Back to Journals » Infection and Drug Resistance » Volume 11
Sera from patients with active pulmonary tuberculosis and their household contacts induce nuclear changes in neutrophils
Authors Juárez-Ortega M, Rojas-Espinosa O , Muñiz-Salazar R , Becerril-Villanueva E , Hernández-Solís A , Arce-Paredes P, Islas-Trujillo S, Cicero-Sabido R
Received 20 April 2018
Accepted for publication 20 June 2018
Published 10 October 2018 Volume 2018:11 Pages 1685—1702
DOI https://doi.org/10.2147/IDR.S171289
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Joachim Wink
Mario Juárez-Ortega,1 Oscar Rojas-Espinosa,1 Raquel Muñiz-Salazar,2 Enrique Becerril-Villanueva,3 Alejandro Hernández-Solís,4 Patricia Arce-Paredes,1 Sergio Islas-Trujillo,1 Raúl Cicero-Sabido4
1Department of Immunology, Escuela Nacional de Ciencias Biológicas, Instituto Politécnico Nacional, Mexico City, Mexico; 2School of Health Sciences, Unidad Ensenada, Universidad Autónoma de Baja California, Ensenada, BC, Mexico; 3Laboratory of Psychoimmunology, Dirección de Investigaciones en Neurociencias, Instituto Nacional de Psiquiatría Ramón de la Fuente, Mexico City, Mexico; 4Pneumology Unit, Hospital General de México “Eduardo Liceaga”, UNAM, Mexico City, Mexico
Background: Resident alveolar macrophages, dendritic cells, and immigrating neutrophils (NEU) are the first cells to contact Mycobacterium tuberculosis in the lung. These cells, and additional lymphoid cells in the developing granuloma, release a series of components that may concentrate in the serum and affect disease progression.
Purpose: The aim of this study was to investigate the effect of the serum from tuberculosis (TB) patients and their household contacts (HHC) on the nuclear morphology of NEU.
Materials and methods: NEU from healthy (HLT) people were incubated with sera from patients with active pulmonary TB, their HHC, and unrelated people. Changes in the nuclear morphology of NEU were analyzed by light and electron microscopy.
Results: Sera from patients with TB induced changes in the nuclear morphology of NEU that included pyknosis, swelling, apoptosis, and netosis in some cases. Sera from some HHC induced similar changes, while sera from HLT people had no significant effects. Bacteria did not appear to participate in this phenomenon because bacteremia is not a recognized feature of nonmiliary TB, and because sera from patients that induced nuclear changes maintained their effect after filtration through 0.22 µm membranes. Neither anti-mycobacterial antibodies, TNFα, IL-6, IFNγ, or IL-8 participated in the phenomenon. In contrast, soluble mycobacterial antigens were likely candidates, as small quantities of soluble M. tuberculosis antigens added to the sera of HLT people led to the induction of nuclear changes in NEU in a dose-dependent manner.
Conclusion: These results might help to detect subclinical TB within HHC, thus leading to a recommendation of prophylactic treatment.
Keywords: tuberculosis, serum, neutrophils, pyknosis, apoptosis, netosis
Introduction
Tuberculosis (TB) is one of the most investigated infectious diseases because it represents one of the deadliest infectious diseases of the last decades.1 TB is caused by a very resilient bacterium (Mycobacterium tuberculosis) that is well adapted to resist both innate and adaptive immunities.2,3 The disease primarily affects the lung and also involves other organs, producing extrapulmonary forms of the disease. It has a high incidence in developing countries but is also not rare in developed nations. The burden of disease is clearly reflected in a 2017 global report from the World Health Organization (WHO) that reported 6.6 million new cases of TB in 2016, with 1.5 million deaths, and an AIDS comorbidity of 0.4 million.1 The immunopathology of TB has been extensively reviewed, and the roles of macrophages, dendritic cells, and T cells in the immunology of the disease are well known.4–7 The role of neutrophils (NEU) in the immunopathology of the disease is less well known, and much of the information to date is controversial.8–10 NEU are recruited early in M. tuberculosis infection, and together with alveolar macrophages and dendritic cells, they ingest mycobacteria.8,11 Microscopic examinations of sputum, bronchoalveolar lavage, and fluid of the pulmonary caverns have revealed that NEU are the major cells infected with M. tuberculosis.12 Although some studies have reported that NEU can kill M. tuberculosis in vitro,13–15 other reports do not support this assertion16–18 and the report by Kisich et al19 in 2002 suggested that the mycobactericidal activity of NEU depends on the donor. The issue of the influence of genetic variability on the mycobactericidal activity of NEU has also been explored in mice. In one study, animals that were susceptible to TB showed higher recruitment of NEU and higher severity of the disease than animals that were resistant to infection.20 NEU influx to the site of infection is determinant in the pathology of the disease, and the depletion of miR-233 (an NEU chemotaxis regulator) in TB-resistant mice leads to progressive disease.21 Importantly, NEU influx depends on cytokines produced locally by macrophages and T cells.22,23 In a BCG (Bacillus of Calmette and Guérin) vaccination model,24 NEU were the first peripheral cells to be recruited to the injection site and they were also able to transport bacilli to regional lymph nodes and other sites, thus contributing to the spread of infection. These observations gave rise to the idea that NEU might function as Trojan horses, facilitating rather than controlling the spread of the infection. This idea was further supported by Zhang et al25 in 2009 who found that the depletion of NEU in the late stages of disease led to decreased colony-forming units (CFU) in the lung, linking neutrophilia to a poor disease prognosis.26,27
Despite these observations, the predominant consensus is that NEU do not play a protective role in TB, although they may facilitate the microbicidal activity of macrophages.28 In addition, apoptotic bodies derived from M. tuberculosis apoptotic NEU are captured by macrophages and dendritic cells and carried to secondary lymphoid organs to initiate the antimycobacterial cell-mediated immune response.29
Here, we describe a previously unknown phenomenon related to NEU in TB: most sera from TB patients and some sera from their household contacts (HHC), but not sera from noncontact healthy (HLT) people, induced nuclear changes compatible with apoptosis (preceded by pyknosis and nuclear swelling), and less frequently with netosis, on NEU from HLT donors.
Materials and methods
Reagents
Unless otherwise specified, chemicals were purchased from Sigma-Aldrich Co. (St Louis, MO, USA).
Biological samples
Serum samples
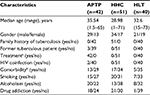
Serum was collected from 42 patients with active pulmonary TB (APTP) and 51 HHC of APTP and from 40 HLT individuals with no known contact with TB patients, all of whom were from Baja California, Mexico, a state with a high incidence of TB. The participants’ demographic characteristics are presented in Table 1.
