Back to Journals » Research and Reports in Tropical Medicine » Volume 10
Serological evidence of dengue fever and its associated factors in health facilities in the Borena Zone, South Ethiopia
Authors Geleta EN
Received 6 June 2019
Accepted for publication 6 August 2019
Published 28 August 2019 Volume 2019:10 Pages 129—136
DOI https://doi.org/10.2147/RRTM.S218586
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Mario Rodriguez-Perez
Eshetu Nigussie Geleta
Department of Medical Laboratory Science, Madda Walabu University, Bale Goba, Ethiopia
Correspondence: Eshetu Nigussie Geleta
Department of Medical Laboratory Science, Madda Walabu University, PO Box 302, Bale Goba 4540, Ethiopia
Tel +251 91 356 0128
Email [email protected]
Background: Dengue fever (DF) is a re-emerging public health threat in Ethiopia. Yet, little is known about the epidemiology and risk factors of dengue infection in the region. In this study, the seroprevalence and associated risk factors of dengue virus infection were assessed in the Borena Zone health facilities.
Methods: A hospital-based cross-sectional study was conducted from July to August 2016. A total of 519 consecutive acute febrile patients attending the outpatient departments of Teltelle Health Center, Yabello and Moyale Hospital were enrolled. Data on socio-demographic and environmental risk factors were collected using a structured questionnaire. Three to five milliliter blood samples were collected from all participants and screened for dengue virus exposure using indirect immunofluorescent assay.
Results: The overall prevalence of anti-DENV IgG and IgM was 22.9% and 7.9%, respectively. DF serostatus was influenced by gender (adjusted odd ratio (AOR)=1.72; 95% CI 1.01–2.94), place of residence (AOR=2.69; 95%CL 1.55–4.64) that had a higher rate of exposure and recalling of a recent mosquito bite (AOR=2.98; 95% CI 1.51–5.89) probably imply recent and/or ongoing active transmission.
Conclusion: This study showed that DF could potentially emerge as a public health threat in the study area. In addition to that, the observed low awareness of participants underlines the urgent need for further community-based studies to determine the environmental, and host factors that determine the extent of exposure to dengue virus infection in the area for appropriate control and prevention planning.
Keywords: Borena, dengue virus, indirect immunofluorescent assay, Ethiopia
Introduction
Dengue fever (DF) is the most rapidly spreading mosquito-borne disease and the major public health problem in the world.1 Dengue is a viral disease caused by dengue virus serotypes (DENV1-4) of the genus flavivirus. Dengue virus is a non-segmented, positive-sense, single-stranded, enveloped RNA virus; transmitted primarily by the bite of Aedes aegypti and Aedes albopictus.2,3 Infections can also be transmitted through blood transfusion, organ transplantation and possibly vertically from mother to child.4,5 The virus is distributed in more than 100 countries in tropical and subtropical areas across the Americas, East Mediterranean, Western Pacific, Africa, South-East Asia and Europe.6 Dengue infection has been reported in most African countries, especially in Eastern Africa, including Ethiopia.3,7,8 More than 390 million people are exposed to DENV each year resulting in 96 million annual cases of viral-associated disease globally.9 The WHO has reported 500,000 people develop severe disease each year, and about 1250 die.10 Although dengue has a global distribution, the majority of cases are from the South-East Asia region together with Western Pacific region bears nearly 75% of the global disease burden.11 Recently, a few cases of dengue infection have been reported in Ethiopia, specifically in the eastern parts of the country Dire Dawa and the Somali regions.3,8,12 Several factors are related to the increase of dengue incidence in Ethiopia. Among the most important ones are uncontrolled urbanization and the absence of standardized public services, such as water supply, sewage and waste disposal.3
Dengue virus infection produces a spectrum of clinical illness, ranging from an asymptomatic or mild febrile illness to classic DF to the most severe form of illness, dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS).13 DHF and DSS cases have also been increasingly recognized in South Asia, Latin America and the Pacific,14,15 with pediatric cases being more common. Also, DF and DHF/DSS have become more common in adults.16 DF is clinically difficult to diagnose, especially in developing countries with no established dengue diagnostic techniques and could easily be mistaken for malaria, typhoid or unknown febrile illnesses.17 Studies have reported that human antibody responses after dengue virus infection are highly cross-reactive with other arboviruses like Zika virus.18,19
Few studies of dengue infection have been carried out in Ethiopia even though unknown causes of acute febrile illnesses are common. A confirmed DF case was reported for the first time in Ethiopia in Dire Dawa city in 2013.8 Later studies were conducted in the Somali region and north-western parts of the country.3,12 However, data are not available on the DF in Southern Ethiopia. Thus, the aim of this study was to generate baseline data on the prevalence of DF and its associated risk factors in acute febrile patients in health facilities with catchments from the Borena Zone. This study will be helpful in providing information on DENV infection to health care authorities for better clinical management of patients and to design and implement appropriate control measures.
