Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
Serum Cystatin C is Associated with Depression After Intracerebral Hemorrhage
Authors Zhu L , Yu C, Chang Y, Sun S, Sun Z
Received 21 February 2023
Accepted for publication 4 May 2023
Published 8 May 2023 Volume 2023:19 Pages 1117—1126
DOI https://doi.org/10.2147/NDT.S409421
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Taro Kishi
Lei Zhu,1,2 Chuanqing Yu,2 Yueyue Chang,2 Shiyu Sun,2 Zhongwu Sun1
1Department of Neurology, First Affiliated Hospital of Anhui Medical University, Hefei, Anhui Province, People’s Republic of China; 2Department of Neurology, First Affiliated Hospital of Anhui University of Science and Technology, First People’s Hospital of Huainan, Huainan, Anhui Province, People’s Republic of China
Correspondence: Zhongwu Sun, Department of Neurology, First Affiliated Hospital of Anhui Medical University, Hefei, Anhui Province, People’s Republic of China, Email [email protected]
Purpose: Cystatins are associated with neuronal degeneration and nervous system healing. Cystatin C (Cys C) has recently been linked to brain injury and immunological inflammation. This study aimed to determine the relationship between serum Cys C levels and depression following intracranial hemorrhage (ICH).
Patients and Methods: Between September 2020 and December 2022, 337 patients with ICH were sequentially recruited and followed up for three months. The post-stroke depression (PSD) and non-PSD groups were separated based on the 17-item Hamilton Depression Rating Scale (HAMD). The PSD diagnosis was established based on the DSM-IV criteria. Cys-C levels were documented within twenty-four hours of admission.
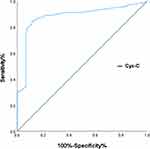
Results: Three months after ICH, 93 (27.6%) of 337 enrolled patients were diagnosed with depression. The Cys C levels were significantly higher in depressed patients than in nondepressed patients after ICH (1.32 vs 1.01; p< 0.001). After adjusting for potential confounding variables, depression after ICH was associated with the highest quartile of Cys C levels (odds ratio (OR) = 3.195, 95% CI: 1.562– 6.536; p=0.001). The receiver operating characteristic curve (ROC) curve predicted that the ideal cut-off for CysC levels as a predictor of depression after ICH would be 0.730, resulting in 84.5% sensitivity and 88.4% specificity, with an area under curve (AUC) of 0.880 (95% CI: 0.843– 0.917; p< 0.0001).
Conclusion: Increased CysC concentrations were independently related to depression three months after ICH, highlighting that CysC levels at admission may be a potential biomarker for predicting the onset of depression following ICH.
Keywords: cystatin C, depression, intracerebral hemorrhage, post-stroke depression
Introduction
The PSD is one of the most common psychosomatic disorders and has been relatively less studied in cases of spontaneous ICH, one of the most disabling and fatal neurological diseases, with approximately 15–23% of ICH survivors experiencing post-stroke depression.1–3 Patients with PSD are more likely to experience memory loss, stroke recurrence, and death.4,5 Therefore, it is crucial to recognize and understand the etiopathogenesis of PSD at the early stages.
A consistent and strong body of practical data indicates that neuronal damage and immune inflammation play important roles in depression, despite their unclear etiology and pathogenesis.6–8 Cys C, encoded by CST3, inhibits cysteine proteases in various tissues and body fluids. It escapes into the extracellular space, blood, and the cerebrospinal fluid.9 In the brain, Cys-C is strongly associated with neuronal damage and immune inflammation.10,11 In addition, there appears to be a higher risk of depression in patients with higher Cys C concentrations.12,13 However, the association between Cys C level and post-ICH depression remains unclear. Accordingly, the purpose of this study was to determine predictive value of Cys-C for depression 3 months post ICH.
Materials and Methods
Study Subjects
From September 2020 to December 2022, patients with ICH were enrolled at First Affiliated Hospital of Anhui University of Science and Technology. The inclusion criteria were: (1) diagnosis of cerebral hemorrhage through CT scans in the first 24 h of symptom onset (2) age greater than 18 years. (3) ability to provide written consent.