Written informed consent
All samples were collected by medical and paramedical personnel of the Instituto Mexicano del Seguro Social (IMSS), Ensenada, BC, Mexico, with written informed consent from the donors. A parent of any donor under the age of 18 years also read and signed a written informed consent. Sera were aseptically separated from 3.0 to 5.0 mL of blood, centrifuged (2,200× g/10 minutes/4°C), divided into 50 µL of aliquots, and stored frozen at -70°C until used.
Approval
Documents regarding the written informed consent of the donors and approval of the project (August 21, 2014) by the Committee of Ethics in Research of the School of Health Sciences at Autonomous University of Baja California are available upon request.
Accordance
Methods were carried out in accordance with relevant national guidelines and regulations.
Characteristics of the study population
The average age of the patients was 35.54 (range 1.5–65) years; 29 patients were males and 13 patients were females. The average age of the HHC was 28.98 (range 1–71) years; 34 HHC were males and 17 HHC were females. The age of the noncontact, HLT individuals was 32.6 (range 15–73) years; 21 HLT individuals were males and 19 HLT individuals were females.
All patients were under anti-TB treatment, but the drug combinations, doses, and times of treatment varied between patients.
Bacteria
M. tuberculosis H37Rv TMC 102 was originally acquired from the Trudeau Mycobacterial Culture Collection (Saranac Lake, NY, USA). It was grown on Proskauer-Beck-Youmans (PBY) medium (asparagine, 0.5%; KH2PO4, 0.5%; K2SO4, 0.05%; glycerol, 2.0%; magnesium citrate, 0.15%) and used as heat-inactivated (10 pounds/10 minutes) whole bacteria or as a soluble extract prepared by the disruption of PBS-washed bacteria in a French press (2,500 psi/5 cycles). Before heat killing, bacteria were quantified using the micro-drop method by counting CFU on Middlebrook 7H10-OADC Plates.30 Bacteria in PBY medium were disaggregated by passing the suspension through a 25 G needle several times, while the mycobacterial extract was dialyzed against PBS for 24 hours at 4°C and passed through a 0.45 µm nitrocellulose filter. Both preparations were separated into small aliquots and stored frozen at -20°C until use.
NEU
Blood from HLT people, who also provided written informed consent (available upon request), was collected from volunteers of the Department of Immunology at our institution. Their ages ranged from 26 to 32 years, and the group included 50% males and 50% females. NEU were separated from heparinized blood by centrifuging 5.0 mL of blood on 5.0 mL of Polymorphprep (Axis-Shield PoC AS, Oslo, Norway) at 200× g for 45 minutes at 25°C. The NEU layer was collected after carefully removing the overlying film of mononuclear cells. NEU were suspended in 12 mL of phosphate buffered saline plus glucose (PBSG) (0.15 M NaCl, 0.01 M Na+K+ phosphate, 0.1% glucose, pH 7.4), washed once by centrifugation (200× g/5 minutes/4°C), and resuspended in 2.0 mL of PBSG for counting; counts were adjusted to 1×106 cells/mL.
Short-term culture of NEU
Cell cultures were prepared on washed and degreased glass slides covered with an adherent vinyl mask casted with three 0.8 mm Ø circular areas on which NEU were deposited for cultivation. Masked glass slides were placed into Petri dishes, and the entire system was sterilized at 10 pounds for 10 minutes.
NEU monolayers were produced by depositing 40 µL of the cell suspension (4×104 cells) on each circular area of the glass slide, and the culture system was incubated for 30 minutes at 37°C and 4% CO2 under a humidified atmosphere. The supernatant was removed and replaced with 40 µL of PBSG, phorbol myristate acetate (PMA; 10 ng/mL), or the test (HLT or TB) sera. Next, the cell monolayers were incubated for 3 or 6 hours at 37°C/5% CO2 in a humidified atmosphere. Finally, the slides were recovered, the supernatant was carefully removed, and 40 µL of 1% paraformaldehyde was added per well. After 10 minutes of fixation, the slides were recovered, washed by dipping three times in distilled water, air dried, and stained for DNA with fluorescent Hoechst stain (1:1,000) for 2 minutes. The vinyl masks were then removed, and the cell preparation was protected with Vecta Shield (Vector Laboratories Inc, Burlingame, CA, USA) for microscopic examination. Changes in the nuclear morphology were semiquantified using the ImageJ software 1.47 v (Wayne Rasband; NIH, Bethesda, MD, USA) on 40× images taken with a Nikon Eclipse 8000 microscope and edited with Corel PaintShop Pro X7 (adjust/effects/edge/trace contour).
Cytokine determination
Quantification of IL-6, IL-8, tumor necrosis factor alpha (TNFα), and interferon gamma (IFNγ) in serum was assessed using sandwich ELISA kits (BioLegend Inc., San Diego, CA, USA) following the manufacturer’s instructions. The cytokine concentrations were calculated using the curve-fitting software (GraphPad Software, Inc., La Jolla, CA, USA) and the recombinant standards included in the kits.
Electron microscopy
Scanning electron microscopy (SEM)
A total of 40,000 glass-adhered cells were incubated (37°C/5% CO2) for 3 or 6 hours in the presence of 40 µL of PBSG (negative control), 40 µL of PMA (0.1 µg/mL of PBSG) (positive control), or 40 µL of normal or TB sera (test samples). At the end of the incubation, the supernatant was removed and the cells were fixed with 40 µL of 2.5% glutaraldehyde in PBS (16520; Electron Microscopy Sciences, Hatfield, PA, USA) for 1 hour. The cell monolayers were rinsed three times (5 minutes each) with Sorensen’s solution (0.01 M Na2HPO4×2H2O/NaH2PO4×H2O, pH 7.4) and fixed with 1% OsO4 (19112; Electron Microscopy Sciences) for 60 minutes. After three more washes (5 minutes each) with Sorensen’s solution, the slides were air-dried and dehydrated in 30%, 40%, 50%, 60%, 70%, 80%, and 90% and three times in 100% ethanol for 10 minutes each time. Finally, the monolayers were placed into a critical point dryer (K-850; Electron Microscopy Sciences) and spread with ionized gold (20 mA) for 60 seconds in a Desk-II machine (Denton Vacuum LLC, Moorestown, NJ, USA). The analysis was performed using the JEM5800LUV SEM (JEOL, Tokyo, Japan).
