Back to Journals » Infection and Drug Resistance » Volume 16
Severe Community-Acquired Pneumonia Caused by Methicillin-Sensitive Staphylococcus aureus: Successfully Treated with Contezolid – A Case Report and Literature Review
Authors Wang K, Hu Y, Duan Z, Fu H, Hu X, Zhao Y, Wen R, Li L, Xie F
Received 1 February 2023
Accepted for publication 24 April 2023
Published 23 May 2023 Volume 2023:16 Pages 3233—3242
DOI https://doi.org/10.2147/IDR.S406799
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Kaifei Wang, Ye Hu, Zhimei Duan, Han Fu, Xingshuo Hu, Ying Zhao, Ruoxuan Wen, Lina Li, Fei Xie
College of Pulmonary and Critical Care Medicine, Chinese PLA General Hospital, Beijing, People’s Republic of China
Correspondence: Fei Xie, College of Pulmonary and Critical Care Medicine, Chinese PLA General Hospital, No. 28 Fuxing Road, Beijing, People’s Republic of China, Tel +86-15001028681, Email [email protected]
Background: Staphylococcus aureus has been well recognized as an important cause of community-acquired pneumonia (CAP), with non-specific characteristics and poor prognosis. In severe CAP (SCAP) guidelines, β-lactam combined with macrolides or fluoroquinolones therapy was recommended, but the efficacy is not satisfactory due to the continued spread of antimicrobial resistance. Contezolid is a new representative of oxazolidinones in clinical development, but no relevant reports have been reported for the treatment of SCAP. This was the first report of a patient with Staphylococcus aureus SCAP who was successfully treated with contezolid combined with other antibiotics and rehabilitation exercise.
Case Presentation: A 44-year-old woman with high blood pressure and diabetes was admitted to our hospital owing to cough, sputum, wheezing for 2 weeks, and aggravation for 2 days. The bronchoscopic alveolar lavage and microorganism-Rapid On Site Evaluation (BAL-mROSE) was used to get pathological data, which were positive for Staphylococcus aureus, in line with blood cultures. During hospitalization, the patient received endotracheal intubation for assisted breathing and anti-infective therapy, including meropenem, linezolid, teicoplanin and tazocin successively. Finally, contezolid obtained excellent result, with platelet recovery to normal levels and significant improvement in pulmonary imaging. Meanwhile, the patient’s swallowing disorder improved after continuous rehabilitation exercise. After discharge, she received contezolid consolidation therapy for 1 week and was free of complaints during the 30-day follow-up without any special treatment for SCAP.
Discussion: Treatment with contezolid combined with other antibiotics and rehabilitation exercise for SCAP has shown remarkable efficacy and good safety; hence, this regimen is a promising treatment strategy for this fatal disease.
Keywords: severe community-acquired pneumonia, methicillin-sensitive Staphylococcus aureus, antibiotic therapy, contezolid, rehabilitation exercise
Introduction
Staphylococcus aureus is one of the most difficult Gram-positive pathogens to treat and is commonly isolated as a cause of serious health care-associated infections, including pneumonia.1 The classification of pneumonia includes community-acquired pneumonia (CAP), hospital-acquired pneumonia and ventilator-associated pneumonia.2 Among the typical causes bacterial of CAP, Staphylococcus aureus accounted for less than 5% of all cases and was associated with worse clinical outcomes.3 In addition, the clinical features of Staphylococcus aureus pneumonia are non-specific, which makes the treatment of infection more complex.2 In recent decades, the number of patients requiring intensive care management due to severe community-acquired pneumonia (SCAP) has increased worldwide, resulting in a severely high clinical burden. Existing data also positively point out that the delay in admission to the intensive care unit (ICU) significantly increases mortality in patients with SCAP.4 These findings suggest that it is urgent to timely identify patients who need intensive care management and give guidance to empirical antibiotic treatment.
Current guidelines recommend the use of β-lactam alone or combination with a macrolide or fluoroquinolone for the treatment of CAP, except in patients admitted to the ICU, patients with concomitant diseases, or patients with risk factors associated with greater resistance to pathogens causing pneumococcal CAP.5 In patients admitted to the ICU, β-lactam is recommended in combination with macrolides or fluoroquinolones.6 However, due to the continued spread of antimicrobial resistance, this has greatly increased incidence rate, mortality and health care costs. Therefore, alternative treatments and novel drugs should be considered.
