |
Table 1 Demographic, Clinical and Procedure-Related Data |
In this study, mild narrowing of the access vein occurred in 19.92%, moderate in 20.69%, severe in 19.92% and total occlusion in 22.49% of the patients. The subclavian vein was the most common site for venous obstruction (38.18%) (Table 2).
 |
Table 2 Preliminary Evaluation of Venous Obstruction in the 3002 Patients |
Analysis of the chest side lead location showed that all veins on the side of lead implantation can be affected. The narrowing of the axillary vein occurred only in patients with left-sided lead location. The subclavian vein was significantly more often obstructed (moderate or severe stenosis, total occlusion when the leads were on the left side of the chest (56.03% vs 33.75%; p<0.001), similarly to the brachiocephalic vein (28.12% vs 18.75%; p=0.086). The left-sided approach for lead implantation was associated with an increased risk and higher degree of LRVSO (68.72 vs 52.50%; p<0.001). If the leads were on both sides of the chest, the risk of LRVSO was comparable. Columns A and B of the table show patency of major thoracic veins on the side of lead placement, whereas columns C and D present venous patency on the side opposite to lead implantation. If the leads were on the left side of the chest, the contralateral subclavian and brachiocephalic veins were rarely stenosed; the incidence of moderate/severe narrowing and total occlusion of the subclavian vein and brachiocephalic vein was 2.57% and 1.33%, respectively. However, when the leads were on the right side of the chest, the contralateral veins were more often affected (25.40% and 13.04%) (Table 3).
 |
Table 3 Chest Side Lead Location and Severity of Venous Obstruction |
Severe stenosis or total occlusion of the superior vena cava (SVC) is a significant complication in patients with permanent pacing and may be symptomatic when collateral circulation is insufficient. In this study, all degrees of SVC flow reduction were rare (1.899%) and differences between cases with left- and right-sided lead placement appeared random. However, moderate/severe SVC stenosis or occlusion occurred in 0.88% of the patients with unilateral lead location, whereas bilateral lead placement was associated with a significantly increased rate of SVC obstruction (3.896%) (Table 4).
 |
Table 4 Superior Vena Cava Obstruction Depending on Chest Side Lead Location |
Discussion
Obstruction of the major veins of the thorax is a well-known complication of permanent transvenous pacing; studies have reported the incidence of obstruction to be as high as 30–45% with an average rate of mild stenosis being 10%,4,10 23%12 and 40%.19 Moderate stenosis has been found in 6–8%,10,16,19 12–13%11,12,18 and 23–50%9,17 of patients, whereas severe stenosis/total occlusion has been reported in 3–9%4,10,11,18,19 and 11–22%9,12,15,17,20 of the patients. The present study demonstrated mild lead-related narrowing of the veins in 19.92%, moderate in 20.69%, severe in 19.92% and total occlusion in 22.49% of the patients. The above cited studies were performed a planned follow-up at 6–12 months after lead implantation1,2,4,5,16 or later,7,16,17,19,20 or at first unit replacement,10–12 and TLE procedure.3,6,13,14 In our patients, candidates for TLE, dwell time of the oldest lead per patient was 102.4 months and cumulative dwell time of the leads was 15.47 years.
Additionally, the present study revealed that the subclavian and brachiocephalic veins were the most frequently narrowed/occluded vessels (38.18% and 24.22%), and the two veins were simultaneously affected in 22.15% of the patients. High-grade obstruction (moderate/severe stenosis or total occlusion) involved one of these veins or two veins simultaneously in 38.37% and 22.15% of the patients, respectively. Moderate and severe stenosis of the superior vena cava was rare (0.338% and 0.270%), similar to total SVC occlusion (0.300%).
