Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
Serum TSH Levels are Associated with Hyperactivity Behaviors in Children with Attention Deficit/Hyperactivity Disorder
Authors Chen G, Gao W, Xu Y, Chen H, Cai H
Received 9 January 2023
Accepted for publication 3 March 2023
Published 7 March 2023 Volume 2023:19 Pages 557—564
DOI https://doi.org/10.2147/NDT.S402530
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Guanru Chen,1,* Wenfan Gao,2– 4,* Yayun Xu,5 Huiying Chen,1 Heping Cai1
1Department of Clinical Pharmacy, Anhui Provincial Children’s Hospital, Hefei, Anhui, People’s Republic of China; 2Psychopharmacology Research Laboratory, Affiliated Psychological Hospital of Anhui Medical University, Hefei, Anhui, People’s Republic of China; 3Department of Pharmacy, Hefei Fourth People’s Hospital, Hefei, Anhui, People’s Republic of China; 4Anhui Clinical Research Center for Mental Disorders, Anhui Mental Health Center, Hefei, Anhui, People’s Republic of China; 5Department of Epidemiology and Biostatistics, School of Public Health, Anhui Medical University, Hefei, Anhui, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Guanru Chen, Department of Clinical Pharmacy, Anhui Provincial Children’s Hospital, 39 Wangjiang Road, Hefei, 230000, People’s Republic of China, Email [email protected]
Objective: Previous studies indicated that maternal thyroid dysfunction increase the offspring’s risk for attention deficit/hyperactivity disorder (ADHD). However, the relationship between thyroid function and symptoms in children with ADHD remains unclear.
Methods: A total of 49 children with ADHD were enrolled. The Conners 3 scale was used to estimate the symptoms associated with ADHD. Correlation between thyroid hormones and the scores of the Conners 3 scale was evaluated by Pearson correlation analysis. Then, ADHD children were divided into two groups according to the hyperactivity index (HI) of the Conners 3 scale: ADHD children with hyperactivity behaviors (HB) (HI > 1.5) and ADHD children without HB (HI < 1.5). The demographic characteristics, thyroid hormones, and routine laboratory parameters between the two groups were collected. To distinguish HI-related factors, a univariate analysis and a binary logistic regression predictive model were used. The discriminative ability of thyroid stimulating hormone (TSH) in predicting ADHD children with HB from ADHD children without HB was investigated using the receiver operating characteristic (ROC) curve method.
Results: The levels of TSH were positively correlated to the scores of the Conners 3 scale (r = 0.338, P = 0.033) and HI (r = 0.371, P = 0.019). Moreover, the levels of TSH, serum ferritin, and lactic acid were significantly increased in ADHD children with HB compared to ADHD children without HB (all P < 0.05). Furthermore, the results of binary logistic regression found that TSH (OR 2.243 (CL 1.052– 4.783)) and lactic acid (OR 1.018 (CI 1.003– 1.032)) were independently associated with HI. Additionally, ROC analysis indicated the potential diagnostic value of TSH in discriminating ADHD children with HB from ADHD children without HB with an AUC of 0.684.
Conclusion: These results suggested that the serum TSH levels may be related to the HB in children with ADHD.
Keywords: thyroid function, children, attention deficit/hyperactivity disorder, thyroid stimulating hormone, lactic acid, hyperactivity behaviors
Introduction
Attention-deficit/hyperactivity disorder (ADHD), one of the most common neurodevelopmental disorders diagnosed in children, has emerged as a major global medical and public health concern.1,2 The incidence of ADHD was estimated to be between 2–7% globally,3 and 6.4% (6.2–7.0%) among children aged 6–16 years old in China was reported to have ADHD.4 It has been demonstrated that ADHD symptoms can persist into adulthood and contribute to a variety of societal issues, such as lower levels of work performance, higher rates of unemployment, smaller social networks, and higher rates of crime.5 However, excluding some medications used to treat specific symptoms of ADHD, there is presently no overall treatment for ADHD.6 Thus, improving current preventive measures requires gaining a better knowledge of the underlying triggers that raise the risk of ADHD.