Materials and methods
Ethical consideration
Ethical clearance was obtained from the institutional review board of Hawassa University College of Medicine and Health Sciences, Oromia Regional Health Bureau Ethics Review Committee and AHRI/ALERT Ethics Review Committee. Before data collection, patients were informed about the objective and purpose of the study, about their right to participate or not in the study or to withdraw at any point in time. Personal privacy and dignity was respected. Data were collected after obtaining participants’/guardians’ informed written consent. Assent was also sought in cases where the study participants were children under 18 years old. All samples and forms containing patient information had no name or information that can identify a particular participant; and data were analyzed and interpreted in aggregate.
Study area
A study was carried in Borena Zone: Yabello Hospital, Moyale Hospital and Teltelle Health Center. Borena Zone is located in southern part of Ethiopia, 565 km from the capital of the country Addis Ababa, and bordering Kenya. A Zone has 2 hospitals and 14 health centers. The population size of the Zone is around 344,648, which majority of the population reside in rural areas comprising market centers and villages with individual homesteads. The climate of the area is arid; mean annual rain fall of 400–700 mm in two rainy seasons (March–May and June–August), and mean annual temperature ranging from 25°C to 37°C.
Study design and patient’s characteristics
A hospital-based cross-sectional study was carried out from July to August 2016. The sample size was estimated to be 519. The study participants consisted of all consecutive patients presenting with acute febrile illness at the outpatient departments during the study period. A febrile patient was defined as a patient who came to either the outpatient and to either the pediatric or medicine unit at the participating hospital with fever ≥38°C.
Data collection and laboratory test
Data collectors interviewed the study participants using a pretested structured questionnaire on socio-demographic and other risk factors such as the use of bed nets, trees around the compound, use of mosquito repellent and presence of stagnant water around the compound. A single 3–5 mL blood samples were collected, clotted and centrifuged at 1300 r/minute. Separated sera were transported using liquid nitrogen (−170°C) to Hawassa University Referral Hospital, and stored in a deep freezer (−80°C). Sera were transported using dry ice and screened in the AHRI laboratory for DENV immunoglobulin G (IgG) and immunoglobulin M (IgM) using EUROIMMUN biochips indirect immunofluorescent assay (IIFA) kit (Medizinische Labordiagnostika AG-Germany) according to the manufacturer’s manual.20 The EUROIMMUN biochips has separate area for each serotype and differentiate all four serotypes.
Data analysis
Data were entered and analyzed using SPSS version 20 software. Simple frequency tables were generated, and categorical variables were compared. A univariate logistic regression analysis was used to identify risk factors associated with the prevalence of anti-DENV IgM and IgG antibodies. Those independent variables found P<0.25 in univariate analysis were then used in multivariate logistic regression analysis. ORs at 95% CIs were calculated to measure the degree of association. A p-value <0.05 was considered as statistically significant and data were presented in the form of tables and figures. Prevalence of DENV infection was defined as the proportion of participants with IgM and/or IgG positive.
Results
Socio-demographic characteristics
A total of 519 participants were investigated during the study period. Two hundred and six (39.7%) of the study participants were from the Teltelle Health Center, 36.6% were from Moyale Hospital, and the remaining study participants (23.7%) were from Yabello Hospital. The median age of the participants was 24 years (range, 1–80 years), and those in the age range of 15–29 accounted 49.3% of participants. Male participants accounted for 51.3%, with a male to female ratio of 1:0.95. A substantial proportion of the study participants were rural residents (51.6%), illiterate (60.7%) and farmers by occupation (33.9%) (Table 1).
