Back to Journals » Infection and Drug Resistance » Volume 15
Serological Evidence of Hepatitis E Virus (HEV) Infection Among Ruminant Farmworkers: A Retrospective Study from Malaysia
Authors Wong LP , Tay ST, Chua KH, Goh XT, Alias H, Zheng Z, Zhao Q, Wu T, Xia N , Hu Z, Lin Y
Received 22 March 2022
Accepted for publication 27 May 2022
Published 19 September 2022 Volume 2022:15 Pages 5533—5541
DOI https://doi.org/10.2147/IDR.S367394
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Li Ping Wong,1,2 Sun Tee Tay,3 Kek Heng Chua,4 Xiang Ting Goh,4 Haridah Alias,1 Zizheng Zheng,5 Qinjian Zhao,6 Ting Wu,5 Ningshao Xia,5,7 Zhijian Hu,2 Yulan Lin2
1Centre for Epidemiology and Evidence-Based Practice, Department of Social and Preventive Medicine, Faculty of Medicine, Universiti Malaya, Kuala Lumpur, 50603, Malaysia; 2Department of Epidemiology and Health Statistics, School of Public Health, Fujian Medical University, Fuzhou, Fujian, 350122, People’s Republic of China; 3Department of Medical Microbiology, Faculty of Medicine, Universiti Malaya, Kuala Lumpur, 50603, Malaysia; 4Department of Biomedical Science, Faculty of Medicine, Universiti Malaya, Kuala Lumpur, 50603, Malaysia; 5State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, National Institute of Diagnostics and Vaccine Development in Infectious Diseases, School of Public Health, Xiamen University, Xiamen, 361102, People’s Republic of China; 6College of Pharmacy, Chongqing Medical University, Chongqing, People’s Republic of China; 7The Research Unit of Frontier Technology of Structural Vaccinology of Chinese Academy of Medical Sciences, Xiamen University, Xiamen, 361102, People’s Republic of China
Correspondence: Yulan Lin, School of Public Health, Fujian Medical University, Fuzhou, Fujian, 350122, People’s Republic of China, Email [email protected] Li Ping Wong, Faculty of Medicine, Universiti Malaya, Kuala Lumpur, Malaysia, Email [email protected]
Background: As scant data are available about Hepatitis E virus (HEV) infection in Malaysia, this study aimed to determine the seroprevalence of HEV amongst ruminant farmworkers in Malaysia.
Methods: A total of 87 farmworkers provided serum samples, which were collected from eight farms. All serum samples were tested for anti-HEV IgG and anti-HEV IgM by an enzyme-linked immunosorbent assay (ELISA) using the Wantai HEV-IgG and HEV-IgM ELISA kits from Beijing Wantai Biological Pharmacy Enterprise Co., Ltd, Beijing, China.
Results: Farmworkers from six cattle farms, one sheep farm and one goat farm were investigated in this study. Only one farm practices zero-grazing, with the rest using rotational grazing. Of the 87 farmworkers, males comprised 83.9%, and almost half (47.1%) were aged 20– 35 years old. By ethnic group, the vast majority were Malay. Most of the farmworkers have good hygiene practices; washing or changing their clothes and showering after dealing with farm animals were common. None of the farmworker serum samples had anti-HEV IgM and IgG detected (95% confidence interval (CI): 0, 0.0415).
Conclusion: The finding suggests that the farmworkers had no previous exposure to Hepatitis E, and were not at risk of occupational exposure to HEV infection. Our findings suggest that a zero seroprevalence of HEV infection among ruminant farmworkers in the Muslim majority country. Good farm management, hygiene practices and the absence of contact with swine-related contamination might have contributed to the no or minimal zoonotic risks of HEV amongst farmworkers surveyed in this study.