The exclusion criteria were: (1) secondary bleeding due to vascular anomalies, intracranial aneurysms, tumors, or hemorrhage transformation and abnormal coagulation were excluded; (2) concurrent diseases that may affect Cys C values, such as a history of organic brain disease, Parkinsonism, cancer, haematological disorders, severe liver and kidney disorders, severe cardiac disease, inflammatory diseases, autoimmune diseases, and infectious diseases; (3) depression or other mental disorders; (4) dementia; (5) severe dysarthria or aphasia; and (6) recurrent stroke or severe critical conditions, dying within the 3- month follow-up period. In total, 337 patients with ICH were enrolled in this study (Figure 1).
 |
Figure 1 Study recruitment profile. |
Ethics Approval and Informed Consent
This study was approved by the Ethics Committee of First Affiliated Hospital of Anhui University of Science and Technology, according to the Declaration of Helsinki. All participants signed informed consent forms (Approval No. 2019B20,2019-1-1).
Collection of Demographic and Clinical Data
The baseline clinical variables, vascular risk factors, and demographic information were recorded on admission. The National Institutes of Health Stroke Scale (NIHSS) measures stroke severity of a stroke.14 The Glasgow Coma Scale (GCS) assesses the degree of coma. Routine blood tests were carried out on the following morning by collecting fasting venous blood and recording laboratory data. An automated haematology analyser was used to measure biochemical parameters. The serum Cys C concentration was determined by an immunoturbidimetric assay using a Siemens Advia 1800 chemistry analyzer. A range of 0.51 to 1.50 mg/L was taken as the reference value for serum Cys C levels.
Assessment of Outcomes
Telephone interviews or clinical appointments were scheduled for all patients 3 months after ICH. The Barthel Index (BI) and modified Rankin Scale (mRS) scores were used to assess functional outcomes. Hamilton Depression Scale (HAMD) 17-item assessment was conducted to assess depression.15 The Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) classifies patients with HAMD scores >7 as depressed after ICH.
Statistical Analysis
Data analysis and processing were performed using SPSS27.0 software (SPSS, USA). In the case of continuous variables, mean ± SD or median and interquartile ranges were provided. Continuous variables among groups were analysed using the Student’s t-test and Mann–Whitney U-test for normal and non-normal data, respectively. Categorical variables are presented as numbers (percentages). Categorical variables were compared using chi-square and Fisher’s exact tests, as appropriate. Multivariate regression analysis was done using three models to identify predictors of PSD, with model 1 adjusted for sex and age; model 2 adjusted for age, sex, and risk factors (smoking history, alcohol consumption, hypertension, diabetes, hyperlipidaemia, atrial fibrillation, and peripheral arterial disease)16,17; and model 3 adjusted for variables in the univariate analysis, p < 0.05. OR with 95% confidence intervals (CI) were used to determine the correlations. In addition, to investigate the optimal cutoff point for serum Cys-C concentrations to predict depression following ICH, a ROC curve was employed. To assess the accuracy of the test, Cys C levels were calculated based on the area under the curve (AUC), and statistical significance was defined at p<0.05.
Results
A total of 450 patients with ICH were screened, and 370 were enrolled between September 2020 and December 2022. 33 individuals were either unavailable for follow-up or refused to complete the scale within three months. Finally, 337 patients were included in this study. They consisted of 101 women (29.97%) and 236 men (70.03%), of whom 93 (27.6%) were diagnosed with depression three months after ICH.