Oxazolidinones are a kind of synthetic antimicrobial agents, which is used to treat serious infections caused by Gram-positive pathogens. They can inhibit the elongation of polypeptide chain during protein synthesis by binding to the A site of bacterial ribosomes.7 The mechanism of oxazolidinone is unique, and there is no cross-resistance with other protein synthesis inhibitors.8 However, due to homology between 23S ribosomal targets and the closely related mitochondrial protein synthesis machinery in mammals, safety concerns have undermined the clinical development of many oxazolidinone compounds.9 The first antibacterial oxazolidinone, linezolid, was introduced in 2000. Contezolid is a new representative of oxazolidinones in clinical development, and was launched in China in June 2021. It is designed to overcome certain serious limitations of linezolid such as myelosuppression, as well as drug–drug and food–drug interaction potential (due to monoamine oxidase inhibition) reflected in the Warnings and Precautions in the Prescribing Information for linezolid by adjusting the B ring, C ring and C-5 domains.10 In addition, the improved activity of contezoid can be largely attributed to this dramatic special change.9 Staphylococcus aureus is susceptible to contezolid, and contezolid is proved to be less likely to cause resistance in Staphylococcus aureus.11 Consequently, Contezolid has the potential to provide a promising alternative therapy for Gram-positive bacterial infections, but there are few studies on the treatment of this deadly disease.
This was the first report of a patient with Staphylococcus aureus SCAP who was successfully treated with contezolid combined with other antibiotics and rehabilitation exercise. This study was approved by ethics committee of Chinese PLA General Hospital. Written informed consent was obtained from the individual for the publication of any potentially identifiable images or data included in this article. In addition, the patient and her family members are aware of her condition and previous treatment history, and know that contezolid used is a newly marketed drug, as well as the situation of other patients who have used this drug. The patient and their family members agree to try using this antibiotic for treatment.
Case Details
On December 2, 2021, a 44-year-old woman, with a history of high blood pressure, diabetes and a cesarean section 15 years ago, developed intermittent cough, expectoration, wheezing accompanied with back pain and hemoptysis without obvious incentive, and her symptoms did not relieve after self-administered oral ibuprofen and cough suppressants. On December 7, she was admitted to a local hospital due to exacerbation of asthma and fever with the highest temperature of 39.5°C. In pertinent laboratory examinations, her white blood count (WBC) was 32.5×109/L, neutrophil (NEUT) was 88.2%, and procalcitonin (PCT) was 32 ng/mL (Table 1). Lung computed tomography (CT) showed multiple patchy shadows and drug sensitivity test for pathogens in blood culture were positive for methicillin-sensitive Staphylococcus aureus (MSSA). The CURB-65 score was 1. Finally, she was clinically diagnosed as MSSA SCAP and was given meropenem (1000 mg by 30 min, intravenous infusion) and moxifloxacin (400 mg/, oral administration) anti-infective therapy. On December 15 (day 1), the patient was transferred to the emergency department of our hospital after endotracheal intubation due to poor treatment effect and progressive decrease of oxygenation combined with renal failure and type I respiratory failure.
 |
Table 1 Laboratory Findings on Admission and Transfer to Our Hospital |
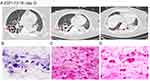
On transfer (December 15, day 1), her initial vital signs included a body temperature of 38.1°C, heart rate of 82 beats/min, blood pressure of 143/64 mmHg, respiratory rate of 20 breaths/min, oxygenation index of 186 and CURB-65 score was 4. The patient was sedated and insensitive to light. In addition, the patient suffered from eyelid edema and lower extremities pitting edema. On emergency department laboratory findings, her WBC was 41.41×109/L with an elevated neutrophil ratio of 90.0%, the PCT was 6.75 ng/mL, C-reactive protein (CRP) was 17.774 mg/dL and IL-6 was 84.18 pg/mL. In arterial blood gas analysis, the PH was 7.28 (reference 7.35–7.45), oxygen partial pressure (PO2) was 88 mmHg, partial pressure of carbon dioxide (PCO2) was 52 mmHg, potassium was 6.8 mmol/L, base excess was (BE) was -3.1mmol/L, blood glucose was 20.4 mmol/L, oxygenation index was 88. Besides, there was significant change in the coagulation panel, showing an increase of D-dimer (1.66 μg/mL), international normalized ratio (1.23), prothrombin time (PT) 15.5 s, and thrombin time (TT) 29.3 s. The patient’s renal function appears abnormal, with urea and creatinine levels as high as 30.79 and 479.9 μmol/L (Table 1). On December 16 (day 2), the lung CT showed multiple patchy shadows with voids in both lungs (Figure 1A) and drug sensitivity test for pathogens in blood culture were positive for MSSA. Meanwhile, the influenza B virus test was weakly positive. BAL-mROSE was used to get pathological data, which were positive for Staphylococcus aureus. Specifically, a large number of centrocytes infiltrates were seen under the staining microscope with the classification count of 58.5%, which suggested suppurative infectious diseases in the lung (Figure 1B). Meanwhile, clusters of Gram-positive cocci were observed, and the morphology and distribution of which were consistent with the characteristics of Staphylococcus aureus (Figure 1C). Further, the Gram-stained smear of sputum showed that Gram-positive cocci underwent phagocytosis by polymorphonuclear leukocytes, indicating a positive cocci infection (Figure 1D). Ultimately, after comprehensive diagnosis, the patient was diagnosed with MSSA SCAP with influenza B and acute kidney injury (III).