Keywords: HEV, farmworkers, seroprevalence, antibody
Introduction
Hepatitis E virus (HEV) is now well recognised as an emerging zoonotic pathogen with an annual estimate of 20 million infections worldwide, of which more than 3 million were acute cases of hepatitis E, resulting in over 57,000 HEV-related deaths.1 HEV transmission is not limited between humans and animals, but can also occur via zoonotic spread from animals to humans. The zoonotic transmission of HEV from animals to humans is of increasing threat. Mounting evidence indicates that the transmission of HEV from animals to humans is mediated by the consumption of uncooked or undercooked animal meat or foods. In European countries, cases of HEV infection related to the consumption of raw seafood and pork liver sausage were reported in Italy and France.2,3 In Germany, HEV viral genome was detected in commercial pork livers and pork meats,4 and a high HEV incidence in Germany was reported to be linked to raw pork intake.5,6 Likewise, in Japan, the consumption of the meat and entrails of pigs, wild boars and deer was linked to HEV infections.7–9 In Thailand, the HEV seroprevalence level was lowest in areas where the majority of the population is Muslim and do not consume pork.10
Apart from foodborne transmission, contact with infected animals is also an important mode of transmission of HEV. In Italy, the HEV subtypes 3e and 3f were detected in wild boars in north-western Italy11 and exposure to wild boars was associated with HEV infection.2 The significance of contact exposure and the zoonotic transmission of HEV has been an important occupational hazard that receives a great deal of attention.12 Farm and agricultural workers represent the occupational population associated with a high risk of zoonotic HEV transmission. In particular, swine farmworkers have been shown to have an increased risk of HEV infection as swine are the principal natural reservoir for HEV.13 A recent meta-analysis found that the pooled prevalence of anti‐HEV IgG in swine farmworkers or people in swine-related occupations compared to the general population was as high as 1.52, suggesting that they are 50% more likely to be infected with HEV than the general population.13
Although swine are the main reservoir, of late, there is an increased recognition that ruminants are also highly susceptible to HEV infection. A study in Lao People’s Democratic Republic reported a worryingly high prevalence of HEV infections in cattle and other ruminants.14 Further, the study also found that anti-HEV IgG seroprevalence was higher in cattle farmers than in villagers that do not own ruminants.14 In Jordan, high HEV seropositivity (37.4%) was reported in dairy cattle.15 HEV seropositivity varied from 4.4% to 6.9% in cattle in India.16 HEV strains have also been found in dairy cows and their milk in China.17,18 In China, HEV seropositivity was also found in cattle (47%) that were bred mainly for meat.19 In Egypt, HEV seropositivity was reported in 21.6%, 14%, 4.4%, and 9.4% of examined cows, buffaloes, sheep, and goats, respectively.20 In a study in Brazil, anti-HEV antibodies were not detected in sheep and goats, however, HEV seropositivity was reported in cows (1.4%).21 All the above evidence indicates the emergence and transmission of zoonotic HEV in farm animals other than swine. In particular, the evidence that HEV circulates in ruminants in Egypt, a Muslim-majority country, has created concerns.
Malaysia is a Muslim majority country in Southeast Asia. HEV genotype 4 is prevalent in Malaysia.22 Genotypes 3 and 4 are related to zoonotic diseases and are transmitted by eating infected animal meat or having close contact with animals.23 The prevalence of anti-HEV antibodies in the general population at large in Malaysia has never been reported. Early studies on HEV infection in Malaysia were published over two decades ago. The first study was conducted on small indigenous communities and blood donors from an urban setting, with the prevalence of anti-HEV IgG among the indigenous people reported to be close to 50%, whereas only 2% was reported in a small sample of urban blood donors.24 The second study dated to 2000, where the prevalence of anti-HEV IgG in a small sample of HIV patients was reported to be 14.5%.25 Over two decades since these two studies, there have been no further reports on HEV seroprevalence in Malaysia. Of late, due to the increasing recognition of the threat of HEV infection to global public health, research into HEV in Malaysia has started to pick up. A recent study in 2019 on a relatively large sample of the indigenous population in Malaysia found an anti-HEV IgG positive rate of 5.9%.26 Most recently, in 2020, HEV-specific IgG antibodies were detected in 3.1% and IgM in 0.9% of blood donor samples in Malaysia.27 Of note, the prevalence of HEV viraemia varies among global blood donors. The overall rates of anti-HEV IgG reactivity among blood donors in Europe ranged from 4.7% to 52.5%.28 Countries such France,29 Germany30 and Netherlands31 reported a considerably higher prevalence of viraemia in the blood donor groups. A recently published systematic review of HEV seroprevalence revealed that Malaysia ranked the lowest (2% among urban blood donors) while Lao PDR ranked highest (77.7% in lowland community) in HEV seroprevalence among the South-Eastern Asia countries.22 In Thailand, seroprevalence of HEV infection among blood donors was near 30%.32 Despite relatively lower HEV seroprevalence compared to neighboring countries, the evidence of HEV seroprevalence in blood donor groups in Malaysia grasps the attention to the possibility of transmission of HEV in blood transfusion. With these worrisome evidence, more research within the area of the seroprevalence of HEV is needed to provide a clearer understanding of HEV infection in the Malaysian population.