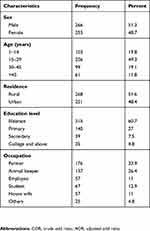 |
Table 1 Socio-demographic characteristics of the study participants, 2016 |
Seroprevalence of DENV infection
The overall prevalence of exposure to DF was found to be 22.9% and 7.9% for IgG and IgM, respectively. Male participants (30.1%) had a higher rate of DENV IgG compared to females (15.4%). With respect to age, the prevalence of DENV IgG was highest (25.2%) in the age group 1–14 years. Further, the rate of DENV infection IgG exposure was higher among urban residents (28.7%), students (32.8%), secondary education level (28.2%) and in participants enrolled from the Yaballo hospital (26.8%) (Table 2). Overall, 7.8% from age group 15–29 years and 10.7% from age group 1–14 years had IgM antibodies which suggests that recent infections with DENV exposure. (Table 4). The prevalence of DENV-3 IgG was the highest (19.8%) and DENV-1 was the lowest (8.3). Regarding IgM, both DENV-2 and DENV-3 were equally the highest (Table 3).
 |
Table 2 Factors associated with the prevalence of anti-DENV IgG seropositivity among the study participants, 2016 |
 |
Table 3 Distribution of dengue infection antibodies by serotypes |
 |
Table 4 Factors associated with the prevalence of anti-DENV IgM seropositivity among the study participants, 2016 |
Factors associated with the seroprevalence of anti-igG DENV antibodies
In bivariate analysis, the association that yielded a P-value <0.25 with DENV IgG was gender and place of residence while age, study area, occupation and educational status of the study participants were not significantly associated. In a multivariable logistic regression analysis, male participants were at higher odds of having DENV IgG infection (AOR 2.68, 95% CI 1.66–4.31) compared to females. Those study participants who were lived in urban areas were 0.37 times (AOR=2.69; 95% 1.55–4.64) more likely to have anti-DENV IgG seropositivity than those who were lived in rural areas (Table 2).
Perceptions and practices of participants with regard to DF
Regarding the general awareness about DF, 38.2% of the participants had heard about this virus infection, and 9.6% responded DENV is transmitted by mosquito but 87.8% of the participants do not know about transmission of the virus. Respondents were asked about the environmental exposures associated with mosquito-borne illnesses in their dwelling areas. Those who reported the existence of stagnant water and trees nearby their dwelling were 31.2% and 64.2%, respectively. Three hundred and thirty-three (63.6%) of the study participants reported that they slept under mosquito nets; of which 20.2% and 41.4% used bed nets always and sometimes, respectively. However, only 4.1% used mosquito repellents at the day or night time (Table 5).
 |
Table 5 Risk factors associated with the prevalence of anti-DENV IgG seropositivity among the study participants, 2016 |
The seropositivity of DENV IgG was 26.3% in those who responded that they had heard about the virus and 32% in those who responded that they were aware that mosquitoes transmit the DENV. The rate of exposure was observed to be 24.3% in those who utilized bed nets. A recent experience of having had a mosquito bite (28%) was the only factor that significantly associated with the rate of DENV IgG seropositivity in bivariate analyses from the knowledge and environmental-related factors. However, use of mosquito repellant, awareness of DF, knowledge of route of transmission and presence of tree around the compound, and the use of bed nets were not significantly associated with history of exposure to DENV (p-value >0.05). The association between a recent mosquito bite and DENV IgG infection was found to be statistically significant in a multivariable logistic regression analysis (AOR=2.23; 95% CI, 1.39–3.56, P=0.001) (Table 5).
Discussion
Recently, DF infection has been considered as an emerging public health problem in several African countries with the accompanying risk of severe infections.21,22 Most febrile cases are routinely diagnosed and treated for typhoid and/or malaria without proper investigation of other conditions, including viral infections. In Ethiopia, where various mosquito-borne diseases are common, little is known about the epidemiology of arboviruses, including DENV. However, the 2013 and 2014 DF outbreaks in Dire Dawa8 and Godey12 that caused a substantial amount of morbidities and mortalities prompts call for more community-based investigations to describe the epidemiology of DF in various localities. Given the impact of continued climate change, which supports the emergence and re-emergence of vector-borne diseases, the need to have a strong surveillance system is critically important. This study assessed the DENV seropositivity and its associated risk factors in health facilities in the Borena Zone where febrile illness is common.
