Back to Archived Journals » Metalloproteinases In Medicine » Volume 7
Sequential Matrix Metalloproteinase-1 Expression Triggered by Infiltrating Monocytic Lineage Cells Modulates Pathophysiological Aspects of Human Nonalcoholic Steatohepatitis
Authors Okazaki I, Shibata S , Ando W , Yanagawa T , Yokomori H, Sonoda A , Suzuki N, Yamanouchi E , Okada S , Kamikura S, Hachimura K, Takaki T, Otori K, Suzuki Y , Okano H, Inagaki Y
Received 8 March 2020
Accepted for publication 19 June 2020
Published 21 July 2020 Volume 2020:7 Pages 1—13
DOI https://doi.org/10.2147/MNM.S252991
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yoshifumi Itoh
Isao Okazaki,1,* Shinsuke Shibata,2,3,* Wataru Ando,4,* Takayo Yanagawa,5,6 Hiroaki Yokomori,7 Akira Sonoda,8 Norihiko Suzuki,9 Eigoro Yamanouchi,10 Shinya Okada,11 Shinichi Kamikura,11 Kazuaki Hachimura,11 Takashi Takaki,12 Katsuya Otori,4 Yutaka Suzuki,9 Hideyuki Okano,2 Yutaka Inagaki5,6
1Department of Internal Medicine, International University of Health and Welfare (IUHW) Hospital, Nasu-Shiobara City, Tochigi Prefecture, Japan; 2Department of Physiology, Keio University School of Medicine, Shinjuku-Ku, Tokyo, Japan; 3Electron Microscope Laboratory, Keio University School of Medicine, Shinjuku-Ku, Tokyo, Japan; 4Department of Clinical Pharmacy, Kitasato University School of Pharmacy, Minato-Ku, Tokyo, Japan; 5Center for Matrix Biology and Medicine, Tokai University Graduate School of Medicine, Isehara City, Kanagawa Prefecture, Japan; 6Department of Innovative Medical Science, Tokai University School of Medicine, Isehara City, Kanagawa Prefecture, Japan; 7Department of Internal Medicine, Kitasato University Medical Center, Kitamoto City, Saitama Prefecture, Japan; 8Collaborative Research Resources, Core Instrumentation Facility, Keio University School of Medicine, Shinjuku-Ku, Tokyo, Japan; 9Department of Surgery; 10Deparment of Radiology; 11Department of Pathology, IUHW Hospital, Nasu-Shiobara City, Tochigi Prefecture, Japan; 12Division of Electron Microscopy, Showa University School of Medicine, Shinagawa-Ku, Tokyo, Japan
*These authors contributed equally to this work
Correspondence: Isao Okazaki
Higashi Nippon International University, Iwaki City, Fukushima Prefecture, Japan
Tel +81-246-35-0001
Fax +81-246-25-9188
Email [email protected]
Background/Aims: Matrix metalloproteinase-1 (MMP-1) is a key enzyme in collagen metabolism and tissue remodeling. We previously reported that MMP-1 expression in monocytes and other types of cells in the liver is associated with disease progression in patients with nonalcoholic steatohepatitis (NASH). To further implicate MMP-1 up-regulation in NASH pathogenesis, we examined dynamic and sequential MMP-1 expression in peripheral blood monocytes and cells in steatotic liver, and attempted to elucidate the mechanism of MMP-1 induction.
Methods: Twenty-nine NASH patients and 26 non-NASH subjects were recruited in the study. Their peripheral blood mononuclear cells were isolated and analyzed by fluorescence-activated cell sorting, and liver specimens were subjected to confocal laser-scanning and immunoelectron microscopic examinations. Peripheral blood monocytic lineage cells were co-cultured with steatotic hepatocytes obtained from biopsy specimens to examine MMP-1 induction.
Results: Infiltrating monocytes were the earliest to acquire an MMP-1-expressing phenotype among all investigated cell types in early-stage NASH liver. On the other hand, circulating monocytes did not express significant levels of MMP-1 mRNA or protein before infiltrating steatotic liver. Co-culture with steatotic hepatocytes induced MMP-1 expression in otherwise MMP-1-negative peripheral blood monocytic lineage cells. As disease progressed, MMP-1 was sequentially expressed by other types of cells in NASH liver. Notably, clusters of OV-6- or CK19-positive hepatic progenitor cells with a morphological feature of ductular reaction showed strong MMP-1 staining in advanced-stage NASH.