Transmission electron microscopy (TEM)
The TEM process was the same as the process for SEM until the ethanol dehydration step, except that cell suspensions were used instead of cell monolayers. Ethanol-treated cell suspensions were separated by centrifugation (200× g/5 minutes) and suspended twice in propylene oxide (20 minutes each) and then in resin (Electron Microscopy Science) propylene oxide at ratios of 1:2, 1:1, 3:1, and two changes of pure resin for 4 hours each before polymerization at 60°C and sectioning. The complete procedure was as described by Graham and Ornstein.31
Apoptosis identification by measurement of exposed phosphatidyl serine
Exposed phosphatidyl serine was measured using an Annexin V FITC Assay kit (Cayman, Ann Arbor, MI, USA) following the manufacturer’s indications. Briefly, 2.5 million NEU/mL were placed into FACS tubes (FALCON; Becton Dickinson, Franklin Lakes, NJ, USA) in the presence of 40 µL of APTP, HHC, or HLT sera or 10 µL of zymosan (50 µg/mL) for 8 hours. A total of 200 µL of binding buffer was then added, and the tubes were centrifuged to remove the supernatants. The cell pellets were resuspended in 50 µL of staining solution (FITC-Annexin V-propidium iodide) for 10 minutes. Next, the cells were analyzed using a Facscalibur flow cytometer (Becton Dickinson) and the data were processed with the FlowJo software for early apoptosis and late apoptosis.
NEU extracellular traps’ (NETs) identification by measurement of released DNA
The procedure for NET characterization was as follows (with PBS washes between steps): 1) fixation of NEU monolayers with 1% paraformaldehyde for 10 minutes; 2) permeabilization with 0.1% Triton X-100 in PBS for 10 minutes; 3) blocking with 1% bovine serum albumin in PBS; 4) incubation for 24 hours at 4°C with polyclonal rabbit antihuman myeloperoxidase (MPO) (Ab9535; Abcam, Cambridge, MA, USA) and goat antihuman histone (HIS) (Ab11946) diluted 1:200 in 1% BSA in PBS; 5) incubation for 2 hours with antibodies to rabbit IgG (Alexa Fluor 488 A21206; Molecular Probes, Eugene, OR, USA) and goat IgG (Alexa Fluor 647 ab1501319), both diluted 1:500 in 1% BSA in PBS; 6) staining with Hoechst reagent 1:1,000 in PBS for 5 minutes; 7) mounting with Vectashield mounting medium (Vector); and 8) inspection on a confocal microscope (LSM5 Pascal; Carl Zeiss Meditec AG, Jena, Germany).
For the semiquantitative assessment of released DNA, 2×105 NEU in complete Iscove’s modified Dulbecco’s medium (IMDM) were placed into wells of a 96-well plate and left to adhere for 30 minutes at 37°C and 5% CO2. The IMDM was carefully removed and replaced with 100 µL of APTP, HHC, or HLT sera; 10 µM of PMA; or plain IMDM as a control. The plate was incubated for 3 hours at 37°C and 5% CO2, and 10 µL of 2 µM Sytox Green (Thermo Fisher Scientific, Waltham, MA, USA) was then added per well. After 10 minutes at 25°C, the plate was centrifuged for 5 minutes at 400× g. Next, after the careful removal of supernatants, 150 µL (0.2 IU in PBS) of micrococcal nuclease (Sigma-Aldrich Co.) was added per well and the plate was incubated for 10 minutes at 37°C. The plate was then read immediately on a fluorometer (Fluoroskan Ascent FL; Thermo Fisher Scientific) at 485/580 nm (excitation/emission), and the blank-corrected readings were registered as relative fluorescence units (RFU) as reported by Hazeldine et al32 in 2014.
Antimycobacterial antibodies
Serum antibodies to mycobacterial antigens were measured by an indirect ELISA test in 96-well array plates (Nunc; Polysorp, Roskilde, Denmark). The assay consisted in 1) coating the ELISA-plate wells with 2 µg of mycobacterial protein in 0.1 mL of 0.01 M carbonate buffer, pH 8.6, for 24 hours; 2) washing three times with 0.2 mL of 0.01 M phosphate buffer (pH 7.4); 3) blocking with 0.2 mL of 3% skimmed milk in phosphate buffer for 60 minutes; 4) adding the test sera diluted 1:100 in PBS and incubating for 120 minutes at 37°C; 5) washing three times with phosphate buffer; 6) adding a peroxidase-labeled goat antihuman immunoglobulin serum (074-1007; KLP Inc., Baltimore, MD, USA) diluted 1:5,000 in PBS and incubating for 60 minutes at room temperature (37°C); 7) washing six times with PBS, adding 0.1 mL of chromogenic substrate (3 mg of ortho-phenylenediamine and 10 µL of 30% hydrogen peroxide dissolved in phosphate buffer), and incubating at 25–26°C for 20 minutes; and 8) stopping the enzymatic reaction with 20 µL/well of 8 N sulfuric acid and reading the absorbance at 492 nm using an ELISA reader (LabSystems Multiskan Plus, Vantaa, Finland).
In vitro infection of NEU with M. tuberculosis
Three independent experiments were performed to show that M. tuberculosis infects NEU and induces nuclear changes in these cells. Monolayers prepared with 4×104 NEU were incubated for 3 hours with M. tuberculosis at multiplicities of infection (MOI) 1, 10, and 100. The monolayers were then recovered, fixed with 2% paraformaldehyde for 10 minutes, washed three times with PBS, and stained with Hoechst reagent for 2 minutes. For these experiments, bacteria were stained for 2 minutes with iris fuchsia (1 µg/mL), a red lipophilic fluorescent (588 nm) dye (Cyane Technologies, Turin, Italy).
In vitro treatment of NEU with soluble antigens extracted from M. tuberculosis
Three independent experiments were performed to assess the capacity of mycobacterial antigens to induce nuclear changes in NEU. Monolayers prepared with 4×104 NEU were incubated for 3 hours in the presence of 40 µL of sera from HLT donors supplemented with 1 µg/mL, 10 µg/mL, 100 µg/mL, or 1 mg/mL of soluble proteins extracted from M. tuberculosis grown on synthetic PBY medium.33 Control cultures included nonstimulated NEU and NEU stimulated with 100 ng of PMA.