There was an estimated 710,481 cattle and 399,045 goat population in Malaysia in the year 2018.33 The Malaysian government plans to double the area of land used for ruminant farming to reduce the country’s dependency on ruminant imports.34 Examining the seroprevalence of HEV in ruminant farmworkers may help to reveal new insights into the occupational health risk of ruminant farmworkers as well as the presence of HEV infection in ruminant farm animals in Malaysia. Findings will provide useful recommendations for HEV prevention in the rapidly growing ruminant farming industry in Malaysia. Therefore, this study aimed to screen the previously archived serum samples of ruminant farmworkers in Malaysia for anti-HEV IgM and IgG and identify potential risk factors for HEV infection.
Materials and Methods
Population and Sample Collection
Archived serum samples of healthy farmworkers from previous studies35–37 were used for this seroprevalence study. The samples were collected between September 2012 and February 2013 from a total of eight ruminant farms throughout Peninsular Malaysia. Inclusion criteria were as follows: (1) Malaysian citizen, (2) 18 years or above, 3) employed to do farm work for ≥6 months. Migrant farmworkers were excluded from the study. The farms were located in the suburban areas of the Southern, Northern, Central and Eastern regions of Peninsular Malaysia (Figure 1). All of the farms were surrounded by either palm oil, rubber plantations or secondary forests. A total of 87 archived serum samples were tested for anti-HEV IgG and anti-HEV IgM between April 2021 and May 2021.
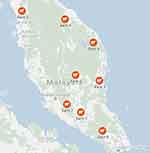 |
Figure 1 Distribution of ruminant farms in the study. |
Anti-HEV Serological Assays
All serum samples were tested for anti-HEV IgG and anti-HEV IgM by an enzyme-linked immunosorbent assay (ELISA) using the Wantai HEV-IgG (catalogue no. WE-7296) and HEV-IgM (catalogue no. WE-7196) ELISA kits from Beijing Wantai Biological Pharmacy Enterprise Co., Ltd, Beijing, China. Testing and calculations (ie, sample to cut-off ratio and determination of equivocal results) were performed following the manufacturer’s instructions. In addition, three negative and two positive controls we included in each round of ELISA assay to validate the results.
The Wantai HEV IgG assay is known for its superior sensitivity38–40 and is also regarded as a “gold standard” for HEV antibody detection.41 The test is currently one of the most commonly used assays with reported specificity and sensitivity of the HEV IgG of 97.96% and 99.6%, respectively.42,43 Analyses were performed in duplicate.
Ethical Approval
This study complies with the World Medical Association Declaration of Helsinki regarding ethical conduct of medical research. The study was approved by the Universiti Malaya Medical Centre MRECID.NO: 2017829-5531. The participants were informed that their participation was voluntary and written informed consent was collected from all involved participants.
Results
Of the total 87 farmworkers, males comprise 83.9% of the total. Almost half (47.1%) were aged 20–35 years old. By ethnic group, the vast majority were Malays. Most of the participants have good hygiene practices, with washing or changing clothes and showering after dealing with farm animals being common (Table 1). Table 2 shows the characteristics of the ruminant farms in which the participants were recruited. The majority of the farms were cattle farms; however, one was a sheep farm and another was a goat farm. Only one farm practiced zero-grazing, as the rest were rotational grazing.
 |
Table 1 Participant Characteristics and Personal Hygiene Practice of Farmworkers Surveyed in This Study (N = 87) |
 |
Table 2 The Characteristics of Ruminant Farms |
None of the study participants had anti-HEV IgM and IgG detected (95% confidence interval (CI): 0, 0.0415).