Compared to nondepressed patients after ICH, those with depression exhibited higher NIHSS (p<0.001), HAMD (p<0.001), and mRS scores (p=0.010) and significantly higher levels of Cys C (p<0.001), creatinine (p<0.001), uric acid (p<0.001), urea nitrogen (p<0.001), and the proportion of basal ganglia lesions (p=0.021). Additionally, individuals with depression had a lower glomerular filtration rate (GFR) (p<0.001), ApoA (p= 0.013), HDL (p= 0.017), and BI scores (p=0.002) than patients without depression. The two groups did not differ significantly in terms of other clinical characteristics. For more details, see Table 1.
 |
Table 1 Clinical and Demographic Characteristics of the Participants According to PSD Status |
The baseline characteristics of the 337 patients with ICH are shown in Table 2. Serum Cys-C levels were divided into four quartiles:0.93 mg/L or less (First quartile, Q1), 0.94–1.08 mg/L (Second quartile, Q2), 1.09–1.27 mg/L (Third quartile, Q3), and more than 1.28 mg/L (Fourth quartile, Q4). In the quartile analysis, increasing quartiles of Cys-C were significantly associated with age (p=0.013), uric acid (p<0.001), urea nitrogen (p<0.001), creatinine (p<0.001), blood glucose (p=0.033), cholesterol (p=0.025), and HDL (p=0.014). They were also significantly associated with higher NIHSS (p<0.001), mRS (p<0.001), and HAND (p<0.001) scores and lower GFR (p<0.001), GCS (p=0.009), and BI (p<0.001) scores. Seven (7.5%), 7 (7.5%), 20 (21.5%), and 59 (63.4%) patients developed depression after ICH in Q1, Q2, Q3, and Q4, respectively. Compared to the three lower quartiles, the highest quartile had a higher percentage of patients with depression.
 |
Table 2 Comparisons of Baseline Characteristics According to Cystatin C Quartiles |
Cys-C was converted to mg/dl to reflect its modest molecular weight and incorporated into the multivariate logistic regression analysis. Patients in the highest quartile of Cys C levels had a greater risk of PSD than those in the lower quartiles (non-adjusted: OR 1.50, 95% CI 1.35–1.66, P < 0.001). After adjusting for potential confounding variables such as age, gender, educational level, marital status, and vascular risk factors (stroke history, coronary artery disease, peripheral arterial disease, hypertension, atrial fibrillation diabetes), BI score, NIHSS score, modified Rankin Scale score, and basal ganglia lesion, GFR, Creatinine (Cr), blood urea nitrogen (BUN), uric acid (UA), BLU, blood glucose (GLU), the highest quartile of Cys C remained significantly associated with depression following ICH (model 1: OR = 6.482, 95% CI =3.422–12.278, P <0.001; model 2: OR = 3.825, 95% CI = 1.832–7.987, P < 0.001; model 3: OR = 3.195 95% CI = 1.562–6.536, P =0.001). (Table 3).
 |
Table 3 Logistic Regression Model of Cystatin C (mg/dl) and Depression After ICH at 3 Month |
The ROC curve predicted that the ideal cut-off for Cys-C levels as a predictor of depression after ICH would be 0.730, which produced 84.5% sensitivity and 88.4% specificity, yielding an AUC of 0.880 (95% CI, 0.843–0.917; p < 0.0001) in (Figure 2).
Discussion
The current investigation showed that elevated serum Cys-C concentrations after ICH were related to depression, and that individuals in the highest quartile of serum Cys-C showed a 3.195-fold greater risk of depression after ICH than those in the lowest quartile after adjusting for potential confounding factors. To the best of our knowledge, this is the first prospective study to investigate the association between serum Cys-C concentrations and depression following ICH. In light of these findings, serum Cys-C level may serve as a possible biomarker for the emergence of depression following ICH.
The prevalence of depression 3 months after ICH in the current study was 27.6%, which is almost consistent with earlier data.3 In addition, this study’s findings support earlier research that indicated that functional outcomes and stroke severity are risk factors for PSD development.18,19 A previous study showed that reduced GFR was associated with the development of PSD in ischaemic stroke.20 However, the present study was not consistent with the above study. In the present study, although GFR levels were significantly decreased in the PSD group after cerebral haemorrhage, and increasing quartiles of Cys C were significantly associated with lower GFR, after adjustment for all variables, the multiple regression model showed that only the highest quartile of Cys C remained significantly associated with depression after ICH, whereas GFR did not show statistical significance. We considered the possibility that the subject of this study was PSD in patients with cerebral haemorrhage, whereas the previous research examined the significant correlation between depression and GFR after ischaemic stroke, so not entirely consistent, which is a good direction of study and we will further investigate in our future studies.