Conclusion: MMP-1 up-regulation in infiltrating monocytic lineage cells represents an initial event in early-stage NASH and, together with the subsequent expression in other types of cells, modulates pathophysiological aspects of NASH.
Keywords: matrix metalloproteinase-1, nonalcoholic steatohepatitis, monocytic progenitor cells, hepatic progenitor cells, liver fibrosis
Introduction
Global trends of overeating and reduced physical activity have increased the prevalence of nonalcoholic fatty liver disease (NAFLD), which affects one-quarter of the global adult population.1 NAFLD may progress to nonalcoholic steatohepatitis (NASH), in which 10% of cases lead to liver cirrhosis and/or hepatocellular carcinoma.1,2 Although several new therapeutic options have been developed, their effects are not promising.1
Matrix metalloproteinases (MMPs) represent a family of specific enzymes that regulate extracellular matrix turnover in various tissues.3,4 We have been studying the roles of MMPs using rodent models of hepatic fibrosis.5–9 In subsequent studies, using human liver samples, we showed that MMP-1 expression levels were increased in both early and advanced stages of NASH, and suggested that MMP-1 up-regulation reflects disease progression.10,11 More recently, we further revealed that serum MMP-1 levels were indicative of disease progression in patients with histologically confirmed NASH.12 From these findings, we hypothesized that MMP-1 expression may accelerate NASH progression to advanced fibrosis. However, the mechanisms underlying MMP-1 up-regulation in human NASH pathogenesis are completely unknown.
In the present study, we reconfirmed sequential MMP-1 up-regulation in different types of cells present in human NASH liver by confocal laser-scanning microscopy and immunoelectron microscopy using antibodies specific for monocytes, Kupffer cells, hepatic stellate cells, hepatocytes, hepatic progenitor cells, and sinusoidal endothelial cells. Altered cell phenotypes resulting from MMP-1 up-regulation in these cells were also examined by confocal laser-scanning microscopy. Among a variety types of cells examined, we focused mostly on monocytic lineage cells as a principal and initial producer of MMP-1 in early-stage NASH. Using flow cytometric analysis, we sought to determine when and how circulating monocytes express MMP-1 and modulate the progression of NASH. Our results indicated that peripheral blood monocytes started to express MMP-1 after their infiltration into the diseased liver. Moreover, co-culture experiments suggested that certain stimuli from steatotic hepatocytes may trigger MMP-1 expression in such monocytic lineage cells.
Patients and Methods
Patient Enrolment Criteria
The study was performed in the Department of Internal Medicine at the International University of Health and Welfare (IUHW) Hospital from April 26, 2017 to June 30, 2019. The study protocols were approved by the IUHW Ethics Committee (13-B-136, February 22, 2016; 13-B-205, November 24, 2016; 13-B-229, April 27, 2017). Collection of blood samples and other clinical examinations were approved by the IUHW Institutional Review Board Full Committee, and written informed consent was obtained from all participants. Twenty-nine histologically proven patients with NASH (23 men and 6 women; mean age 48.0 ± 10.2 years) and 26 control subjects without NASH (18 men and 8 women; mean age 56.6 ± 11.6 years) were enrolled in this study (Supporting Tables S1 and S2). The exclusion criteria for NASH patients were applied according to the guidelines for the diagnosis and management of NAFLD by the American Association for the Study of Liver Diseases.13,14
Clinical Characteristics of the Study Cohort
Clinical and laboratory data of NASH and non-NASH subjects are shown in Supporting Tables S1 and S2, respectively. Twenty-nine patients with NASH were admitted to the Department of Surgery in IUHW Hospital, in agreement with the NASH Education Program; they underwent computed tomography or magnetic resonance imaging of the liver, liver needle biopsy, and a standard oral glucose tolerance test to evaluate and diagnose potential complications with type 2 diabetes mellitus according to the 2003 American Diabetes Association diagnostic criteria.15 Control non-NASH subjects were selected from outpatients who visited the Division of Gastroenterology and Hepatology, Department of Internal Medicine in IUHW Hospital. They were diagnosed as chronic gastritis, gastroesophageal regurgitation, gallbladder polyps, hepatic hemangioma, colon polyps, and other diseases shown in Supporting Table S2. All non-NASH subjects exhibited normal liver function values when tested (Supporting Table S2). Diagnosis of metabolic syndrome was made according to the following criteria of eight Japanese Medical Associations16 for Japanese subjects that is based on the Third National Cholesterol Education Program:17 waist circumference (WC) ≥85 cm for males and 90 cm for females, satisfying any three or more of the following criteria: triglyceride (TG) ≥ 150 mg/dL, high-density lipoprotein (HDL) cholesterol < 40 mg/dL, fasting glucose ≥ 116 mg/dL or HbA1c ≥ 6.5%, and blood pressure (BP) ≥ 130/85 mm Hg (or taking antihypertensive drug treatment).