Discussion
This study was conducted to determine the seroprevalence of HEV among ruminant farmworkers to fill the knowledge gap concerning occupational risk due to HEV infection in Malaysia. Our finding of zero seroprevalence suggests that the ruminant farmworkers had no previous exposure to HEV. This may also imply that ruminants might not serve as HEV reservoirs in the farms studied and hence do not pose a major health risk to the farmworkers. A study from Belgium similarly reported the absence of HEV seroprevalence in dairy cows and HEV RNA in milk samples collected from dairy farms in various districts.44 Good management of farms and the low likelihood of direct or indirect contact between pigs and cows were reported as being associated with the absence of HEV infection.44 Likewise, good farm management and a lack of contact with swine perhaps result in the absence of HEV infection in the ruminants and workers on the farms in the present study. In addition, the farms in our study neither practiced mixed farming nor were located near pig farms. Contrary to our results, the presence of HEV in ruminants has been reported in farms practicing the mixed farming of ruminants and pigs or in farms neighboring pig farms.17 A mixed-breeding environment has also been associated with the possibility of cross-species transmission of HEV from pigs to rabbits.45,46
Grazing-contaminated pasture or pasture that is fertilised with pig manure or animal waste has been reported to harbour HEV and various infections from farm animals.44,47,48 Pig manure slurry is an emergent health and environmental problem and a potential source of HEV infection.49 The presence of HEV found in pig manure slurry was found to be infectious.49 In the present study, despite the majority of farms practicing rotational grazing, the absence of HEV seropositivity among the farmworkers perhaps implies the absence of HEV infection in the ruminants due to those animals on the investigated farms grazing on pasture that is not contaminated. Furthermore, there was no pig farming close to the ruminant farms in this study. Malaysia is a Muslim majority country, so pig management and slurry management in pig farming are given great emphasis. This perhaps results in a low risk of HEV infection of swine-origin, and hence could be the reason for the absence of HEV seroprevalence in the ruminants and workers despite the practice of rotational grazing.
In the Lao People’s Democratic Republic, ruminants from villages with free-roaming animals and animals that have open access to river water were found to have a higher prevalence of HEV infections.14 Animal manure contamination in drinking water was also assumed to be the main source of zoonotic HEV infection in humans and animals.46,50 Therefore, both farmworkers and livestock should avoid contact or drink from unsafe water sources. In our study, the ruminants were not free-roaming, perhaps explaining the lower risk of acquiring HEV infections. A recent study reported that interaction between wild and domestic ruminants may contribute to the spread of HEV.51 Another recent study in Portugal revealed that high HEV antibodies in sheep sera (16.6%) correspond to higher HEV seropositivity in workers occupationally exposed to sheep (29.3%) compared to the control population (16.1%).52 Therefore, domestic ruminants must avoid or minimize close contact with wildlife animals.
It is also important to note that alternatively, good hygiene practices among the farmworkers in this study could also contribute to no risk or minimal risk of HEV infection. In this study, most of the farmworkers reported changing clothes or showers after dealing with farm animals. HEV could be transmitted from farm animals via contact with farmworkers or their contaminated workwear. A study from Lao People’s Democratic Republic revealed that lower hygiene practices were found to result in an increased risk of testing seropositive for HEV among farmworkers.53 Training about personal hygiene and infection prevention should be provided to all farmworkers to prevent or reduce the risk of contracting zoonotic diseases. Wearing appropriate personal protective equipment (PPE) and proper farm hygiene practices by farmworkers are important to reduce the risk of occupational-associated zoonotic infections.54 It is also important to create awareness among farmworkers about the risk and sequelae of HEV exposure and infection.
It is also important to note that the majority of the farmworkers in this study are of Malay ethnicity and most of the Malaysian Malays are Muslims. The Syariah laws in Malaysia prohibit the consumption of pork among Muslims. In our previous study on serum samples of blood donors, none of the Malay blood donors were found to be positive for IgG or IgM against HEV.27 This could also be a contributing factor to the absence of IgG and IgM HEV antibodies in the current study samples.