Although the volume and location of hematomas in PSD have received considerable attention from researchers, no consensus has been reached. According to Hadidi et al, PSD morbidity is unrelated to location of the lesion.21 Based on the findings of Nys et al.19 PSD morbidity was determined solely by the lesion size rather than lesion location.22 The volume of hematoma had no effect on the outcome, although some locations had an impact on depression after ICH, according to this study. The brain’s emotional network is centred on the basal ganglia and left frontal lobe, and numerous investigations have discovered that the precise site of the lesion may impact the etiology of PSD, especially when the lesion is close to the basal nucleus or left frontal region.23 Similar findings were obtained in the current investigation, indicating a strong relationship between ganglia lesions and depression after ICH. However, the results of this investigation also revealed no significant differences between frontal lobe lesions in depressed patients following ICH and nondepressed patients, which might be related to the relatively small sample size of our study. Therefore, additional validation using a larger sample size is required.
Based on current findings, it remains unknown how serum Cys-C levels promote depression following ICH. Several potential mechanisms can explain this phenomenon. First, CysC participates in the pathophysiology of atherosclerosis.24 At the beginning of a stroke, a significant amount of Cys C in CSF fluid crosses the diseased blood-brain barrier and enters the bloodstream. High serum levels of cysteine protease inhibitor Cys C disrupt the equilibrium between metalloproteinase and antiproteolytic activity, which has an impact on vascular wall remodeling.25 This could be a contributing factor to elevated serum Cys-C concentrations following ICH. Second, haemorrhagic stroke has the potential to cause inflammation, and there is a link between serum Cys C levels and inflammation and its influence on neutrophil migration. This encourages the release of pro-inflammatory chemokines and cytokines, which adversely affect the brain’s serotonin system and stimulate the hypothalamic, pituitary, and adrenal axes, ultimately leading to inflammatory symptoms of depression after ICH.26–29 Third, apoptosis is another mechanism through which Cys-C contributes to depression. Depression may be triggered by this directly or indirectly.30 Animal studies have demonstrated that as a result of Cys C enhancing caspase-9 activity and lowering B cell leukemia 2 (Bcl-2) concentrations, neuronal death occurs, possibly contributing to depression.31 Fourth, Cys C is associated with oxidative stress, which has been found to upregulate Cerebral Cys C concentrations, and may be a putative mechanism. Oxidative stress is a major contributor to depression.32 Finally, in the walls of the cerebral microvasculature, cysteine C, an amyloid protein variant, can be found alongside-amyloid, thereby enhancing cerebrovascular damage.33 These amyloid deposits are associated with depression, implying that Cys-C may function via amyloid deposition in the brain, causing depression after ICH. In the current study, Cys-C levels and depression were associated with ICH. The mechanisms described above may explain this relationship. However, the exact mechanism requires further investigation.
In this study, there are several limitations that need to be addressed. First, patients were only tested for Cys-C levels once upon admission, limiting the study’s dynamics regarding the correlation between Cys-C levels over time and depression after ICH. Second, it was a single-centre trial with a relatively small sample size, which reduces its generalisability. To support our findings and contribute to replication, future studies should increase the sample size and include multicentre longitudinal studies.
Conclusion
In summary, higher Cys C concentrations were independently associated with depression 3 months after ICH, indicating that Cys C concentration at admission may be an important biomarker for predicting the onset of depression after ICH and may help guide clinical management accurately.
Funding
This research was supported by the Key Project of Anhui Province’s Education Department (KJ2019A0096), China International Medical Exchange Foundation Chinese Neurology Special Fund for Young Innovators in Cerebrovascular Diseases (Z-2016-20-2101-15), and the Huainan Technology and Science Planning Project (2021146).