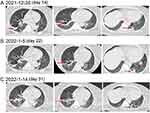
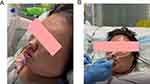
On December 16 (day 2), under the guidance of the clinicians, the patient continued to receive endotracheal intubation for assisted breathing and anti-infective therapy with meropenem, linezolid (600 mg every 8 h, intravenously), voriconazole (6 mg/kg twice daily on day 1, and 4 mg/kg twice daily from day 2 onwards, intravenously) and oseltamivir (75 mg twice a day, orally). Among them, voriconazole was discontinued after 1, 3-β-D glucan test (G test) and galactomannan test (GM test) were negative on December 20, 2021 (day 6), and Oseltamivir was discontinued after B nucleic acid reexamination was negative on December 19, 2021 (day 5). After 1 week of therapy, the patient’s body temperature, infection, and inflammatory biomarkers improved (Figure 2). The lung CT showed multiple patchy shadows and the pulmonary cavity caused by infection narrows (Figure 3A). In addition, in view of the significant improvement of the oxygenation and positive for spontaneous breathing trial (SBT) and cuff leak test (CLT), the tracheal intubation was removed and rehabilitation exercise was activated. On December 30, 2021 (day 16), the platelets (PLT) of patient decreased to 54×109/L; therefore, linezolid was discontinued. Subsequently, we found that the patient’s body temperature increased, fluctuating at 37.1–37.8°C, and the peak body temperature was as high as 38°C. After comprehensive consideration, the treatment drug was adjusted to teicoplanin (400 mg once a day, intravenously) plus tazocin (4500 mg by 30 min intravenous infusion every 8 h). During the observation period, the patient’s temperature showed a downward trend, but CT showed no obvious absorption of the pulmonary cavity (Figure 3B). Therefore, on January 05, 2022 (day 22), we decided to discontinue teicoplanin and replace it with contezolid. On January 14, 2022 (day 31), the patient’s pulmonary imaging improved significantly (Figure 3C), her body temperature decreased, and PLT returned to normal levels (Figure 4) after contezolid (800 mg every 12 h, orally) plus tazocin treatment. In addition, the patient’s swallowing disorder improved and the severe cough caused by intaking food or water has also disappeared after continuous rehabilitation exercise (Figure 5). In view of the good treatment effect, the patient was discharged by a wheelchair 3 days after (day 34). The patient received contezolid consolidation therapy for 1 week after discharge, and was free of complaints in the 30-day follow-up without any special treatment for this disease. The administration process was shown in Figure 6.