The findings of this study should be interpreted cautiously owing to some limitations. First, the study was limited to only eight ruminant farms and only 87 workers consented to provide their blood samples for analyses. We recognise that the small sample size of this study may underestimate the magnitude of undiagnosed HEV infection among ruminant farmworkers in Malaysia in general. Participation in the study was voluntary and only 87 farmworkers consented to provide blood samples; as such, the farmworkers who refused to participate or were not sampled might be seropositive for HEV antibodies, so the findings should be interpreted with caution. Also, the serum samples in the study were collected eight years ago and may not be reflective of the current HEV infection among ruminant farmworkers. Future analyses on more recent farmworker samples are warranted. It is important to note that HEV serology should be interpreted with caution. Currently, detection of HEV RNA by reverse transcriptase polymerase chain reaction (rtPCR) is the gold standard for demonstration of active viremia. Overall, the diagnostic utility of HEV antigen (Ag) demonstrated a very good correlation with HEV rtPCR.55 Therefore, the absence of HEV seropositivity among farmworkers in this study does not rule out the absence of HEV infection in the ruminants. Furthermore, direct serological evidence of HEV infection from ruminants or testing of HEV RNA of raw meat and milk samples of the ruminants are warranted to accurately gauge the HEV infection in ruminants.
Conclusion
In conclusion, we could not find any evidence of active or past HEV infection in samples of ruminant farmworkers in Malaysia. Our findings suggest a zero seroprevalence of HEV infection among ruminant farmworkers in the Muslim majority country. This could be attributable to the absence of exposure to contamination associated with swine during farming. This study suggests the important role of farm management and hygiene in reducing the risk of zoonotic HEV infection in the ruminant farming sector. The occupational risk groups, mainly farm and agricultural workers, represent an occupational population associated with zoonotic risks of HEV. Therefore, apart from testing farm animals for HEV, it is also essential to conduct health screening or surveillance for the farmworkers periodically.
Data Sharing Statement
The datasets generated and/or analysed during the current study are not publicly available due to privacy or ethical restrictions but are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The study was approved by the Universiti Malaya Medical Centre MRECID.NO: 2017829-5531. The participants were informed that their participation was voluntary and written informed consent was collected from all involved participants.
Acknowledgment
The analyses of HEV were performed using Wantai HEV IgG ELISA, of which all the HEV ELISA kits were sponsored by Beijing Wantai Biological Pharmacy Enterprise Co., Ltd, Beijing, China. The authors would like to thank Blood Bank Malaysia/National Blood Centre for their support during this study.
Funding
This work is supported by 1) Newton-Medical Research Council grant (IF077-2019), 2) National Natural Science Foundation of China (No. 81871247, No.82071783), and 3) Research Unit of Frontier Technology of Structural Vaccinology of Chinese Academy of Medical Sciences (CAMS), Innovation Fund for Medical Sciences (No. 2019RU022). The funders were not involved in study design, in the collection, analysis and interpretation of data; in the writing of the manuscript; nor in the decision to submit the manuscript for publication.
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization (WHO). Hepatitis E; 2020. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-e.
2. La Rosa G, Muscillo M, Vennarucci VS, et al. Hepatitis E virus in Italy: molecular analysis of travel-related and autochthonous cases. J Gen Virol. 2011;92(7):1617–1626. doi:10.1099/vir.0.031278-0
3. Berto A, Grierson S, Hakze-van der Honing R, et al. Hepatitis E virus in pork liver sausage, France. Emerg Infect Dis. 2013;19(2):264. doi:10.3201/eid1902.121255
4. Pallerla SR, Schembecker S, Meyer CG, et al. Hepatitis E virus genome detection in commercial pork livers and pork meat products in Germany. J Viral Hepatitis. 2021;28(1):196–204. doi:10.1111/jvh.13396
5. Healio. High hepatitis E incidence in Germany linked to raw pork intake; 2018. Available from: https://www.healio.com/news/hepatology/20180322/high-hepatitis-e-incidence-in-germany-linked-to-raw-pork-intake.