Soluble antigens from M. tuberculosis were obtained by disrupting a heavy bacterial suspension in PBS (containing 1 mM n-ethyl maleimide and 1 mM phenylmethanesulfonyl fluoride as protease inhibitors) with a French pressure cell press (FA-078 SLM-AMINCO) at 2,500 psi. Five pressure cycles were applied. In the end, the extract was collected, dialyzed for 24 hours at 4°C against PBS, centrifuged (10,000× g/10 min/4°C), and membrane (0.22 µm) sterilized for protein determination (Lowry method). Protein concentration was adjusted to 1.0 mg protein/mL using bovine serum albumin (1 mg/mL) as a reference standard.
Statistical analysis
The results were analyzed with the GraphPad Prism software Version 6 using the one-way ANOVA with Tukey’s post hoc test for data with normal distributions and the Kruskal–Wallis test for data with non-normal distributions. A P-value of ≤0.05 was considered the limit of statistical significance. Normality and correlations were determined by the Shapiro–Wilk and Pearson tests.
Data availability
Original and additional data are available upon request.
Results
We suspected phagocytic defects in two patients with APTP, so tested the response of these patients’ NEU (TB-NEU) to the stimuli of PMA and complement-opsonized yeast in the presence of nitro blue tetrazolium (NBT). We found that the leukocytes of these patients responded strongly to both stimuli by reducing NBT (meaning reactive oxygen species [ROS] production), phagocytosing yeast, and releasing nuclear material (chromatin) within 30 minutes of incubation. These changes were suggestive of netosis, which was corroborated when “netotic” NEU stained positively for DNA, elastase, MPO, and HIS.
To discern whether this strong response of TB-NEU to PMA and yeast was due to some factor present in the serum of APTP, we incubated NEU from three HLT people (HLT-NEU) with the APTP sera for 3 hours, a time usually used to induce NETs in vitro. The result was notable as the APTP sera induced marked changes in the nuclear morphology of HLT-NEU characterized by massive release of DNA as stained with the Hoechst reagent. We therefore hypothesized that sera from tuberculous patients might be able to induce similar changes in HLT NEU. Thus, collections of sera from APTP, from some of their HHC, and from unrelated HLT people were tested for their abilities to induce changes in the nuclear morphology of NEU. The findings are discussed below.
Sera from HLT people do not induce changes in the nuclear morphology of NEU after 3 hours of incubation
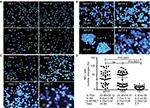
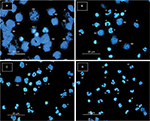
When sera from HLT people were added to HLT-NEU monolayers, no significant changes in the nuclear morphology of NEU were observed (changes were not observed in >80% of NEU monolayers treated with HLT sera; when observed, they were attributed to the normally short half-life of NEU in vitro). Figure 1A depicts the results of four of the 40 sera from noncontact HLT people; similar results were observed with the remaining HLT sera tested. A semiquantitative assessment of changes made by direct observation of the NEU monolayer is shown in Figure 1D.
Sera from patients with APTP induce a variety of changes in the nuclear morphology of NEU after 3 hours of incubation
The images in Figure 1B illustrate the changes induced by APTP sera on HLT-NEU and depict the effect of four of the 42 APTP sera; the remaining sera induced similar changes in the nuclear morphology of HLT-NEU. As shown in the image, these sera induced a variety of changes, including various degrees of pyknosis, nuclear swelling, cell aggregation, apoptosis, and netosis. The average percentage of changes induced by APTP sera was 44.59±31.1 (SD), compared to only 5.7±4.7% in the HLT group. A semiquantitative assessment of the changes as determined by direct counts of the NEU monolayers is shown in Figure 1D.
Several sera from household APTP contacts induce nuclear changes in HLT-NEU similar to those induced by sera from APTP after 3 hours of incubation
A high proportion of sera (34.85%) from the HHC group induced nuclear changes in NEU. Figure 1C illustrates the effect of 4 of the 51 sera from household APTP contacts (HHC) on the nuclear morphology of HLT NEU. Changes included nil, swelling, aggregation, apoptosis, and netosis. The results of a semiquantitative assessment of the changes as determined by direct counts on the NEU monolayers are shown in Figure 1D.
Sera from APTP, HHC, and HLT people induce different extents of nuclear changes in NEU
Although all sera induced changes in the nuclear morphology of NEU, these changes differed in their frequency depending on the group. The frequencies of changes induced by APTP sera were 29.88±24.12% for pyknosis, 8.42±13.88% for nuclear swelling, and 6.29±13.67% for apoptosis/netosis (mean changes, 44.59%). The changes induced by HHC sera were pyknosis (20.36±16.31%), nuclear swelling (7.75±11.14%), and apoptosis/netosis (6.74±13.08%, mean changes, 34.85%). The changes induced by HLT sera were pyknosis (5.30±4.06%), nuclear swelling (0.31±0.93%), and apoptosis/netosis (0.09±0.036%, mean changes, 5.7%). These results are illustrated in Figure 1D. Each serum was tested three times in independent experiments.
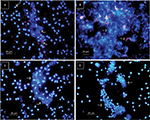
Sera from HHC and APTP increase the nuclear size of NEU
To more precisely assess the changes induced by HLT, APTP, and HHC sera, photographic images taken at 40× were edited with Corel PaintShop ProX7 (adjust/effects/edge/trace contour) and the images were processed with the ImageJ software. The results are given in micrometers. The average areas were 24.67±0.62 µm2 standard error (SE) in the HLT group, 53.70±10.56 µm2 in the APTP group, and 42.29±8.37 µm2 in the HHC group. A highly significant difference with the HLT group was observed in both cases (P=0.031 and 0.044, respectively). A representative result of the effect of sera from the three groups tested (Figure 2A–C) and the global result with all sera tested (Figure 2D) are summarized in the upper panel of Figure 2. It is clear the increment in the nuclear size of NEU induced by the sera from APTP and HHC.
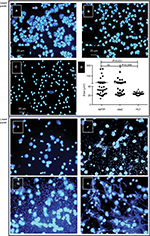
Sera from patients with APTP and some of their HHC induce extreme changes in the nuclear morphology of NEU after 6 hours of incubation
Sera that induced significant nuclear changes after 3 hours of incubation showed extreme nuclear changes after 6 hours of incubation. All APTP sera and some of their HHC had some effect on the nuclear morphology of NEU, although the effects were highly variable as shown in the lower panel of Figure 2. The most frequent changes were pyknosis (p) (15.93±23.79%), nuclear swelling (s) (25.22±24.52%), apoptosis (ap), and netosis (n) (12.88±29.06%) in the HHC group (Figure 2E and F) and pyknosis (6.75±14.66%), nuclear swelling (25.53±17.49%), and apoptosis/netosis (19.06±29.79%) in the APTP group (Figure 2G and H). Apoptosis bodies (ab) were also frequent in HHC and APTP groups at this incubation time.