Histopathological Diagnosis of Liver Biopsy Specimens
According to the American Association for the Study of Liver Diseases guidelines,13 S.O. (a pathologist) and H.Y. (an internist and pathologist) rechecked all biopsy specimens from 29 NASH patients. The histological features defined by the NASH Clinical Research Network and fibrosis staging14 were also used for histopathological evaluation.
Immunohistochemistry and Confocal Laser-Scanning Microscopy
Immunohistochemical staining and confocal laser-scanning microscopy were performed as described previously,10,11 using specific antibodies recognizing different cell markers such as glial fibrillary acidic protein (GFAP) and α-smooth muscle actin (α-SMA) for quiescent and activated hepatic stellate cells, respectively,18,19 CD11b for monocytes, F4/80 for Kupffer cells, hepatocyte nuclear factor 4α (HNF4α) for hepatocytes, OV-6 and cytokeratin (CK) 19 for hepatic progenitor cells, and CD31 for sinusoidal endothelial cells, as described previously.20 Detailed information regarding the primary and secondary antibodies used is shown in Supporting Table S3.
Immunoelectron Microscopy
Immunoelectron microscopy was performed to determine the ultrastructural localization of MMP-1 as described previously.10 Liver biopsy specimens were immediately immersed in phosphate-buffered saline (PBS; pH 7.4) at 37°C, and perfusion-fixed with periodate-lysine-paraformaldehyde (PLP). Then, PLP was further injected from multiple sides using a 26-G syringe until discoloration and hardening of tissues were achieved. After incubation with PLP overnight at 4°C, semi-thin 5-mm sections were prepared. Sections were then incubated with anti-MMP-1 antibody (1:50) in 0.01% PBS containing 1% bovine serum albumin overnight at 4°C in a moisture chamber, washed three times in PBS, incubated with 1.4-nm colloidal gold-conjugated anti-mouse IgG antibody (1:40; Nanoprobes Inc., Yaphank, NY, USA) for 40 min. After washing three times in 10 mM citrate buffer (pH 6.0), they were physically developed using a silver enhancement kit (Nanoprobes Inc.) for 5 min as previously described.10 After being fixed in 2.5% glutaraldehyde in 0.01% PBS (pH 7.4) for 1 h at 4°C and washed using a graded series of ethanol solutions, samples were post-fixed with 2% osmium tetroxide in 0.01% PBS (pH 7.4) for 90 min at 4°C. After embedding in Epon, ultrathin sections were cut using a diamond knife and an ultramicrotome (LKB Bromma, Stockholm, Sweden), stained with uranyl acetate, and observed using transmission electron microscopy (JEM-1200 EX; JEOL, Tokyo, Japan).
Flow Cytometric Analysis
Peripheral blood mononuclear cells (PBMNCs) were obtained from NASH patients and the control non-NASH subjects using Lymphoprep tubes (Abbott Diagnostics Technologies AS, Oslo, Norway). They were incubated with PC7-conjugated anti-CD45 antibody, FITC-conjugated anti-CD14 antibody, and PE-conjugated anti-CD34 antibody (Beckman Coulter, Brea, CA, USA) in Dulbecco’s modified Eagle’s medium (DMEM) containing exosome-free 5% fetal bovine serum (FBS; Invitrogen/Thermo Fisher Scientific, Carlsbad, CA, USA). After incubation for 1 h at 4°C, cells were subjected to flow cytometry using a MoFlo XDP (Beckman Coulter) to isolate CD45+CD14−CD34− (R1), CD45+CD14+CD34− (R2), CD45+CD14+CD34+ (R3), and CD45+CD14−CD34+ (R4) cell fractions. PC7-conjugated anti-mouse IgG1, FITC-conjugated anti-mouse IgG2, and PE-conjugated anti-mouse IgG1 antibodies (Beckman Coulter) were used as isotype controls. In some experiments, anti-MMP-1 antibody conjugated with Alexa Fluor 647 (Abcam, Cambridge, UK) was used to examine MMP-1 expression in each cell fraction.