6. Slot E, Zaaijer HL, Molier M, et al. Meat consumption is a major risk factor for hepatitis E virus infection. PLoS One. 2017;12(4):e0176414. doi:10.1371/journal.pone.0176414
7. Masuda JI, Yano K, Tamada Y, et al. Acute hepatitis E of a man who consumed wild boar meat prior to the onset of illness in Nagasaki, Japan. Hepato Res. 2005;31(3):178–183. doi:10.1016/j.hepres.2005.01.008
8. Miyashita K, Kang JH, Saga A, et al. Three cases of acute or fulminant hepatitis E caused by ingestion of pork meat and entrails in Hokkaido, Japan: zoonotic food‐borne transmission of hepatitis E virus and public health concerns. Hepato Res. 2012;42(9):870–878. doi:10.1111/j.1872-034X.2012.01006.x
9. Tei S, Kitajima N, Ohara S, et al. Consumption of uncooked deer meat as a risk factor for hepatitis E virus infection: an age‐and sex‐matched case‐control study. J Med Virol. 2004;74(1):67–70. doi:10.1002/jmv.20147
10. Gonwong S, Chuenchitra T, Khantapura P, et al. Pork consumption and seroprevalence of hepatitis E virus, Thailand, 2007–2008. Emerg Infect Dis. 2014;20(9):1531. doi:10.3201/eid2009.140418
11. Caruso C, Modesto P, Bertolini S, et al. Serological and virological survey of hepatitis E virus in wild boar populations in northwestern Italy: detection of HEV subtypes 3e and 3f. Arch Virol. 2015;160(1):153–160. doi:10.1007/s00705-014-2246-5
12. Vonesch N, Binazzi A, Bonafede M, et al. Emerging zoonotic viral infections of occupational health importance. Pathog Dis. 2019;77(2):ftz018. doi:10.1093/femspd/ftz018
13. Huang X, Huang Y, Wagner AL, et al. Hepatitis E virus infection in swine workers: a meta‐analysis. Zoonoses Public Health. 2019;66(1):155–163. doi:10.1111/zph.12548
14. Tritz SE, Khounvisith V, Pommasichan S, et al. Evidence of increased Hepatitis E virus exposure in Lao villagers with contact to ruminants. Zoonoses Public Health. 2018;65(6):690–701. doi:10.1111/zph.12483
15. Obaidat MM, Roess AA. Individual animal and herd level seroprevalence and risk factors of Hepatitis E in ruminants in Jordan. Infect Genet Evol. 2020;81:104276. doi:10.1016/j.meegid.2020.104276
16. Arankalle VA, Joshi MV, Kulkarni AM, et al. Prevalence of anti‐hepatitis E virus antibodies in different Indian animal species. J Viral Hepat. 2001;8(3):223–227. doi:10.1046/j.1365-2893.2001.00290.x
17. Huang F, Li Y, Yu W, et al. Excretion of infectious hepatitis E virus into milk in cows imposes high risks of zoonosis. Hepatology. 2016;64(2):350–359. doi:10.1002/hep.28668
18. Hu GD, Ma X. Detection and sequences analysis of bovine hepatitis E virus RNA in Xinjiang Autonomous Region. Bing du Xue Bao. 2010;26:27–32.
19. Yan B, Zhang L, Gong L, et al. Hepatitis E virus in yellow cattle, Shandong, Eastern China. Emerg Infect Dis. 2016;22(12):2211. doi:10.3201/eid2212.160641
20. El‐Tras WF, Tayel AA, El‐Kady NN. Seroprevalence of hepatitis E virus in humans and geographically matched food animals in Egypt. Zoonoses Public Health. 2013;60(3):244–251. doi:10.1111/j.1863-2378.2012.01516.x
21. Vitral CL, Pinto MA, Lewis-Ximenez LL, Khudyakov YE, Dos Santos DR, Gaspar AM. Serological evidence of hepatitis E virus infection in different animal species from the Southeast of Brazil. Mem Inst Oswaldo Cruz. 2005;100:117–122. doi:10.1590/S0074-02762005000200003
22. Raji YE, Toung OP, Mohd Taib N, Sekawi ZB. A systematic review of the epidemiology of Hepatitis E virus infection in South–Eastern Asia. Virulence. 2021;12(1):114–129. doi:10.1080/21505594.2020.1865716
23. Goel A, Aggarwal R. Advances in hepatitis E–II: epidemiology, clinical manifestations, treatment and prevention. Expert Rev Gastroenterol Hepatol. 2016;10(9):1065–1074. doi:10.1080/17474124.2016.1185365
24. Seow HF, Mahomed NM, Mak JW, et al. Seroprevalence of antibodies to hepatitis E virus in the normal blood donor population and two aboriginal communities in Malaysia. J Med Virol. 1999;59:164–168. doi:10.1002/(SICI)1096-9071(199910)59:2<164::AID-JMV7>3.0.CO;2-J
25. Ng KP, He J, Saw TL, et al. A seroprevalence study of viral hepatitis E infection in human immunodeficiency virus type 1 infected subjects in Malaysia. Med J Malaysia. 2000;55:58–64.