The seroprevalence of DENV IgG among febrile patients in the study area was 22.9%. This result is in agreement with findings reported in Djibouti, 21.8%23 and in the Northern Province of Sudan, 24%.24 However, the observed rate of DENV exposure was lower than results in Dire Dawa, 56.8%,8 northern Ethiopia, 33.3%,3 Eritrea, 33.3%,25 Kassala, Eastern Sudan, 71.7%,26 and El-Gadarif state, Sudan, 47.6%.27 In contrast, the prevalence of anti-DENV IgG seropositivity in this study was higher compared to the rates 7.7% and 12.5% in Tanzania and Kenya, respectively.28,29 These discrepancies may be due to the difference in the distribution of risk factors and the variable climatic conditions by geographical regions, the diversity of the studied populations, and the difference in the diagnostic performance of the employed laboratory methods. For example, some studies analyzed samples using laboratory techniques such as ELISA, Plague Reduction Neutralization Test (PRNT) and PCR which are more sensitive and specific compared to IIFA technique used in the current study. The high circulation of DENV in the study area could be attributed to several factors, including misdiagnosis of febrile cases, the movement of migrants from endemic countries and the proliferation of breeding sites of Aedes mosquitoes. Also one-fourth of the study participants had antibody against DENV infection, dengue was under recognized and under reported in Ethiopia, which is in line with an earlier report in Africa.7 The overall prevalence of anti-DENV IgM seropositivity was 7.9% which indicates recent infection with DENV. Since IgM against the DENV infection can usually be detected after the first 5–7 days of infection.30,31 However, the possibility that as the IgM antibodies remain negative for the first few days, and also the IgM reactivity was non-specific; thus, there is cross-reactive due to infection with another flavivirus.32
This study showed that gender significantly influenced the rate of anti-DENV IgG exposure status where male participants were disproportionately infected, which is in agreement with the study conducted elsewhere.3,33 It might be due to that males are more likely to work in outdoor forested areas where they come into contact with vectors for DENV. In this study, those individuals who were dwelling in urban areas were more affected than those in the rural areas. This is in agreement with the studies conducted elsewhere.3,34,35 It was previously reported that the seropositivity rate for DENV, which is carried by common vectors Ae. aegypti and Ae. albopictus, was higher in the geographically central sites (urban centers) than villages.36 The recent mosquito bite was significantly associated with anti-DENV IgG seropositivity. This is in line with the fact that mosquito bite exposes individuals to DF, and it may be the main mode of transmission in the study area. However, factors such as age, study site, occupation and educational status have little significance in influencing the rate of exposure to DENV in the current study.
Although this is the first study of seroprevalence and risk factors associated with DENV infection in Southern Ethiopia, the study has several limitations. IIFA was shown to have good performance as compared to PRNT; its inherent cross-reactivity to other flaviviruses could not be ruled out. Moreover, no febrile community controls or convalescent sera, and as any health institutional-based study that used consecutive voluntary cases only the risk of introducing bias is unavoidable. Thus, the findings of this study may not be generalized to the population in the study area.
In conclusion, this study showed high prevalence of DENV IgG and IgM. Factors like gender, residence and recent mosquito bites have had association with DF. It also showed low awareness among participants and the potential that DENV could likely to be public health significance in the study area. Thus, we recommended that a community-based survey in the study area and adjacent communities would be conducted to verify our findings and take appropriate public health measures. Further studies should be conducted to determine the environmental, and host factors that determine the extent of exposure to DENV infection in the area for appropriate control and prevention planning.
Abbreviations
AHRI, Armauer Hansen Research Institute; IIFA, indirect immunofluorescent assay; IgG, immunoglobulin G; IgM, immunoglobulin M; PRNT, Plague Reduction Neutralization Test; DF, dengue fever; DENV, dengue virus.
Acknowledgment
I thank the Oromia Regional Health Bureau, Health Bureaus and the responsible officials and professionals in the study area for their cooperation. I am also most grateful to the study participants for volunteering.