Thyroid status, which is determined by free triiodothyronine (fT3), free thyroxine (fT4), and thyroid stimulating hormone (TSH), is crucial for the control of motor, cognitive, and affective activities.7 Several lines of evidence indicate that fetal brain development may be harmed by maternal thyroid dysfunction.8 Specifically, the risk of ADHD in the offspring is increased by maternal (overt) hyperthyroidism,9,10 high TSH concentration during pregnancy,11 and maternal autoimmune thyroiditis12 in the early stages of pregnancy. More recently, it has been reported that in contrast to children without hyperthyroidism, the prevalence ratio of ADHD in children who have hyperthyroidism is 1.7.13 Moreover, individuals with hyperthyroidism may experience symptoms similar to ADHD including anxiety, nervousness, irritability, and physical hyperactivity.14 These findings point to a possible relationship between thyroid dysfunction and ADHD in children.
In the present study, to test this hypothesis that thyroid function may be related to the symptoms in children with ADHD, a total of 49 children with ADHD were enrolled. The Conners 3 scale was used to estimate the symptoms associated with ADHD. Correlation between thyroid hormones and the scores of the Conners 3 scale was evaluated by Pearson correlation analysis. Then, ADHD children were divided into two groups according to the hyperactivity index (HI) of the Conners 3 scale: ADHD children with hyperactivity behaviors (HB) (HI > 1.5) and ADHD children without HB (HI < 1.5). The demographic characteristics, thyroid hormones, and routine laboratory parameters between the two groups were collected and compared to explore potential indicators related to HB. To distinguish HI-related factors, a univariate analysis and a binary logistic regression predictive model were used. The discriminative ability of thyroid stimulating hormone (TSH) in predicting ADHD children with HB from ADHD children without HB was investigated using the receiver operating characteristic (ROC) curve method.
Materials and Methods
Study Design and Participants
Children with ADHD at Anhui Provincial Children’s Hospital from October 2021 and May 2022 were selected retrospectively. According to the standards of the structured clinical interview and the Diagnostic and Statistical Manual for Psychiatric Disorders-Fifth Edition (DSM-V), all participants were given an ADHD diagnosis. The criteria for joining the group were as follows: (1) age 6 to 14 years; (2) IQ scores > 80; (3) receiving no treatment with ADHD. The exclusion criteria were as follows: (1) patients with nervous system diseases; (2) patients with serious somatic disease; (3) patients with other mental disorders, such as schizophrenia, mental retardation, and depression; (4) patients with a history of severe brain trauma. The Ethics Committee of Anhui Provincial Children’s Hospital approved this study. Informed consent was obtained from each parent/legal guardian of eligible participants prior to enrolment in accordance with the Declaration of Helsinki.
Clinical Data Collection
Retrospective research was done on the individuals, and electronic medical records were used to get the subjects’ actual data (clinical and laboratory). The following clinical information was gathered: age, gender, and routine laboratory parameters including fT3, fT4, TSH, total protein, albumin, globulin, prealbumin (PAB), total bilirubin (TBIL), direct bilirubin (DBIL), indirect bilirubin (IBIL), anion gap, plasma osmolarity, serum ferritin, lactic acid, aspartate aminotransferase (AST), alanine aminotransferase (ALT), AST/ALT, alkaline phosphatase (ALP), γ-glutamyl transpeptidase (γ-GT), glucose, uric acid, creatinine, blood urea nitrogen (BUN), BUN/creatinine, Na+, Mg2+, Cl−, phosphorus, Ca2+, K+, CO2, Cystatin C, and cholinesterase.