Some sera from APTP and HHC induce apoptosis in NEU from HLT individuals
Apoptotic changes were observed after 3 hours of incubation, but the phenomenon was stronger at 6 hours of incubation, with also the observation of membrane ruffling, cell disintegration, and the emergence of apoptotic bodies. This was corroborated by SEM and annexin-V staining after 12 hours of incubation. At this time, the percentage of annexin-V-positive (apoptotic) cells was 43.72±11.66 (SD) for the HLT group, 52.04±10 for the HHC group, and 53.25±10.61 for the APTP group, with the statistical significances of P=0.0056 (HHC) and P=0.013 (APTP) with respect to the HLT group. The study was performed after 12 hours of incubation to clarify the separation between apoptotic and nonapoptotic cells. However, at this time, a significant proportion of NEU incubated with HLT sera exhibited late apoptosis likely because of their naturally short life span. Figure 3 depicts the nuclear morphology of NEU incubated with HHC (Figure 3A), APTP (Figure 3B), and HLT (Figure 3C) sera. The global percentage of annexin-V-positive (apoptotic) cells in each group is illustrated in Figure 3D.
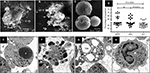
Pyknosis, apoptosis, nuclear fragmentation, and vacuolation are observable by TEM
Nuclear NEU alterations observed by fluorescence and SEM were also observed by TEM. Figure 3E–H illustrates the most frequent changes induced by HLT, HHC, and APTP sera. These changes included nuclear condensation in pyknotic cells (p; Figure 3E), membrane ruffling and nuclear fragmentation with apoptotic bodies in apoptotic cells (ap; Figure 3F), and chromatin extrusion and lysosomes’ degranulation leaving empty spaces or vacuoles in postnetotic cells (vac, Figure 3G). For comparison, the normal cell morphology of NEU treated with HLT serum is illustrated in Figure 3H (nl).
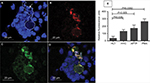
DNA release
Some sera from HHC and APTP induced the release of DNA compatible with NETs. NETs were identified directly on NEU monolayers treated with the sera and characterized by Hoechst staining (DNA) and antibodies against NEU elastase (NEL), MPO, and HIS. Figure 4 shows an NEU aggregate where released DNA (the outlined area in Figure 4A) was also stained for HIS (red in Figure 4B) and MPO (green in Figure 4C). A merged image is shown in Figure 4D. In addition, the release of DNA was quantified in NEU monolayers incubated in the presence of all HLT, APTP, and HHC sera.32 The average amount of DNA released was 130.3±14.52 RFU in the HHC group and 171.3±25.44 RFU in the APTP group, with highly significant differences (P=0.036 and 0.005, respectively) from that in the HLT group (35.33±15.41 RFU). These results are shown in Figure 4E.
Nature of the serum factor(s) responsible for NEU alterations
Several candidates were analyzed: M. tuberculosis in circulating blood, soluble mycobacterial antigens, antimycobacterial antibodies, and cytokines.
Circulating M. tuberculosis as possible inducers of nuclear changes in NEU
This possibility was rejected because although M. tuberculosis can induce NEU alterations in vitro, bacteremia at the level of septicemia would be necessary to achieve the drastic changes observed and this had not occurred in the blood of TB patients or their HHC. Figure 5A and B shows the results when an NEU monolayer (4×104 cells) is treated with M. tuberculosis (stained with iris fuchsia) at an MOI of 10:1 (4×105 bacteria) and 100:1 (4×106 bacteria). While the MOI of 10:1 induced some changes (netosis) in the nuclear morphology of NEU, changes such as those induced by APTP sera were evident only at the MOI of 100:1. The improbability of this number of bacteria in APTP sera and more so in HHC sera is discussed later. In addition, sera from APTP retained their capacity to induce nuclear changes in NEU after they were centrifuged (8,000× g/20 minutes) and passed through 0.22 µm sterilizing filters (Figure 5C and D). This definitively eliminated the possibility of bacteria in serum as the cause of nuclear changes in NEU.
Soluble mycobacterial antigens (SMA) induce changes in NEU
The possibility that SMA might be the cause of nuclear changes in NEU was confirmed when concentrations of SMA ranging from 1 µg/mL to 1 mg/mL was used to induce proportional nuclear changes in NEU (Figure 6A–D). Although 1 µg/mL (40 ng in the test) of SMA did not produce obvious changes (Figure 6D), changes were evident with SMA at concentrations 10 µg/mL (400 ng in the test) (Figure 6C), 100 µg/mL (4 µg in the test) (Figure 6B), and 1 mg/mL (40 µg in the test) (Figure 6A). Except for this latter concentration, concentrations ranging from 4 ng/mL to 4 µg/mL of antigen may exist in APTP and HHC sera. Soluble circulating mycobacterial antigens have been detected in the blood of TB patients,34,35 but no reference data on the amount of circulating antigens are available as this depends on each subject.
Antimycobacterial antibodies
Most TB sera contain high levels of free antimycobacterial antibodies.35 In our study, sera from APTP contained the highest amounts of anti-M. tuberculosis antibodies (0.405±0.141 OD), but sera from HLT individuals (0.213±0.072 OD) and HHC (0.246±0.089 OD) also had significant levels of antibodies. This finding was expected for a region with endemic TB. Nevertheless, no correlation was observed between the antibody levels in APTP (r=-0.110, P=0.55) or HHC (r=-0.067, P=0.72) sera and their capacity to induce nuclear changes in NEU (Figure S1).
Cytokines
TNFα and IL-6
TNFα and IL-6 are cytokines that have proven effects on NEU.36,37 Compared to TNFα levels in the sera from HLT individuals (14.76±4.82 pg/mL), sera from APTP contained low but detectable levels of TNFα (21.31±21.9 pg/mL, P=0.026; the data in parenthesis are the mean values ± SD), but no correlation was found between the levels of TNFα and the proportion of nuclear changes observed (r=-0.03, P=0.86). Additionally, compared to the IL-6 levels in HLT sera (71.08±27.91 pg/mL), sera from APTP contained slightly elevated levels of IL-6 (94.92±36.48 pg/mL, P=0.04); again, no correlation between the IL-6 levels in APTP sera and their capacity to induce nuclear changes in NEU was observed (r=-0.111, P=0.57). Similar results were obtained with HHC sera. These findings are illustrated in Figure S2.