 |
Figure 6 The administration process of this patient. |
Discussion
SCAP is still associated with substantial morbidity and mortality, especially patients with comorbidities and the immunocompromised. Staphylococcus aureus is a rare pathogen that causes SCAP, and the clinical characteristics of Staphylococcus aureus pneumonia are not specific, leading to a significant increase in the barriers to diagnosis and treatment.12 The patient reported here was a middle-aged woman who developed SCAP, and she suffered from respiratory failure, renal failure and other complications, which required intubation and mechanical ventilation. Besides, due to the poor physical condition, the patient could not tolerate complicated examination methods. So how to obtain a specific infectious pathogen efficiently became a priority. Blood cultures are the “gold standard” to diagnose bloodstream infections. However, with only 4–7% of the cultures being truly positive.13 In addition, it takes some time to obtain the results of blood culture. In view of the urgency of the disease, BAL-mROSE was performed to further identify the pathogen in addition to blood culture. BAL is a useful method of obtaining microbial specimens with relatively low risk. Moreover, smear BAL fluid (BALF) is not easy to be polluted by respiratory tract bacteria, contains less non-pathogenic bacteria, and has high detection accuracy.14 mROSE is a real-time, rapid cytological interpretation technique, which can quickly indicate sample characteristics and evaluate their quality, greatly avoid sample contamination, and improve the diagnostic accuracy.15 Additionally, bacteria have natural advantages in morphology, which can be directly observed under the microscope to judge the type of bacteria, which increases the advantage of mROSE. Here, this patient was confirmed to be positive for Staphylococcus aureus by the combination of BAL-mROSE and culture results, which provided us with more solid evidence for medication.
In CAP patients admitted to ICU, β-lactam combined with macrolides or fluoroquinolones is recommended.6 However, the patient did not respond well to treatment meropenem and moxifloxacin. Considering that oxazolidinones are mostly used to treat serious infections caused by Gram-positive pathogens, clinical administration focused on these drugs. Linezolid, the first member of the oxazolidinone class of antibiotics, has been demonstrated in multiple studies to have a high concentration in alveolar tissue so as to be a potential drug for SCAP patients.16,17 Therefore, meropenem plus linezolid was administered to improve her body condition. 1 week later, her temperature and infection indexes became normal, which suggested that linezolid was effective. However, 2 weeks post-treatment, the patient developed severe thrombocytopenia, which was consistent with previous reports of severe adverse reactions caused by linezolid,18 and the drug was immediately discontinued. Coherently, teicoplanin was administered due to plasma protein binding rate, but it was discontinued after 5 days post-treatment because it was judged to be ineffective in improving the patient’s clinical course. However, considering that the blood pressure was stable, the body temperature decreased, and the infection index did not increase, teicoplanin was not considered to be completely ineffective. It may be difficult to assess the effectiveness of antimicrobial agents because radiographic improvement is delayed due to reduced antibiotic penetration into lung lesions.
Contezolid is a new class of oxazolidinones with a different structure from linezolid, which can improve its binding to bacterial targets and reduce the adverse effects on mitochondria.19 Compared to linezolid, contezolid has a good safety profile, and appears to increase the clinical appeal of contezolid. A 28-day multiple-dose exploratory study did not identify any haematological abnormalities in contezolid-treated healthy subjects, but did report 3 cases of haematological abnormalities in 2 healthy subjects treated with linezolid. In the same study, linezolid was associated with a significantly higher incidence of haematological laboratory adverse events than contezolid, eg thrombocytopenia (50% vs 5%). Besides, in the Phase III, multicentre, randomized, double-blind trial of skin and soft tissue infections patients, the incidence of both leucopenia and neutropenia was 0.3% (1/354) without thrombocytopenia (0/354) in the contezolid group. In contrast, the incidences of leucopenia, neutropenia, and thrombocytopenia were 3.4% (12/351), 1.7% (6/351), and 2.3% (8/351), respectively, in linezolid-treated patients. Here, after the antibiotic was adjusted to contezolid, the platelet levels of the patient returned to normal. Correspondingly, the patient’s body temperature, infection indicators, and lung CT findings were significantly improved, and no other adverse events occurred during the treatment. Collectively, it suggested that contezolid is a promising drug for the Staphylococcus aureus SCAP patients.
It’s important to note that, broad-spectrum combined with MRSA antibiotics were used in the treatment of MSSA pneumonia. In fact, the choice also has been proven to be correct and effective. The following reasons may explain our medication strategy. In clinical treatment, there is a discrepancy between in vitro drug sensitivity and in vivo antibiotic treatment, mainly due to the difference of internal and external environment. Sometimes bacteria are sensitive outside the body but can use substances in the body to develop antibiotic resistance. Besides, the individual differences, different levels of disease severity, and heterogeneous drug resistance may affect drug efficacy and choice. This suggests that, as the course of antibiotic treatment increases, it may be necessary to consider covering resistant bacteria to achieve efficacy.