Measurement of the Conners 3 Scale
The symptoms associated with ADHD was estimated by the Conners 3 scale, a widely used and validated screening instrument to evaluate behavioral issues associated with ADHD in children between the ages of 3 and 16 years old.15 The tool’s Chinese translation demonstrated high validity and reliability.16 It has 48 items in total, which are summarized into 6 subscales: impulsivity-hyperactivity, anxiety, hyperactivity index (HI), learning problems, behavioral problems, psychosomatic problems. Depending on how true each statement is to the children’s behavior, each item receives a score between 0 and 3, with 0 representing never, 1 occasionally, 2 regularly, and 3 representing frequently. Children’s hyperactivity behaviors (HB) were measured by the HI subscale.15 The HI measure is comprised of 10 items and the average score, with a maximum possible value of 3, was then calculated. The HB measurement was a continuous variable with a score between 0 and 3, with a higher score indicating a higher level of HB. In order to distinguish between children with and without HB, a cut-off score of 1.5 was used.16
Statistical Analysis
SPSS (version 17.0; IBM Corp., Armonk, NY, USA) was used for statistical analysis. The statistical significance level was set at 0.05, and the data are presented as mean ± standard error of the mean (SEM). The one-sample Kolmogorov–Smirnov test was used to determine whether the distribution of continuous variables was normally distributed. Correlation between thyroid hormones and the scores of the Conners 3 scale was evaluated by Pearson correlation analysis. Student’s t-test or Mann–Whitney U-test for independent samples was used to compare the age, thyroid hormones, routine laboratory parameters between ADHD children with HB and ADHD children without HB. The gender differences between the groups were examined using the chi-square test. The variable with a P-value of 0.1 or less in the Student’s t-test, Mann–Whitney U-test, or chi-squared test were entered into binary logistics regression model to evaluate the risk factors related to HI. The discriminative ability of TSH in predicting ADHD children with HB from ADHD children without HB was investigated using the receiver operating characteristic (ROC) curve method.
Results
Correlation Between Thyroid Hormones and the Scores of the Conners-3 Scale
Figure 1 summarizes the relationship between thyroid hormones and the scores of the Conners-3 scale in ADHD children. The levels of TSH were positively correlated to the scores of the Conners-3 scale (r = 0.338, P = 0.033) and HI (r = 0.371, P = 0.019). In terms of fT3 and fT4, there was no significant relationship between either fT3 or fT4 and the scores of the Conners-3 scale.
 |
Figure 1 Correlation between thyroid hormones and the scores of the Conners-3 scale. ×: no significance. |
Comparison of Demographic Characteristics, Thyroid Hormones, Routine Laboratory Parameters Between ADHD Children with HB and Without HB
Children with and without HB are distinguished between by using HI at a cut-off score of 1.5. In order to explore potential indicators related to HB, the demographic characteristics, thyroid hormones, and routine laboratory parameters between the two groups were collected and compared. ADHD children were divided into two groups according to the HI: ADHD children with HB (HI > 1.5) and ADHD children without HB (HI < 1.5). As shown in Table 1, the levels of TSH, serum ferritin, and lactic acid were significantly increased in ADHD children with HB compared to ADHD children without HB (all P< 0.05).
 |
Table 1 Demographic Characteristics, Thyroid Hormones, Routine Laboratory Parameters Between ADHD Children with HB and without HB |
Binary Logistic Regression Evaluating the Factors Related to HI
The variable with a P-value of 0.1 or less in the univariate analysis were entered into binary logistics regression model to evaluate the factors related to HI. The results of binary logistic regression found that TSH (OR 2.243 (CL 1.052–4.783)) and lactic acid (OR 1.018 (CI 1.003–1.032)) were independently associated with HI (Table 2).
 |
Table 2 Binary Logistics Regression Analysis of Factors Associated with HI |
Diagnostic Values of TSH in Discriminating ADHD Children with HB from ADHD Children Without HB
The diagnostic performance of TSH in discriminating ADHD children with HB from ADHD children without HB was performed by ROC curve analysis. As shown in Figure 2, the AUC value of TSH was 0.684, with a sensitivity of 65.4% and a specificity of 78.3%.