26. Wong LP, Alias H, Choy SH, et al. The study of seroprevalence of hepatitis E virus and an investigation into the lifestyle behaviours of the aborigines in Malaysia. Zoonoses Public Health. 2020;67(3):263–270. doi:10.1111/zph.12681
27. Wong LP, Lee HY, Khor CS, et al. The risk of transfusion-transmitted hepatitis E virus: evidence from seroprevalence screening of blood donations. Indian J Hematol Blood Tranfus. 2021;2021:1–8.
28. Petrik J, Lozano M, Seed CR, et al. Hepatitis E. Vox Sang. 2016;110(1):93–130. doi:10.1111/vox.12285
29. Mansuy JM, Gallian P, Dimeglio C, et al. A nationwide survey of hepatitis E viral infection in French blood donors. Hepatology. 2016;63(4):1145–1154. doi:10.1002/hep.28436
30. Denner J, Pischke S, Steinmann E, Blümel J, Glebe D. Why all blood donations should be tested for hepatitis E virus (HEV). BMC Infect Dis. 2019;19(1):1–5. doi:10.1186/s12879-019-4190-1
31. Slot E, Hogema BM, Riezebos-Brilman A, Kok TM, Molier M, Zaaijer HL. Silent hepatitis E virus infection in Dutch blood donors, 2011 to 2012. Eurosurveillance. 2013;18(31):20550. doi:10.2807/1560-7917.ES2013.18.31.20550
32. Jupattanasin S, Chainuvati S, Chotiyaputta W, et al. A nationwide survey of the seroprevalence of hepatitis E virus infections among blood donors in Thailand. Viral Immunol. 2019;32(7):302–307. doi:10.1089/vim.2018.0146
33. Department of Veterinary Services. DVS Perangkaan Ternakan. Department of Veterinary Services, Malaysia; 2019. Available from: http://www.dvs.gov.my/dvs/resources/user_1/2019/BP/Perangkaan%20Ternakan/3._Msia__Perangkaan_ternakan_M_Surat_1-15_pdf.
34. The Malaysian Reserve. Govt to double cattle farming area by 2025; 2020. Available from: https://themalaysianreserve.com/2020/02/19/govt-to-double-cattle-farming-area-by-2025/.
35. Kho KL, Koh FX, Jaafar T, et al. Prevalence and molecular heterogeneity of Bartonella bovis in cattle and Haemaphysalis bispinosa ticks in Peninsular Malaysia. BMC Vet Res. 2015;11(1):1–9. doi:10.1186/s12917-015-0470-1
36. Kho KL, Koh FX, Hasan LI, et al. Rickettsial seropositivity in the indigenous community and animal farm workers, and vector surveillance in Peninsular Malaysia. Emerg Microbes Infect. 2017;6(1):1–9. doi:10.1038/emi.2017.4
37. Shukri MM, Kho KL, Kisomi MG, et al. Seroprevalence report on tick-borne encephalitis virus and Crimean-Congo hemorrhagic fever virus among Malaysian’s farm workers. BMC Public Health. 2015;15(1):1–6. doi:10.1186/1471-2458-15-1
38. Park HK, Jeong SH, Kim JW, et al. Seroprevalence of anti-hepatitis E virus (HEV) in a Korean population: comparison of two commercial anti-HEV assays. BMC Infect Dis. 2012;12(1):1–6. doi:10.1186/1471-2334-12-142
39. Wenzel JJ, Preiss J, Schemmerer M, et al. Test performance characteristics of Anti-HEV IgG assays strongly influence hepatitis E seroprevalence estimates. J Infect Dis. 2013;207:497–500. doi:10.1093/infdis/jis688
40. Sommerkorn FM, Schauer B, Schreiner T, et al. Performance of hepatitis E virus (HEV)-antibody tests: a comparative analysis based on samples from individuals with direct contact to domestic pigs or wild boar in Germany. Med Microbiol Immun. 2017;206:277–286. doi:10.1007/s00430-017-0503-4
41. Kmush BL, Labrique AB, Dalton HR, et al. Two generations of “gold standards”: the impact of a decade in hepatitis E virus testing innovation on population seroprevalence. Am J Trop Med Hyg. 2015;93(4):714–717. doi:10.4269/ajtmh.15-0159
42. Capai L, Falchi A, Charrel R. Meta-analysis of human IgG anti-HEV seroprevalence in industrialized countries and a review of literature. Viruses. 2019;11:84. doi:10.3390/v11010084
43. Yan Q, Du HL, Wang YB, et al. Comparison of two diagnostic reagents to detect anti-hepatitis E virus IgG antibodies. Chin J Zoonoses. 2008;24:1087–1089.