Approximately 13–20 million critically ill patients are intubated in the ICU every year, but are prone to dysphagia after extubation.20 An early study estimated that the incidence of dysphagia was as high as 62% among critically ill patients who had been intubated for a long time recently.21 Importantly, existing data showed that 87% of the patients with dysphagia failed to recover at ICU, and 64% of those patients had persistent swallowing disorders at hospital discharge.22 Additionally, dysphagia can negatively impact patient outcomes, leading to longer hospital length of stay, aspiration pneumonia, re-intubation, need to place feeding tubes, and higher mortality rate.23 A multicenter retrospective cohort study showed that, starting rehabilitation exercise within 24 h after extubation may be the preferred option to prevent this important complication.24 Moreover, there is evidence that rehabilitation exercise for swallowing function after extubation can effectively improve the clinical symptoms.25 The patient was in critical condition at the time of admission, and mechanical ventilation lasted nearly half a month. Thus, the intubation results in incomplete glottic closure. After eating, food or water strays into the airway, causing severe coughing. Besides, intracranial infection in patients also leads to transient hemiplegia, which is one of the reasons for eating difficulties. In view of this situation, timely rehabilitation exercise of swallowing function was started within 24 after extubation, and after 20 days of training, the patient could eat autonomously and speak fluently before discharge, indicating that early initiation of rehabilitation exercise has a positive impact on the recovery of swallowing function.
There are some limitations in the manuscript. First, the study only evaluated clinical efficacy and lacked evidence of lung cultures that consistently indicated the presence of Staphylococcus aureus in lung infections. Secondly, the patient was treated with contezolid and other antibiotics at the same time, so it is difficult to explain the therapeutic effect of a single antibiotic. Besides, in the early treatment, the lung lesions have been absorbed, and the continued absorption of the lung lesions in the late stage may be the natural course of the disease. Finally, there was only one patient in this study, and contezolid needs to be tested in a larger group of people.
Conclusion
This was a successful exploration of contezolid in the treatment of Staphylococcus aureus SCAP in the study, suggesting that this regimen can be considered as a promising option for this deadly disease. However, large-scale randomised controlled trials are necessary to validate the efficacy and safety of contezolid in patients with Staphylococcus aureus SCAP.
Data Sharing Statement
All data generated or analyzed were included in this published article.
Ethics Statement
This study was approved by ethics committee of Chinese PLA General Hospital. Written informed consent was obtained from the individual for the publication of any potentially identifiable images or data included in this article.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The study was supported by National Natural Science Foundation of China (61976223) and Beijing Municipal Natural Science Foundation (No. 7222181).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Sader HS, Castanheira M, Arends SJR, et al. Geographical and temporal variation in the frequency and antimicrobial susceptibility of bacteria isolated from patients hospitalized with bacterial pneumonia: results from 20 years of the SENTRY Antimicrobial Surveillance Program (1997–2016). J Antimicrob Chemother. 2019;74(6):1595–1606. doi:10.1093/jac/dkz074
2. Patil SM, Beck PP, Patel TP, et al. Electronic vaping-induced methicillin-sensitive Staphylococcus aureus pneumonia and empyema. Case Rep Infect Dis. 2021;2021:6651430. doi:10.1155/2021/6651430
3. Self WH, Wunderink RG, Williams DJ, et al. Staphylococcus aureus community-acquired pneumonia: prevalence, clinical characteristics, and outcomes. Clin Infect Dis. 2016;63(3):300–309. doi:10.1093/cid/ciw300
4. Woodhead M, Welch CA, Harrison DA, et al. Community-acquired pneumonia on the intensive care unit: secondary analysis of 17,869 cases in the ICNARC Case Mix Programme Database. Crit Care. 2006;10(Suppl2):S1.