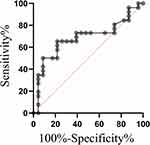 |
Figure 2 ROC curve of serum TSH levels in identification of ADHD children with HB from ADHD children without HB. |
Discussion
The purpose of this study was to investigate the connection between ADHD symptoms and thyroid function. Four major findings emerged in the present study. Firstly, the levels of TSH were positively correlated to the scores of the Conners 3 scale and HI. Secondly, the levels of TSH, serum ferritin, and lactic acid were significantly increased in ADHD children with HB compared to ADHD children without HB. Thirdly, TSH and lactic acid were independently associated with HI. Fourthly, TSH achieved a relatively high accuracy in discriminating ADHD children with HB from ADHD children without HB.
The Conners 3 scale is a questionnaire for parents that is intended to evaluate behavioral issues associated with ADHD and related disorders.17 The scale consists of six content scales: behavioral problems, HI, hyperactivity/impulsivity, anxiety, somatic symptom disorder. Due to its good reliability and validity, the Conners 3 scale is widely used to assess ADHD related symptoms in a numerous of studies.18,19 Thus, this scale was selected to evaluate the symptoms associated with ADHD.
Several studies have evaluated the association between TSH and ADHD. It has been shown that TSH levels, although within normal limits, were significantly lower in methylphenidate-treated boys with ADHD than in the drug-naive and control groups.20 Another study has demonstrated that in children, but not in adolescents, increased serum TSH levels were associated with a decreased risk of ADHD diagnosis.8 More recently, no significant correlation was found between serum TSH levels and the symptoms of ADHD in India.21 This finding is not in concordance with our results. In the present study, positive relationship was found between the levels of TSH and the scores of the Conners 3 scale and HI, indicating that TSH may be related to the symptom severity of ADHD, especially HB. The reason for these contradictory findings is still not entirely clear. There are several possible explanations: (1) these differences could be attributed to the different populations studied, since Indian populations were included in their study and Chinese populations were included in the present study; (2) gender ratio difference. There were 28 boys and 2 girls in ADHD group in their study, while there were 38 boys and 11 girls in the present study. (3) the correlation between TSH and symptoms was analyzed in the total subjects including ADHD patients and healthy controls in their study, and our study was to analyze this correlation in ADHD patients.
Children’s HB were measured by the HI subscale. HI at a cut-off score of 1.5 is used to identify the children with and without HB.16 Therefore, in order to further explore the relationship between thyroid function and symptoms in children with ADHD, ADHD children were divided into two groups according to the HI of the Conners 3 scale: ADHD children with HB (HI > 1.5) and ADHD children without HB (HI < 1.5). The demographic characteristics, thyroid hormones, and routine laboratory parameters between the two groups were compared and the results showed that the levels of TSH were significantly increased in ADHD children with HB compared to ADHD children without HB. Further results of binary logistic regression found that TSH was independently associated with HI. We further explored the potential value of TSH as diagnostic biomarker of HB in ADHD. The AUC value of TSH was 0.684, with a sensitivity of 65.4% and a specificity of 78.3% in discriminating ADHD children with HB from ADHD children without HB. These findings further link a close relationship between TSH and the HB of ADHD children.
Iron deficiency (ID) has been proposed as a potential etiopathophysiological component in a subsample of people with ADHD due to iron’s involvement in neurocognitive and behavioral functioning.22 Serum ferritin, a measure of peripheral iron status, has been the main focus of the majority of investigations evaluating ID in ADHD. Several lines of evidence discovered a link between low ferritin levels and the prevalence of ADHD,23–26 and one study27 was unable to support this finding. To the best of our knowledge, we firstly compared the serum ferritin levels between ADHD children with HB and without HB. The results showed that the levels of serum ferritin were significantly increased in ADHD children with HB compared to ADHD children without HB. This finding suggests that ferritin, as a marker for iron stores, may be included in the baseline evaluations of children with clinical presentation of ADHD.