IFNγ and IL-8
IFNγ and IL-8 are also cytokines that have direct effects on NEU. Compared to the levels of IL-8 in sera from HLT people (10.70±5.95 pg/mL), sera from APTP contained lower levels of IL-8 (6.16±3.91 pg/mL, P=0.033) and their levels were not correlated with the capacity of APTP sera to induce nuclear changes in NEU (r=-0.131, P=0.48). Additionally, compared to the levels of IFNγ in sera from HLT people (71.85±71.45 pg/mL), sera from APTP contained detectable levels of IFNγ (54.81±65.01 pg/mL, P=0.61), but these levels did not correlate with the capacity of sera to induce nuclear changes in NEU (r=-0.080, P=0.68). Similar results were observed with the levels of IFNγ and IL-8 in the HHC group. These findings are illustrated in Figure S3.
Discussion
The participation of lymphocytes, macrophages, and dendritic cells in the immunopathology of TB is well known,4,6,7 but the participation of NEU is controversial, with some studies reporting beneficial effects and others reporting no role16–18 or negative effects.38–41 The effect of the disease on the physiology of NEU is, however, a neglected subject. In this article, we report that sera from patients with APTP induce a series of changes in the nuclear (and hence cellular) morphology of NEU from HLT donors. The dominant global changes were apoptosis (preceded by pyknosis and karyorrhexis)42 and netosis (with extrusion of DNA intermingled with elastase, MPO and HIS)43 in some cases.
All sera from APTP induced some changes, and changes were also frequent in a significant number of sera from APTP HHC. Sera from noncontact HLT individuals produced no changes or minor changes (mainly pyknosis or nuclear swelling but not apoptosis or netosis). These changes were attributed to the normal short life span of NEU in vitro. Recently, in 2017, Schechter et al40 reported a high frequency of NETs (as DNA–MPO complexes) in the sera of patients with active TB whose frequency diminished following treatment and other studies reported the ability of sera from patients with autoimmune disorders to induce netosis, but not apoptosis.44,45 In our study, pyknosis and swelling as a prelude to apoptosis and netosis were induced by sera from APTP and several of their HHC.
From these observations, we concluded that APTP and several HHC sera contained factors responsible for the observed nuclear alterations. We first considered circulating mycobacteria, but this possibility was discarded because 1) large numbers of bacteria would be needed to reach the same level of alterations seen with the APTP sera and 2) marked alterations were also induced by sera from some HHC who showed no clinical sign of disease. In vitro experiments with M. tuberculosis at an MOI of 10:1 induced significant changes in NEU (4×104 cells), and these were more evident at an MOI of 100:1. For the MOI of 10:1, the number of bacteria contained in 10 µL used for infection was 4×105 in the test, which means that 4×107 (40 million) bacteria existed per milliliter of serum, an amount unimaginable for a TB patient (without septicemia) and impossible for an HHC. The induction of nuclear changes with bacteria at an MOI of 100:1, although possible in vitro, is inconceivable in vivo as 4×108 (400 million) bacteria per milliliter of serum would be necessary to attain the observed changes in vitro. Therefore, bacteria in APTP or HHC sera were not responsible for the changes observed in this study.
In contrast, soluble mycobacterial antigens in serum (both the secreted antigens as well as the ones released during in vivo destruction of the bacteria by macrophages, dendritic cells and NEU in the incipient active granuloma) seemed to be the main, although perhaps not only, cause of the nuclear changes observed. Concentrations as low as 10 µg protein/mL (400 ng in the test) enabled the induction of NEU changes in sera from HLT people, and this was more apparent with the higher amounts used (4 and 40 µg in the test). Although in this report, no purified or recombinant antigens from M. tuberculosis were tested, an obvious next step will be the identification of the antigens involved, probably the most immunogenic or abundant ESAT-6, CFP-10, MPT-64, Ag85A, and Ag85B and also the antigens expressed during preclinical TB PirG (Rv3810), polymorphic GC-repetitive sequence (PE-PGRS, Rv 3367), and proline–threonine repetitive protein (PTRP, Rv0538).46 Among these, ESAT-6 seems to be the most relevant candidate as this protein provokes necrosis and NET production in NEU stimulated in vitro with the protein.18 Analysis of ESAT-6 will be soon initiated in our laboratory.
Other candidates initially proposed were circulating antimycobacterial antibodies and cytokines with documented effects on NEU. Regarding antibodies, significant levels of circulating antimycobacterial antibodies were found in APTP sera, but HHC and noncontacts also had detectable levels of antimycobacterial antibodies. While this was an expected finding in a region with endemic TB,47 the presence of antimycobacterial antibodies was not related to the nuclear changes observed in NEU.
Concerning cytokines, IL-6, TNFα, and IFNγ are recognized to have effects on NEU. Apart from its chemotactic activity, IL-6 might participate in the NEU-monocyte shift during inflammation, favoring the resolution of the neutrophilic infiltrate and the initiation of the adaptive immune response.37 IFNγ modulates many aspects of NEU function; NEU respond to IFNγ by increasing their phagocytic capacity and the release of reactive oxygen species, proinflammatory cytokines (TNFα and IL-6), and granular enzymes.48 TNFα also has many effects on NEU, but a particular effect is its capacity to stimulate apoptosis in these cells.36,49 Several studies on cytokines in TB have shown that most proinflammatory cytokines are elevated in untreated patients and tended to diminish after treatment without following a precise pattern.50–52 In our study, compared to HLT donors, APTP sera contained marginally elevated levels of TNFα and IL-6, but these levels did not correlate with the ability of sera to induce nuclear changes in NEU. Furthermore, the levels of IFNγ and IL-8 did not significantly differ from those found in HLT sera; again, no correlation was observed between the levels of these cytokines and the capacity of sera to induce changes in NEU. Patients were undergoing variable treatments and therefore had a variable disease status, something which might have influenced our results.
Apart from mycobacterial antigens, other mediators identified as participants in the pathology of TB such as exosomes and damage-associated molecular patterns (DAMPs) might also be involved in the phenomenon. Exosomes released by macrophages infected with M. tuberculosis contain mycobacterial antigens that might influence the NEU’s physiology.53 Also, DAMPs released from disrupted cells by effect of the infection could directly interact with NEU modulating their recruitment to the sites of infection and subsequent activation.54 The role of DAMPs and exosomes deserves further investigation as possible inducers of the nuclear changes in NEU described in this article.