Among the 22 reports, there are many studies looking at the risk of LRVSO1–7,9,11,13–16,18–20 but they were performed in relatively small cohorts of patients. The investigators searched for modifiable risk factors to predict the frequency and severity of LRVSO. Low LVEF,1,4,12 atrial fibrillation,4,14 no antiplatelet treatment or anticoagulation5,11 were considered as risk factors for LRVSO. All investigators agreed that gender had no influence1,3–8,10,11,15,16,18,20 on the development of LRVSO. There is much debate among investigators over the importance of the number of leads (lead burden) as a risk factor for LRVSO. Some of them support the view,3,5,11,14,18 whereas others argue against.1,2,6,7,9,15,16,19 Similarly, several authors consider lead caliber as a risk factor for LRVSO10,11,14,17,18 but the others do not.1–7,15,16,19,20 Still another factor is lead implant duration, and all investigators agree that it has no significant influence on the risk of LRVSO.1,11,15,16,20
Nobody has considered the influence of chest side lead location on LRVSO so far. We showed that left-sided lead implantation is associated with an increased risk and higher occurrence of LRVSO of all grades (68.72% vs 52.50%). This phenomenon may be related to the anatomical variability documented in the literature.34–36 Our findings show patency of large veins on the contralateral chest side. If the lead is on the left side, the subclavian and brachiocephalic veins on the right side are rarely obstructed; the incidence of high-grade narrowing in the two veins (moderate/severe stenosis or total occlusion) was 2.566% and 1.333%, respectively. But if the lead is on the right side of the chest, the veins on the contralateral side are more often affected ie 25.40% and 13.04%. This seemingly strange phenomenon can be accounted for by a history of right-sided vein use, probably after previous left side access. It appears to be a very important message in all patients with implanted leads who need access for urgent hemodialysis or creation of the arteriovenous fistula for dialysis. Such patients more often than the others may need acute intervention with additional transvenous device implantation, and the results of widely available ultrasound may be misleading in the context of venous stenosis and proximal patency. In our opinion, every attempt at placement of permanent or temporary pacing leads, central lines, venous ports, hemodialysis catheters, or creation of dialysis fistula in patients with left-sided leads needs previous venography, especially in subjects with long implant duration. In acute conditions, when venography is not available, in our judgement, it is best to avoid central vein cannulation ipsilaterally to the existing PM system. This piece of knowledge may be clinically important for anesthesiologists, vascular surgeons, cardiologists and nephrologists. If it is planned to use the opposite side of the chest, venography is also recommended.
Study Limitations
Our study has several limitations worth noting. Routine venography before TLE was performed in patients without contraindications for using contrast agents (mainly renal failure). Thus, this subpopulation was not included in the study. Analysis was performed retrospectively using the database that was prospectively collected and integrated. In most patients, venography was performed on one side only, ie, on the side of lead insertion to avoid contrast overload.
Conclusions
- The degree of venous stenosis in this large population of patients with cardiac implantable electronic devices ranges from mild lead-related venous obstruction in 19.92%, through moderate in 20.69%, and severe in 19.92% to total occlusion in 22.49% of the patients.
- The subclavian and brachiocephalic veins are the most frequently narrowed/occluded vessels. Moderate and severe superior vena cava stenosis and total SVC occlusion are rare.
- Left-sided approach for lead placement is associated with higher occurrence, higher severity of LRVSO and larger extent of significant vein narrowing. Bilateral lead location is an important risk factor for lead-related venous stenosis/occlusion.
- Large thoracic veins on the opposite side to lead insertion may be obstructed in varying degrees. If the lead is on the left side, the right subclavian and brachiocephalic veins are rarely obstructed.
- Contrast venogram should be obtained before placement of permanent or temporary pacing leads, central lines, venous ports, hemodialysis catheters, or creation of dialysis fistula in patients with left-sided leads. If it is planned to use the opposite side of the chest, venography is also recommended.
Data Sharing Statement
Readers can access the data supporting the conclusions of the study at www.usuwanieelektrod.pl.
Funding
This research received no external funding.
Disclosure
The authors declare no conflict of interest.