Disclosure
No conflicts of interest have been reported by the authors.
References
1. Stern-Nezer S, Eyngorn I, Mlynash M, et al. Depression one year after hemorrhagic stroke is associated with late worsening of outcomes. Neuro Rehabil. 2017;41:179–187. doi:10.3233/NRE-171470
2. Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001;344(19):1450–1460. doi:10.1056/NEJM200105103441907
3. Koivunen R-J, Harno H, Tatlisumak T, Putaala J. Depression, anxiety, and cognitive functioning after intracerebral hemorrhage. Acta Neurol Scand. 2015;132(3):179–184. doi:10.1111/ane.12367
4. Bartoli F, Di Brita C, Crocamo C, et al. Early post-stroke depression and mortality: meta-analysis and meta-regression. Front Psychiatry. 2018;9:530. doi:10.3389/fpsyt
5. Tu J, Wang LX, Wen HF, et al. The association of different types of cerebral infarction with post-stroke depression and cognitive impairment. Medicine. 2018;97:e10919. doi:10.1097/MD.0000000000010919
6. Zeng Q, Huang Z, Wei L, Fang J, Lin K. Correlations of serum cystatin C level and gene polymorphism with vascular cognitive impairment after acute cerebral infarction. Neurol Sci. 2019;40(5):1049–1054. doi:10.1007/s10072-019-03777-8
7. Kim JW, Szigethy EM, Melhem NM, Saghafi EM, Brent DA. Inflammatory markers and the pathogenesis of pediatric depression and suicide: a systematic review of the literature. J Clin Psychiatry. 2014;75(11):1242–1253. doi:10.4088/JCP.13r08898
8. Anjum S, Qusar MMAS, Shahriar M, Islam SMA, Bhuiyan MA, Islam MR. Altered serum interleukin-7 and interleukin-10 are associated with drug-free major depressive disorder. Ther Adv Psychopharmacol. 2020;10:2045125320916655. doi:10.1177/2045125320-916655
9. Wilson ME, Boumaza I, Bowser R. Measurement of cystatin C functional activity in the cerebrospinal fluid of amyotrophic lateral sclerosis and control subjects. Fluids Barriers CNS. 2013;10(1):15. doi:10.1186/2045-8118-10-15
10. Nagai A, Terashima M, Sheikh AM, et al. Involvement of cystatin C in pathophysiology of CNS diseases. Front Biosci. 2008;13(13):3470–3479. doi:10.2741/2941
11. Warfel AH, Zucker-Franklin D, Frangione B, Ghiso J. Constitutive secretion of cystatin C (gamma-trace) by monocytes and macrophages and its downregulation after stimulation. J Exp Med. 1987;166(6):1912–1917. doi:10.1084/jem.166.6.1912
12. Wu L, Yan Z, Jiang H, Xing H, Li H, Qiu C. Serum cystatin C, impaired kidney function, and geriatric depressive symptoms among older people living in rural area: a population-based study. BMC Geriatr. 2018;18(1):265. doi:10.1186/s12877-018-0957-2
13. Evgueni M, Mark U, Michael G, et al. Association of cystatin C and depression in healthy elders: the health, aging and body composition study. Association of cystatin C and depression in healthy elders: the health, aging and body composition study. Nephron Clin Pract. 2010;116(3):c241–c246. doi:10.1159/000317205
14. Brott T, Adams HP, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20:864–870. doi:10.1161/01.STR.20.7.864
15. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi:10.1136/jnnp.23.1.56
16. O’Hare AM, Newman AB, Katz R, et al. Cystatin C and incident peripheral arterial disease events in the elderly-Results from the cardiovascular health study. Arch Intern Med. 2005;165:2666–2670. doi:10.1001/archinte.165.22.2666