Conclusion
Two subpopulations within the HHC group could be segregated, one comparable to the HLT group and the other similar to the APTP group (likely subclinical TB). Looking for NEU nuclear changes induced by sera from TB HHC may help to identify individuals with subclinical disease at the risk of developing active disease, thus leading to the recommendation of prophylactic treatment. However, to validate and attain clinical applications of the test, a wider standardized study should be performed. This will take time and involve health authorities in charge of TB control programs. A capture (sandwich) ELISA with monoclonal antibodies to a major antigen of M. tuberculosis will be developed to complement the findings with NEU.
Acknowledgments
This study was supported by Secretaría de Investigación y Posgrado (SIP) del Instituto Politécnico Nacional (IPN), Mexico, Comisión de Operación y Fomento de las actividades académicas (COFAA) del IPN, Programa de Estímulos al Desempeño en Investigación (EDI) del IPN, Universidad Autónoma de Baja California (Mex), Sistema Nacional de Investigadores (SNI) (Mex), and Consejo Nacional de Ciencia y Tecnología (CONACyT) (Mex). The authors thank Dr Enrique Espinosa from the Instituto Nacional de Enfermedades Respiratorias (INER) for providing the IL-8 ELISA kit used in this investigation, Dr Edgar O López Villegas for his assistance in the electron microscopy studies, and Claudia Pérez Dionisio for her technical help. The authors specially thank Dr Simon Kolstoe (senior lecturer and university ethics advisor), University of Portsmouth, UK, for checking the final version of the manuscript.
Author contributions
ORE directed the investigation and wrote the article, MJO, EBV, and SIT carried out the experiments, PAP statistically analyzed the data, and RMZ and AHS handled the patients and provided the serum samples. All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work
References
World Health Organization. Global Tuberculosis Report 2017. Switzerland: WHO; 2017. | ||
Gupta A, Kaul A, Tsolaki AG, Kishore U, Bhakta S. Mycobacterium tuberculosis: immune evasion, latency and reactivation. Immunobiology. 2012;217(3):363–374. | ||
Korb VC, Chuturgoon AA, Moodley D. Mycobacterium tuberculosis: Manipulator of Protective Immunity. Int J Mol Sci. 2016;17(3):131. | ||
O’Garra A, Redford PS, Mcnab FW, Bloom CI, Wilkinson RJ, Berry MP. The immune response in tuberculosis. Annu Rev Immunol. 2013;31(1):475–527. | ||
Schwander S, Dheda K. Human lung immunity against Mycobacterium tuberculosis: insights into pathogenesis and protection. Am J Respir Crit Care Med. 2011;183(6):696–707. | ||
Tascon RE, Soares CS, Ragno S, Stavropoulos E, Hirst EM, Colston MJ. Mycobacterium tuberculosis-activated dendritic cells induce protective immunity in mice. Immunology. 2000;99(3):473–480. | ||
Torrado E, Robinson RT, Cooper AM. Cellular response to mycobacteria: balancing protection and pathology. Trends Immunol. 2011;32(2):66–72. | ||
Dallenga T, Schaible UE. Neutrophils in tuberculosis--first line of defence or booster of disease and targets for host-directed therapy? Pathog Dis. 2016;74(3):ftw012. | ||
Kumar V, Sharma A. Neutrophils: Cinderella of innate immune system. Int Immunopharmacol. 2010;10(11):1325–1334. | ||
Martineau AR, Newton SM, Wilkinson KA, et al. Neutrophil-mediated innate immune resistance to mycobacteria. J Clin Invest. 2007;117(7):1988–1994. | ||
Filio-Rodríguez G, Estrada-García I, Arce-Paredes P, et al. In vivo induction of neutrophil extracellular traps by Mycobacterium tuberculosis in a guinea pig model. Innate Immun. 2017;23(7):625–637. | ||
Eum SY, Kong JH, Hong MS, et al. Neutrophils are the predominant infected phagocytic cells in the airways of patients with active pulmonary TB. Chest. 2010;137(1):122–128. | ||
Brown AE, Holzer TJ, Andersen BR. Capacity of human neutrophils to kill Mycobacterium tuberculosis. J Infect Dis. 1987;156(6):985–989. | ||
Jones GS, Amirault HJ, Andersen BR. Killing of Mycobacterium tuberculosis by neutrophils: a nonoxidative process. J Infect Dis. 1990;162(3):700–704. | ||
Pedrosa J, Saunders BM, Appelberg R, Orme IM, Silva MT, Cooper AM. Neutrophils play a protective nonphagocytic role in systemic Mycobacterium tuberculosis infection of mice. Infect Immun. 2000;68(2):577–583. | ||
Aston C, Rom WN, Talbot AT, Reibman J. Early inhibition of mycobacterial growth by human alveolar macrophages is not due to nitric oxide. Am J Respir Crit Care Med. 1998;157(6 Pt 1):1943–1950. | ||
Denis M. Human neutrophils, activated with cytokines or not, do not kill virulent Mycobacterium tuberculosis. J Infect Dis. 1991;163(4):919–920. | ||
Francis RJ, Butler RE, Stewart GR. Mycobacterium tuberculosis ESAT-6 is a leukocidin causing Ca2+ influx, necrosis and neutrophil extracellular trap formation. Cell Death Dis. 2014;5(10):e1474. | ||
Kisich KO, Higgins M, Diamond G, Heifets L. Tumor necrosis factor alpha stimulates killing of Mycobacterium tuberculosis by human neutrophils. Infect Immun. 2002;70(8):4591–4599. | ||
Eruslanov EB, Lyadova IV, Kondratieva TK, et al. Neutrophil responses to Mycobacterium tuberculosis infection in genetically susceptible and resistant mice. Infect Immun. 2005;73(3):1744–1753. | ||
Dorhoi A, Iannaccone M, Farinacci M, et al. MicroRNA-223 controls susceptibility to tuberculosis by regulating lung neutrophil recruitment. J Clin Invest. 2013;123(11):4836–4848. | ||
Appelberg R, Interferon-Gamma AR. Interferon-gamma (IFN-gamma) and macrophage inflammatory proteins (MIP)-1 and -2 are involved in the regulation of the T cell-dependent chronic peritoneal neutrophilia of mice infected with mycobacteria. Clin Exp Immunol. 1992;89(2):269–273. | ||
Appelberg R. T cell regulation of the chronic peritoneal neutrophilia during mycobacterial infections. Clin Exp Immunol. 1992;89(1):120–125. | ||