References
1. Da Costa SS, Scalabrini NA, Costa R, et al. Incidence and risk factors of upper extremity deep vein lesions after permanent transvenous pacemaker implant: a 6-month follow-up prospective study. Pacing Clin Electrophysiol. 2002;25(9):1301–1306. doi:10.1046/j.1460-9592.2002.01301.x
2. Korkeila P, Mustonen P, Koistinen J, et al. Clinical and laboratory risk factors of thrombotic complications after pacemaker implantation: a prospective study. Europace. 2010;12(6):817–824. doi:10.1093/europace/euq075
3. Li X, Ze F, Wang L, et al. Prevalence of venous occlusion in patients referred for lead extraction: implications for tool selection. Europace. 2014;16(12):1795–1799. doi:10.1093/europace/euu124
4. Korkeila P, Nyman K, Ylitalo A, et al. Venous obstruction after pacemaker implantation. Pacing Clin Electrophysiol. 2007;30(2):199–206. doi:10.1111/j.1540-8159.2007.00650.x
5. van Rooden CJ, Molhoek SG, Rosendaal FR, et al. Incidence and risk factors of early venous thrombosis associated with permanent pacemaker leads. J Cardiovasc Electrophysiol. 2004;15(11):1258–1262. doi:10.1046/j.1540-8167.2004.04081.x
6. Boczar K, Zabek A, Haberka K, et al. Venous stenosis and occlusion in the presence of endocardial leads in patients referred for transvenous lead extraction. Acta Cardiol. 2017;72(1):61–67. doi:10.1080/00015385.2017.1281545
7. Oginosawa Y, Abe H, Nakashima Y. The incidence and risk factors for venous obstruction after implantation of transvenous pacing leads. Pacing Clin Electrophysiol. 2002;25(11):1605–1611. doi:10.1046/j.1460-9592.2002.01605.x
8. Abu-El-Haija B, Bhave PD, Campbell DN, et al. Venous stenosis after transvenous lead placement: a study of outcomes and risk factors in 212 consecutive patients. J Am Heart Assoc. 2015;4(8):e001878. doi:10.1161/JAHA.115.001878
9. Goto Y, Abe T, Sekine S, et al. Long-term thrombosis after transvenous permanent pacemaker implantation. Pacing Clin Electrophysiol. 1998;21(6):1192–1195. doi:10.1111/j.1540-8159.1998.tb00177.x
10. Lickfett L, Bitzen A, Arepally A, et al. Incidence of venous obstruction following insertion of an implantable cardioverter defibrillator. A study of systematic contrast venography on patients presenting for their first elective ICD generator replacement. Europace. 2004;6(1):25–31. doi:10.1016/j.eupc.2003.09.001
11. Haghjoo M, Nikoo MH, Fazelifar AF, et al. Predictors of venous obstruction following pacemaker or implantable cardioverter-defibrillator implantation: a contrast venographic study on 100 patients admitted for generator change, lead revision, or device upgrade. Europace. 2007;9(5):328–332. doi:10.1093/europace/eum019
12. Albertini CMM, Silva KRD, Filho L, et al. Usefulness of preoperative venography in patients with cardiac implantable electronic devices submitted to lead replacement or device upgrade procedures. Arq Bras Cardiol. 2018;111(5):686–696. doi:10.5935/abc.20180164
13. Arora Y, Carrillo RG. Lead-related superior vena cava syndrome: management and outcomes. Heart Rhythm. 2021;18(2):207–214. doi:10.1016/j.hrthm.2020.09.006
14. Bulur S, Vural A, Yazıcı M, et al. Incidence and predictors of subclavian vein obstruction following biventricular device implantation. J Interv Card Electrophysiol. 2010;29(3):199–202. doi:10.1007/s10840-010-9516-2
15. Sohal M, Williams S, Akhtar M, et al. Laser lead extraction to facilitate cardiac implantable electronic device upgrade and revision in the presence of central venous obstruction. Europace. 2014;16(1):81–87. doi:10.1093/europace/eut163
16. Bar-Cohen Y, Berul CI, Alexander ME, et al. Age, size, and lead factors alone do not predict venous obstruction in children and young adults with transvenous lead systems. J Cardiovasc Electrophysiol. 2006;17(7):754–759. doi:10.1111/j.1540-8167.2006.00489.x
17. Figa FH, McCrindle BW, Bigras JL, et al. Risk factors for venous obstruction in children with transvenous pacing leads. Pacing Clin Electrophysiol. 1997;20(8):1902–1909. doi:10.1111/j.1540-8159.1997.tb03594.x
18. Safi M, Akbarzadeh MA, Azinfar A, et al. Upper extremity deep venous thrombosis and stenosis after implantation of pacemakers and defibrillators; a prospective study. Rom J Intern Med. 2017;55(3):139–144. doi:10.1515/rjim-2017-0018