17. Nagy EE, Puskás A, Kelemen P, et al. Elevated serum cystatin C and decreased cathepsin S/Cystatin C ratio are associated with severe peripheral arterial disease and polyvascular involvement. Diagnostics. 2022;12(4):833. doi:10.3390/diagnostics12040833
18. Lu X, Duan J, Cheng Q, et al. The association between serum growth differentiation factor-15 and 3-month depression after acute ischemic stroke. J Affect Disord. 2020;260:695–702. doi:10.1016/j.jad.2019.09.037
19. Tsai CS, Wu CL, Hung TH, et al. Incidence and risk factors of poststroke depression in patients with acute ischemic stroke: a 1-year prospective study in Taiwan. Biomed J. 2016;39:195–200. doi:10.1016/j.bj.2015.10.004
20. Lin S, Luan X, He W, et al. Post-stroke depression and estimated glomerular filtration rate: a prospective stroke cohort. Neuropsychiatr Dis Treat. 2020;16:201–208. doi:10.2147/NDT.S225905
21. Hadidi N, Treat-Jacobson DJ, Lindquist R. Poststroke depression and functional outcome: a critical review of literature. Heart Lung. 2009;38(2):151–162. doi:10.1016/j.hrtlng.2008.05.002
22. Nys GM, van Zandvoort MJ, van der Worp HB, et al. Early depressive symptoms after stroke: neuropsychological correlates and lesion characteristics. J Neurol Sci. 2005;228:27–33. doi:10.1016/j.jns.2004.09.031
23. Jastorff J, Huang YA, Giese MA, et al. Common neural correlates of emotion perception in humans. Hum Brain Mapp. 2015;36:4184–4201. doi:10.1002/hbm.22910
24. Xu Z, Leng C, Yang B, Wang H, Zhu J. Serum cystatin C is associated with large cerebral artery stenosis in acute ischemic stroke. Oncotarget. 2017;8(40):67181–67188. doi:10.18632/oncotarget.18061
25. Liu J, Sukhova GK, Sun JS, Xu WH, Libby P, Shi GP. Lysosomal cysteine proteases in atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24(8):1359. doi:10.1161/01.atv.0000134530.27208.41
26. Guo J, Wang J, Sun W, Liu X. The advances of post-stroke depression: 2021 update. J Neurol. 2021;269:1236–1249. doi:10.1007/s00415-021-10597-4
27. Arpegård J, Ostergren J, de Faire U, Hansson LO, Svensson P. Cystatin C—a marker of peripheral atherosclerotic disease? Atherosclerosis. 2008;199(2):397–401. doi:10.1016/j.atherosclerosis.2007.11.025
28. Yalcin S, Ulas T, Eren MA, et al. Relationship between oxidative stress parameters and cystatin C levels in patients with severe preeclampsia. Medicina. 2013;49(3):118–123. doi:10.3390/medicina.49030019
29. Zi M, Xu Y. Involvement of cystatin C in immunity and apoptosis. Immunol Lett. 2018;196:80–90. doi:10.1016/j.imlet.2018.01.006
30. Ghidoni R, Paterlini A, Albertini V, et al. Cystatin C is released in association with exosomes: a new tool of neuronal communication which is unbalanced in Alzheimer’s disease. Neurobiol Aging. 2011;32(8):1435–1442. doi:10.1016/j.neurobiolaging.2009.08.013
31. Xu Y, Ding Y, Li X, Wu X. Cystatin C is a disease-associated protein subject to multiple regulation. Immunol Cell Biol. 2015;93(5):442–451. doi:10.1038/icb.2014.121
32. Islam MR, Ali S, Karmoker JR, et al. Evaluation of serum amino acids and non-enzymatic antioxidants in drug-naïve first episode major depressive disorder. BMC Psychiatry. 2020;20(1):333. doi:10.1186/s12888-020-02738-2
33. Conejero I, Navucet S, Keller J, Olié E, Courtet P, Gabelle A. A complex relationship between suicide, dementia, and amyloid: a narrative review. Front Neurosci. 2018;12:371. doi:10.3389/fnins.2018.00371
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.