44. Vercouter AS, Sayed IM, Lipkens Z, et al. Absence of zoonotic hepatitis E virus infection in Flemish dairy cows. Int J Food Microbiol. 2018;281:54–59. doi:10.1016/j.ijfoodmicro.2018.05.009
45. Liu L, Wang L, Xia J, et al. Mix‐breeding with HEV‐infected swine induced inapparent HEV infection in SPF rabbits. J Med Virol. 2016;88(4):681–685. doi:10.1002/jmv.24374
46. Khuroo MS, Khuroo MS, Khuroo NS. Transmission of hepatitis E virus in developing countries. Viruses. 2016;8(9):253. doi:10.3390/v8090253
47. Morgan ER, Charlier J, Hendrickx G, et al. Global change and helminth infections in grazing ruminants in Europe: impacts, trends and sustainable solutions. Agriculture. 2013;3(3):484–502. doi:10.3390/agriculture3030484
48. Meester M, Tobias TJ, Bouwknegt M, et al. Infection dynamics and persistence of hepatitis E virus on pig farms–a review. Porc Health Manag. 2021;7(1):1–16. doi:10.1186/s40813-021-00189-z
49. Kasorndorkbua C, Opriessnig T, Huang FF, et al. Infectious swine hepatitis E virus is present in pig manure storage facilities on United States farms, but evidence of water contamination is lacking. Appl Environ Microbiol. 2005;71(12):7831–7837. doi:10.1128/AEM.71.12.7831-7837.2005
50. Yugo DM, Meng XJ. Hepatitis E virus: foodborne, waterborne and zoonotic transmission. Int J Environ Res Public Health. 2013;10(10):4507–4533. doi:10.3390/ijerph10104507
51. Palombieri A, Robetto S, Di Profio F, et al. Surveillance STUDY of hepatitis E virus (HEV) in domestic and wild ruminants in northwestern Italy. Animals. 2020;10(12):2351. doi:10.3390/ani10122351
52. Mesquita JR, Santos‐Ferreira N, Ferreira AS, et al. Increased risk of hepatitis E virus infection in workers occupationally exposed to sheep. Transbound Emerg Dis. 2020;67(5):1918–1921.
53. Holt HR, Inthavong P, Khamlome B, et al. Endemicity of zoonotic diseases in pigs and humans in lowland and upland Lao PDR: identification of socio-cultural risk factors. PLoS Negl Trop Dis. 2016;10(4):e0003913. doi:10.1371/journal.pntd.0003913
54. Odo NU, Raynor PC, Beaudoin A, et al. Personal protective equipment use and handwashing among animal farmers: a multi-site assessment. J Occup Environ Hyg. 2015;12(6):363–368. doi:10.1080/15459624.2015.1006635
55. Gupta E, Pandey P, Pandey S, Sharma MK, Sarin SK. Role of hepatitis E virus antigen in confirming active viral replication in patients with acute viral hepatitis E infection. J Clin Virol. 2013;58(2):374–377. doi:10.1016/j.jcv.2013.07.019
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