Disclosure
The author reports no conflicts of interest in this work.
References
1. Yong YK, Thayan R, Chong HT, Tan CT, Sekaran SD. Rapid detection and serotyping of dengue virus by multiplex RT-PCR and real-time SYBR green RT-PCR. Singapore Med J. 2007;48(7):662.
2. Dhar-Chowdhury P, Paul KK, Haque CE, et al. Dengue seroprevalence, seroconversion and risk factors in Dhaka, Bangladesh. PLoS Negl Trop Dis. 2017;11(3):e0005475. doi:10.1371/journal.pntd.0005475
3. Ferede G, Tiruneh M, Abate E, et al. A serologic study of dengue in northwest Ethiopia: suggesting preventive and control measures. PLoS Negl Trop Dis. 2018;12(5):e0006430. doi:10.1371/journal.pntd.0006430
4. Stramer SL. The potential threat to blood transfusion safety of emerging infectious disease agents. Clin Adv Hematol Oncol. 2015;13:420–422.
5. Weerakkaody RM, Palangasinghe DR, Dalpatadu KP, Rankothkumbura JP, Cassim MR, Karunanayake P. Dengue fever in a liver-transplanted patient: a case report. J Med Case Reports. 2014;8(1):378. doi:10.1186/1752-1947-8-378
6. Kyle J, Harris E. Global spread and persistence of dengue. Annu Rev Microbiol. 2008;62:71–92. PMID: 18429680. doi:10.1146/annurev.micro.62.081307.163005
7. Ananda A, Joel N, Kuritsky G, William L, Harold S, Margolis H. Dengue virus infection in Africa. Emerg Infect Dis. 2011; 17:1349–54. doi:10.3201/eid1708.101515. PMID: 21801609
8. Abyot BW, Mesfin M, Wubayehu K, et al. The first acute febrile illness investigation associated with dengue fever in Ethiopia, 2013: a descriptive analysis. Ethiop J Health Dev. 2014;28:155–161.
9. Guzman MG, Harris E. Dengue. Lancet. 2015;385:453–465. doi:10.1016/S0140-6736(14)60572-9
10. World Health Organization. Global Strategy for Dengue Prevention and Control 2012– 2020. Geneva, Switzerland: World Health Organization; 2012.
11. WHO-TDR. Dengue: Guidelines for Diagnosis, Treatment, Prevention, and Control- New Edition. Geneva, Switzerland: World Health Organization; 2009.
12. Yusuf MA, Ali AS. Epidemiology of dengue fever in Ethiopian Somali region: retrospective health facility-based study. CAJPH. 2016;2:51–56.
13. Martina BE, Koraka P, Osterhaus A. Dengue virus pathogenesis, an integrated view. Clin Microbiol Rev. 2009;22:564–581. doi:10.1128/CMR.00035-09
14. Gupta N, Srivastava S, Jain A, Chaturvedi UC. Dengue in India. Indian J Med Res. 2012;136:373–390. PMID:23041731.
15. Humayoun MA, Waseem T, Jawa AA, Hashmi MS, Akram J. Multiple dengue serotypes and high frequency of dengue hemorrhagic fever at two tertiary care hospitals in Lahore during the 2008 dengue virus outbreak in Punjab, Pakistan. Int J Infect Dis. 2010;14:e54–e59. doi:10.1016/j.ijid.2009.10.008
16. Teixeira MG, Costa MC, Coelho G, Barreto ML. Recent shift in age pattern of dengue hemorrhagic fever, Brazil. Emerg Infect Dis. 2008;14(10):1663. doi:10.3201/eid1410.071164
17. Baba M, Saron MF, Vorndam A, Adeniji J, Diop O, Olaleye D. Dengue virus infections in patients suspected of malaria/typhoid in Nigeria. J Am Sci. 2009;5(5):129–134.
18. Priyamvada L, Quicke KM, Hudson WH, et al. Human antibody responses after dengue virus infection are highly cross-reactive to zika virus. Proc Natl Acad Sci USA. 2016;113:7852–7857. doi:10.1073/pnas.1607931113
19. Dejnirattisai W, Supasa P, Wongwiwat W, et al. Dengue virus sero-cross-reactivity drives antibody dependent enhancement of infection with zika virus. Nat Immunol. 2016;17:1102–1108. doi:10.1038/ni.3515
20. EUROIMMUN AG. Biochips mosaics and profile for detection of flavivirus infections instructions for indirect immunoflourescent test. 2013.