Higher lactic acid levels were found in ADHD children with HB compared to ADHD children without HB in the present study. The results of binary logistic regression found that lactic acid was independently associated with HI. It is well known that ADHD children with HB have an excess of activity, which may lead to accumulation of lactic acid in serum. Since this study is a single-center study and the sample size is relatively small, this relationship should be confirmed by multicentric studies.
Several limitations should be highlighted. First, the small sample size and single-center design of the current study may indicate sampling bias. There are two other reasons that limit the sample size: (1) in order to rule out the effects of drugs, patients receiving no treatment with ADHD were enrolled; (2) the sample size is limited due to the impact of COVID-19 epidemic. Second, the causal relationship between TSH and hyperactivity in ADHD children cannot be examined in this cross-sectional study. Third, due to a retrospective study, the BMI was not included.
In summary, the present study reveals that the serum TSH levels may be related to the HB in children with ADHD. To validate this potential relationship, multicentric studies are clearly required, and to examined the causal relationship between thyroid function and symptoms in children with ADHD, longitudinal studies and randomized controlled trials (RCTs) are warranted.
Data Sharing Statement
The raw data supporting the conclusions of this article will be made available by the corresponding author, without undue reservation.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Rajaprakash M, Leppert ML. Attention-deficit/hyperactivity disorder. Pediatr Rev. 2022;43(3):135–147. doi:10.1542/pir.2020-000612
2. Antshel KM, Barkley R. Attention deficit hyperactivity disorder. Handb Clin Neurol. 2020;174:37–45.
3. Sayal K, Prasad V, Daley D, et al. ADHD in children and young people: prevalence, care pathways, and service provision. Lancet Psychiatry. 2018;5(2):175–186. doi:10.1016/S2215-0366(17)30167-0
4. Li F, Cui Y, Li Y, et al. Prevalence of mental disorders in school children and adolescents in China: diagnostic data from detailed clinical assessments of 17,524 individuals. J Child Psychol Psychiatry. 2022;63(1):34–46. doi:10.1111/jcpp.13445
5. Thapar A, Cooper M. Attention deficit hyperactivity disorder. Lancet. 2016;387(10024):1240–1250. doi:10.1016/S0140-6736(15)00238-X
6. Caye A, Swanson JM, Coghill D, et al. Treatment strategies for ADHD: an evidence-based guide to select optimal treatment. Mol Psychiatry. 2019;24(3):390–408. doi:10.1038/s41380-018-0116-3
7. Chambers T, Anney R, Taylor PN, et al. Effects of thyroid status on regional brain volumes: a diagnostic and genetic imaging study in UK biobank. J Clin Endocrinol Metab. 2021;106(3):688–696. doi:10.1210/clinem/dgaa903
8. Albrecht D, Ittermann T, Thamm M, et al. The association between thyroid function biomarkers and attention deficit hyperactivity disorder. Sci Rep. 2020;10(1):18285. doi:10.1038/s41598-020-75228-w
9. Andersen SL, Laurberg P, Wu CS, et al. Attention deficit hyperactivity disorder and autism spectrum disorder in children born to mothers with thyroid dysfunction: a Danish nationwide cohort study. BJOG. 2014;121(11):1365–1374. doi:10.1111/1471-0528.12681
10. Andersen SL, Andersen S, Vestergaard P, et al. Maternal thyroid function in early pregnancy and child neurodevelopmental disorders: a Danish Nationwide Case-Cohort Study. Thyroid. 2018;28(4):537–546. doi:10.1089/thy.2017.0425