5. Martin-Loeches I, Torres A. New guidelines for severe community-acquired pneumonia. Curr Opin Pulm Med. 2021;27(3):210–215. doi:10.1097/MCP.0000000000000760
6. Martin-Loeches I, Lisboa T, Rodriguez A, et al. Combination antibiotic therapy with macrolides improves survival in intubated patients with community-acquired pneumonia. Intensive Care Med. 2010;36(4):612–620. doi:10.1007/s00134-009-1730-y
7. Carvalhaes CG, Duncan LR, Wang W, et al. In vitro activity and potency of the novel oxazolidinone contezolid (MRX-I) tested against gram-positive clinical isolates from the United States and Europe. Antimicrob Agents Chemother. 2020;64(11). doi:10.1128/AAC.01195-20
8. Diekema DJ, Jones RN. Oxazolidinone antibiotics. Lancet. 2001;358(9297):1975–1982. doi:10.1016/S0140-6736(01)06964-1
9. Gordeev MF, Yuan ZY. New potent antibacterial oxazolidinone (MRX-I) with an improved class safety profile. J Med Chem. 2014;57(11):4487–4497. doi:10.1021/jm401931e
10. Zhao X, Huang H, Yuan H, et al. A Phase III multicentre, randomized, double-blind trial to evaluate the efficacy and safety of oral contezolid versus linezolid in adults with complicated skin and soft tissue infections. J Antimicrob Chemother. 2022;77(6):1762–1769. doi:10.1093/jac/dkac073
11. Huang Y, Xu Y, Liu S, et al. Selection and characterisation of Staphylococcus aureus mutants with reduced susceptibility to the investigational oxazolidinone MRX-I. Int J Antimicrob Agents. 2014;43(5):418–422. doi:10.1016/j.ijantimicag.2014.02.008
12. Martin-Loeches I, Garduno A, Povoa P, et al. Choosing antibiotic therapy for severe community-acquired pneumonia. Curr Opin Infect Dis. 2022;35(2):133–139. doi:10.1097/QCO.0000000000000819
13. Long B, Koyfman A. Best clinical practice: blood culture utility in the emergency department. J Emerg Med. 2016;51(5):529–539. doi:10.1016/j.jemermed.2016.07.003
14. Hogea SP, Tudorache E, Pescaru C, et al. Bronchoalveolar lavage: role in the evaluation of pulmonary interstitial disease. Expert Rev Respir Med. 2020;14(11):1117–1130. doi:10.1080/17476348.2020.1806063
15. Izumo T, Matsumoto Y, Sasada S, et al. Utility of rapid on-site cytologic evaluation during endobronchial ultrasound with a guide sheath for peripheral pulmonary lesions. Jpn J Clin Oncol. 2017;47(3):221–225. doi:10.1093/jjco/hyw180
16. Wei Y, Zhang H, Fu M, Ma R, Li R, Kong L. Plasma and intrapulmonary pharmacokinetics, and dosage regimen optimization of linezolid for treatment of gram-positive cocci infections in patients with pulmonary infection after cerebral hemorrhage. Infect Drug Resist. 2022;15:1733–1742. doi:10.2147/IDR.S357300
17. De Pascale G, Fortuna S, Tumbarello M, et al. Linezolid plasma and intrapulmonary concentrations in critically ill obese patients with ventilator-associated pneumonia: intermittent vs continuous administration. Intensive Care Med. 2015;41(1):103–110. doi:10.1007/s00134-014-3550-y
18. Vinh DC, Rubinstein E. Linezolid: a review of safety and tolerability. J Infect. 2009;59(Suppl 1):S59–S74. doi:10.1016/S0163-4453(09)60009-8
19. Kang Y, Ge C, Zhang H, et al. Compassionate use of contezolid for the treatment of tuberculous pleurisy in a patient with a leadless pacemaker. Infect Drug Resist. 2022;15:4467–4470. doi:10.2147/IDR.S373082
20. Macht M, Wimbish T, Clark BJ, et al. Diagnosis and treatment of post-extubation dysphagia: results from a national survey. J Crit Care. 2012;27(6):578–586. doi:10.1016/j.jcrc.2012.07.016
21. Skoretz SA, Flowers HL, Martino R. The incidence of dysphagia following endotracheal intubation: a systematic review. Chest. 2010;137(3):665–673. doi:10.1378/chest.09-1823
22. Schefold JC, Berger D, Zürcher P, et al. Dysphagia in mechanically ventilated ICU patients (DYnAMICS): a prospective observational trial. Crit Care Med. 2017;45(12):2061–2069. doi:10.1097/CCM.0000000000002765
23. Macht M, King CJ, Wimbish T, et al. Post-extubation dysphagia is associated with longer hospitalization in survivors of critical illness with neurologic impairment. Crit Care. 2013;17(3):R119. doi:10.1186/cc12791
24. Regala M, Marvin S, Ehlenbach WJ. Association between postextubation dysphagia and long-term mortality among critically ill older adults. J Am Geriatr Soc. 2019;67(9):1895–1901. doi:10.1111/jgs.16039
25. El Gharib AZG, Berretin-Felix G, Rossoni DF, Seiji Yamada S. Effectiveness of therapy on post-extubation dysphagia: clinical and electromyographic findings. Clin Med Insights. 2019;12:1179550619873364. doi:10.1177/1179550619873364
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.