21. Amarasinghe A, Kuritsk JN, Letson GW, Margolis HS. Dengue virus infection in Africa. Emerg Infect Dis. 2011;17(8):1349–1354. doi:10.3201/eid1701.100876
22. Shepard DS, Undurraga EA, Halasa YA. Economic and disease burden of dengue in Southeast Asia. PLoS Negl Trop Dis. 2013;7(2):e2055. doi:10.1371/journal.pntd.0002055
23. Andayi F, Charrel R, Kieffer A, Richet H, Pastorino B, Leparc I. A sero-epidemiological study of arboviral fevers in Djibouti, Horn of Africa. PLoS Negl Trop Dis. 2014;8:e3299. PMID: 2550269. doi:10.1371/journal.pntd.0003299
24. Watts D, EI-Tigani A, Botros B, Salib A, Olmn J, McCarthy M. Arthropod-borne viral infections associated with a fever outbreak in the northern province of Sudan. J Trop Med Hyg. 1994;97:228–230. PMID: 8064945.
25. Abdulmumini U, Jacob D, Diana R, et al. Dengue fever outbreaks in Eritrea, 2005–2015. A case for strengthening surveillance, control, and reporting. Glob Health Res Policy. 2016;1:17. doi:10.1186/s41256-016-0016-5. PMID: 29202065.
26. Tajeldin A, AbdelAziem A, Mubarak K, Ishag A. Epidemiology of dengue infections in Kassala, Eastern Sudan. J Med Virol. 2012;84:500–503. PMID: 22246838. doi:10.1002/jmv.23218
27. Eldigail MH, Adam GK, Babiker RA, et al. Prevalence of dengue fever virus antibodies and associated risk factors among residents of El-Gadarif state, Sudan. BMC Public Health. 2018;18(1):921. doi:10.1186/s12889-018-5853-3
28. Francesco V, Emanuele N, Silvia M, et al. Seroprevalence of dengue infection: a cross-sectional survey in mainland Tanzania and on Pemba Island, Zanzibar. Int J Infect Dis. 2012;16:e44–e6. doi:10.1016/j.ijid.2011.09.018. PMID: 22088862.
29. Caroline O, Petronella A, Aymond N, Stella G, Cyrus W. Seroprevalence of infections with dengue, rift valley fever and chikungunya viruses in Kenya. PLoS One. 2015;10:e0132645. 10. 1371/journal.pone.0132645 PMID: 26177451. doi:10.1371/journal.pone.0132645. .
30. Maria G, Scott B, Harvey A, et al. Dengue: a continuing global threat. Nat Rev Microbiol. 2010;8:S7–S16. doi:10.1038/nrmicro2460. PMID: 21079655.
31. Madara AA, Abdulraheem NO. Relative abundance of adult mosquitoes in University of Abuja Main Campus, Abuja FCT, Nigeria. Nig J Parasitol. 2013;34(2):1–5.
32. Calisher C, Karabatsos N, Dalrymple J, et al. Antigenic relationships between flaviviruses as determined by cross-neutralization tests with polyclonal antisera. J Gen Virol. 1989;70:37–43. doi:10.1099/0022-1317-70-1-37
33. Yik W, Tun Y, Li W, et al. Seroepidemiology of dengue virus infection among adults in Singapore. Ann Acad Med Singapore. 2009;38:667–675. PMID: 19736569.
34. Mohammed S, Syed M, Omrana P, et al. Dengue fever in a border state between Sudan and Republic of South Sudan: epidemiological perspectives. J Public Health Epidemiol. 2013;5:319–24.56.
35. Sadia N, Muhammad A, Muhammad A, Ahmad R, Bahar M. The epidemiology of dengue fever in district Faisalabad, Pakistan. Int J Sci Res. 2015;5(3):1–6.
36. Gubler D. Dengue, urbanization and globalization: the Unholy Trinity of the 21(st) century. Trop Med Health. 2011;39:3–11. doi:10.2149/tmh.2011-S05
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