11. Ghassabian A, Bongers-Schokking JJ, Henrichs J, et al. Maternal thyroid function during pregnancy and behavioral problems in the offspring: the generation R study. Pediatr Res. 2011;69(5 Pt 1):454–459. doi:10.1203/PDR.0b013e3182125b0c
12. Ghassabian A, Bongers-Schokking JJ, de Rijke YB, et al. Maternal thyroid autoimmunity during pregnancy and the risk of attention deficit/hyperactivity problems in children: the Generation R Study. Thyroid. 2012;22(2):178–186. doi:10.1089/thy.2011.0318
13. Zader SJ, Williams E, Buryk MA. Mental health conditions and hyperthyroidism. Pediatrics. 2019;144(5). doi:10.1542/peds.2018-2874
14. Ahmed OM, El-Gareib AW, El-Bakry AM, et al. Thyroid hormones states and brain development interactions. Int J Dev Neurosci. 2008;26(2):147–209. doi:10.1016/j.ijdevneu.2007.09.011
15. Conners CK, Sitarenios G, Parker JDA, et al. The revised Conners’ Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol. 1998;26(4):257–268. doi:10.1023/A:1022602400621
16. Lin Q, Hou X-Y, Yin X-N, et al. Prenatal exposure to environmental tobacco smoke and hyperactivity behavior in Chinese young children. Int J Environ Res Public Health. 2017;14(10):1132. doi:10.3390/ijerph14101132
17. Izzo VA, Donati MA, Primi C. Conners 3-Self-Report Scale: an empirical support to the dimensionality of the content scales. Clin Child Psychol Psychiatry. 2018;23(4):556–566. doi:10.1177/1359104518757289
18. Izzo VA, Donati MA, Novello F, et al. The Conners 3-short forms: evaluating the adequacy of brief versions to assess ADHD symptoms and related problems. Clin Child Psychol Psychiatry. 2019;24(4):791–808. doi:10.1177/1359104519846602
19. Abdullah M, Jowett B, Whittaker PJ, et al. The effectiveness of omega-3 supplementation in reducing ADHD associated symptoms in children as measured by the Conners’ rating scales: a systematic review of randomized controlled trials. J Psychiatr Res. 2019;110:64–73. doi:10.1016/j.jpsychires.2018.12.002
20. Wang LJ, Huang Y-H, Chou W-J, et al. Growth hormone and thyroid function in children with attention deficit hyperactivity disorder undergoing drug therapy. J Clin Endocrinol Metab. 2022;107(7):2047–2056. doi:10.1210/clinem/dgac139
21. Kuppili PP, Pattanayak RD, Sagar R, et al. Thyroid and cortisol hormones in attention deficit hyperactivity disorder: a case-control study. Asian J Psychiatr. 2017;28:73–77. doi:10.1016/j.ajp.2017.03.005
22. Cortese S, Angriman M. Attention-deficit/hyperactivity disorder, iron deficiency, and obesity: is there a link? Postgrad Med. 2014;126(4):155–170. doi:10.3810/pgm.2014.07.2793
23. Sever Y, Ashkenazi A, Tyano S, et al. Iron treatment in children with attention deficit hyperactivity disorder. A preliminary report. Neuropsychobiology. 1997;35(4):178–180. doi:10.1159/000119341
24. Konofal E, Lecendreux M, Arnulf I, et al. Iron deficiency in children with attention-deficit/hyperactivity disorder. Arch Pediatr Adolesc Med. 2004;158(12):1113–1115. doi:10.1001/archpedi.158.12.1113
25. Juneja M, Jain R, Singh V, et al. Iron deficiency in Indian children with attention deficit hyperactivity disorder. Indian Pediatr. 2010;47(11):955–958. doi:10.1007/s13312-010-0160-9
26. Konofal E, Lecendreux M, Deron J, et al. Effects of iron supplementation on attention deficit hyperactivity disorder in children. Pediatr Neurol. 2008;38(1):20–26. doi:10.1016/j.pediatrneurol.2007.08.014
27. Millichap JG, Yee MM, Davidson SI. Serum ferritin in children with attention-deficit hyperactivity disorder. Pediatr Neurol. 2006;34(3):200–203. doi:10.1016/j.pediatrneurol.2005.09.001
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