19. Antonelli D, Turgeman Y, Kaveh Z, et al. Short-term thrombosis after transvenous permanent pacemaker insertion. Pacing Clin Electrophysiol. 1989;12(2):280–282. doi:10.1111/j.1540-8159.1989.tb02660.x
20. Zuber M, Huber P, Fricker U, et al. Assessment of the subclavian vein in patients with transvenous pacemaker leads. Pacing Clin Electrophysiol. 1998;21(12):2621–2630. doi:10.1111/j.1540-8159.1998.tb00039.x
21. Czajkowski M, Jacheć W, Polewczyk A, et al. The influence of lead-related venous obstruction on the complexity and outcomes of transvenous lead extraction. Int J Environ Res Public Health. 2021;18(18):1–16. doi:10.3390/ijerph18189634
22. Czajkowski M, Jacheć W, Polewczyk A, et al. Risk factors for lead-related venous obstruction: a study of 2909 candidates for lead extraction. J Clin Med. 2021;10(21):51–58. doi:10.3390/jcm10215158
23. Rozmus G, Daubert JP, Huang DT, et al. Venous thrombosis and stenosis after implantation of pacemakers and defibrillators. J Interv Card Electrophysiol. 2005;13(1):9–19. doi:10.1007/s10840-005-1140-1
24. Saad TF, Ahmed W, Davis K, et al. Cardiovascular implantable electronic devices in hemodialysis patients: prevalence and implications for arteriovenous hemodialysis access interventions. Semin Dial. 2015;28(1):94–100. doi:10.1111/sdi.12249
25. Spatola L, Rivera RF, Migliore F, et al. Cardiovascular implantable electronic devices in hemodialysis patients: an updated review. J Cardiovasc Med. 2021;22(12):867–873. doi:10.2459/JCM.0000000000001214
26. Tan CS, Jie C, Joe J, et al. The impact of transvenous cardiac devices on vascular access patency in hemodialysis patients. Semin Dial. 2013;26(6):728–733. doi:10.1111/sdi.12073
27. Wilkoff BL, Love CJ, Byrd CL, et al.; Heart Rhythm Society; American Heart Association. Transvenous lead extraction: Heart Rhythm Society expert consensus on facilities, training, indications, and patient management: this document was endorsed by the American Heart Association (AHA). Heart Rhythm. 2009;6(7):1085–1104. doi:10.1016/j.hrthm.2009.05.020.
28. Bongiorni MG, Burri H, Deharo JC, et al. 2018 EHRA expert consensus statement on lead extraction: recommendations on definitions, endpoints, research trial design, and data collection requirements for clinical scientific studies and registries: endorsed by APHRS/HRS/LAHRS. Europace;2018. 1–11. doi:10.1093/europace/eux086
29. Kusumoto FM, Schoenfeld MH, Wilkoff B, et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm. 2017;14(12):e503–e551. doi:10.1016/j.hrthm.2017.09.001
30. Starck CT, Gonzalez E, Al-Razzo O, et al. Results of the Patient-Related Outcomes of Mechanical lead Extraction Techniques (PROMET) study: a multicentre retrospective study on advanced mechanical lead extraction techniques. Europace. 2020;22(7):1103–1110. doi:10.1093/europace/euaa103
31. Morani G, Bolzan B, Valsecchi S, Morosato M, Ribichini FL. Chronic venous obstruction during cardiac device revision: incidence, predictors, and efficacy of percutaneous techniques to overcome the stenosis. Heart Rhythm. 2020;17(2):258–264. doi:10.1016/j.hrthm.2019.08.012
32. Bracke F, Meijer A, Van Gelder B. Venous occlusion of the access vein in patients referred for lead extraction: influence of patient and lead characteristics. Pacing Clin Electrophysiol. 2003;26(8):1649–1652. doi:10.1046/j.1460-9592.2003.t01-1-00247.x
33. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–483. doi:10.1016/0021-9681(87)90171-8
34. Chowdhury UK, Anderson RH, Sankhyan LK, et al. Surgical management of lesions encountered in the setting of the retroaortic left brachiocephalic vein. J Card Surg. 2021;36(11):4280–4429. doi:10.1111/jocs.15907
35. Gaeta G, Fesslova V, Villanacci R, et al. Prenatal diagnosis and postnatal outcomes of left brachiocephalic vein abnormalities: systematic review. J Clin Med. 2022;11(7):1805. doi:10.3390/jcm11071805
36. Strachinaru M, Papadopoulou B, Damry N, Laureys M, Samyn S. Contrast echocardiography detecting a rare abnormal venous connection. Eur Heart J Cardiovasc Imaging. 2015;16(7):813. doi:10.1093/ehjci/jev062
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.