Abadie V, Badell E, Douillard P, et al. Neutrophils rapidly migrate via lymphatics after Mycobacterium bovis BCG intradermal vaccination and shuttle live bacilli to the draining lymph nodes. Blood. 2005;106(5):1843–1850. | ||
Zhang X, Majlessi L, Deriaud E, Leclerc C, Lo-Man R. Coactivation of Syk kinase and MyD88 adaptor protein pathways by bacteria promotes regulatory properties of neutrophils. Immunity. 2009;31(5):761–771. | ||
Barnes PF, Leedom JM, Chan LS, et al. Predictors of short-term prognosis in patients with pulmonary tuberculosis. J Infect Dis. 1988;158(2):366–371. | ||
Lowe DM, Bandara AK, Packe GE, et al. Neutrophilia independently predicts death in tuberculosis. Eur Respir J. 2013;42(6):1752–1757. | ||
Braian C, Hogea V, Stendahl O. Mycobacterium tuberculosis- induced neutrophil extracellular traps activate human macrophages. J Innate Immun. 2013;5(6):591–602. | ||
Blomgran R, Ernst JD. Lung neutrophils facilitate activation of naive antigen-specific CD4+ T cells during Mycobacterium tuberculosis infection. J Immunol. 2011;186(12):7110–7119. | ||
Arana I, Orruño M, Barcina I. Basic methods for microbial enumeration. In: How To Solve Practical Aspects of Microbiology; 2013:1–6. Available from: https://ocw.ehu.eus/file.php/253/Temas/2_BASIC_METHODS_FOR_THE_ENUMERATION_OF_MICROORGANISMS.pdf. Accessed July 31, 2018. | ||
Graham L, Orenstein JM. Processing tissue and cells for transmission electron microscopy in diagnostic pathology and research. Nat Protoc. 2007;2(10):2439–2450. | ||
Hazeldine J, Harris P, Chapple IL, Jon H, Phillipa H L. CI, et al. Impaired neutrophil extracellular trap formation: a novel defect in the innate immune system of aged individuals. Aging Cell. 2014;13(4):690–698. | ||
Youmans GP, Karlson AG. Streptomycin sensitivity of tubercle bacilli; studies on recently isolated tubercle bacilli and the development of resistance to streptomycin in vivo. Am Rev Tuberc. 1947;55(6):529–535. | ||
Tucci P, González-Sapienza G, Marin M. Pathogen-derived biomarkers for active tuberculosis diagnosis. Front Microbiol. 2014;5:549. | ||
El-Masry S, El-Kady I, Zaghloul MH, Al-Badrawey MK. Rapid and simple detection of a mycobacterium circulating antigen in serum of pulmonary tuberculosis patients by using a monoclonal antibody and Fast-Dot-ELISA. Clin Biochem. 2008;41(3):145–151. | ||
Cross A, Moots RJ, Edwards SW. The dual effects of TNFalpha on neutrophil apoptosis are mediated via differential effects on expression of Mcl-1 and Bfl-1. Blood. 2008;111(2):878–884. | ||
Kaplanski G, Marin V, Montero-Julian F, Mantovani A, Farnarier C. IL-6: a regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends Immunol. 2003;24(1):25–29. | ||
Ben AH, Koubaa M, Smaoui F, et al. Elevated neutrophil-to-lymphocyte ratio is an effective prognosis indicator in extra-pulmonary tuberculosis. Open Forum Infect Dis. 2017;4(suppl_1):S618–S618. | ||
Niazi MK, Dhulekar N, Schmidt D, et al. Lung necrosis and neutrophils reflect common pathways of susceptibility to Mycobacterium tuberculosis in genetically diverse, immune-competent mice. Dis Model Mech. 2015;8(9):1141–1153. | ||
Schechter MC, Buac K, Adekambi T, et al. Neutrophil extracellular trap (NET) levels in human plasma are associated with active TB. PLoS One. 2017;12(8):e0182587. | ||
Yin Y, Kuai S, Liu J, et al. Pretreatment neutrophil-to-lymphocyte ratio in peripheral blood was associated with pulmonary tuberculosis retreatment. Arch Med Sci. 2017;13(2):404–411. | ||
Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516. | ||
Fuchs TA, Abed U, Goosmann C, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176(2):231–241. | ||
Sur Chowdhury C, Giaglis S, Walker UA, Buser A, Hahn S, Hasler P. Enhanced neutrophil extracellular trap generation in rheumatoid arthritis: analysis of underlying signal transduction pathways and potential diagnostic utility. Arthritis Res Ther. 2014;16(3):R122. | ||
Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med. 2013;5(178):178ra40-178ra40. | ||
Singh KK, Zhang X, Patibandla AS, Chien P, Laal S. Antigens of Mycobacterium tuberculosis expressed during preclinical tuberculosis: serological immunodominance of proteins with repetitive amino acid sequences. Infect Immun. 2001;69(6):4185–4191. | ||
Centro Nacional de Programas Preventivos y Control de Enfermedades (CENAPRECE S). DIRECCION DE MICOBACTERIOSIS Casos Nuevos de Tuberculosis Otras Formas Estados Unidos Mexicanos 1990-2015. México; 2015. | ||
Marchi LF, Sesti-Costa R, Ignacchiti MD, Chedraoui-Silva S, Mantovani B. In vitro activation of mouse neutrophils by recombinant human interferon-gamma: increased phagocytosis and release of reactive oxygen species and pro-inflammatory cytokines. Int Immunopharmacol. 2014;18(2):228–235. | ||
Kettritz R, Gaido ML, Haller H, Luft FC, Jennette CJ, Falk RJ. Interleukin-8 delays spontaneous and tumor necrosis factor-α-mediated apoptosis of human neutrophils. Kidney Int. 1998;53(1):84–91. | ||
Verbon A, Juffermans N, van Deventer SJ, Speelman P, van Deutekom H, van der Poll T. Serum concentrations of cytokines in patients with active tuberculosis (TB) and after treatment. Clin Exp Immunol. 1999;115(1):110–113. | ||
Deveci F, Akbulut HH, Turgut T, Muz MH. Changes in serum cytokine levels in active tuberculosis with treatment. Mediators Inflamm. 2005;2005(5):256–262. | ||
Chowdhury IH, Ahmed AM, Choudhuri S, et al. Alteration of serum inflammatory cytokines in active pulmonary tuberculosis following anti-tuberculosis drug therapy. Mol Immunol. 2014;62(1):159–168. | ||
Kruh-Garcia NA, Wolfe LM, Dobos KM. Deciphering the role of exosomes in tuberculosis. Tuberculosis. 2015;95(1):26–30. | ||
Pittman K, Kubes P. Damage-associated molecular patterns control neutrophil recruitment. J Innate Immun. 2013;5(4):315–323. |
Supplementary materials
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.