Detection of MMP1 Gene Expression in R1, R2, R3, and R4 Populations Using Quantitative Reverse Transcription-PCR (RT-PCR)
After sorting R1, R2, R3, and R4 cell fractions from 8 NASH samples and 8 control non-NASH samples by flow cytometry, total RNA was extracted from each cell fraction using TRIzol reagent (Invitrogen/Thermo Fisher Scientific) and reverse-transcribed using a High-Capacity cDNA reverse transcription kit with RNase inhibitor (Applied Biosystems/Thermo Fisher Scientific). Quantitative RT-PCR analysis of human MMP1 expression was performed as previously described21,22 using Fast SYBR Green master mix (Applied Biosystems/Thermo Fisher Scientific) with specific primers as follows: (forward primer) 5′-TACACGGATACCCCAAGGAC-3′, and (reverse primer) 5′-AACTTTGTGGCCAATTCCAG-3′ (Gene ID: 4312, the National Center for Biotechnology Information, Bethesda, MD, USA). MMP1 mRNA levels were normalized to that of glyceraldehyde-3-phosphate dehydrogenase gene (GAPDH) in each RNA preparation.
Examination of MMP-1 Protein in Plasma Exosomes
Exosomes were isolated from heparinized blood of 8 NASH patients and 2 non-NASH subjects using the ExoQuick Plasma prep and Exosome precipitation kit (System Biosciences, Mountain View, CA, USA). ExoQuick PLUS exosome purification kit for serum and plasma (System Biosciences) was used to purify exosomes and exclude other proteins. Presence of MMP-1 protein in the obtained exosomes was assessed using protein blot analysis with anti-MMP-1 antibody and secondary horseradish peroxidase-conjugated anti-mouse F(ab’)2 fragment antibody (GE Healthcare, Chicago, IL, USA). Signals were detected using Immobilon Western chemiluminescent kit (MERCK-Millipore, Darmstadt, Germany). Cell lysates from a hepatocellular carcinoma cell line HLE that express MMP-121 were used as a positive control.
Preparation of Steatotic Hepatocytes from NASH Liver
To remove exosomes, FBS was incubated with a 1:5 volume of Total Exosome Isolation Reagent (Invitrogen/Thermo Fisher Scientific) at 4°C for 30 min, followed by centrifugation at 10,000 × g for 10 min at 4°C. Obtained exosome-free FBS was then used in the following co-culture study. After being subjected to histopathological diagnosis, the remaining small pieces of liver tissue (3–4 pieces of 2–3 mm3 each) were homogenized in DMEM containing 5% exosome-free FBS, 0.3 mg/mL collagenase type II (WOR CLS2; Worthington, Funakoshi, Co., Tokyo, Japan), and 10 units/mL DNase I (New England BioLabs, Ipswich, MA, USA) using a glass homogenizer. Samples were then incubated in a water bath with gentle shaking at 37°C for 1 h, filtered through sterilized gauze to remove undigested materials, and centrifuged at 500 × g for 5 min to separate steatotic hepatocytes.
Co-Culture of Steatotic Hepatocytes and Monocytic Lineage Cells from NASH Patients
Steatotic hepatocytes (10,000 cells) and peripheral blood monocytic lineage cells (R2 and R3 population, 10,000 cells for each) obtained from 3 NASH patients were co-cultured in DMEM containing 10% exosome-free FBS and 4 U/mL penicillin/streptomycin on Lab-Tek-Chamber Slide (Nunc, Roskilde, Denmark) at 37°C under 5% CO2. Poly-L-ornithine (0.01%; Sigma-Aldrich, St. Louis, MO) was used as a cell adhesive agent in some experiments. After 7-day co-culture, cells were fixed with 4% paraformaldehyde in PBS, incubated with a mixture of anti-CD11b and anti-MMP-1 antibodies, and observed using confocal laser-scanning microscopy.
Statistical Analyses
Values were expressed as the means ± standard deviation (SD). Statistical analyses were performed using GraphPad Prism 7 for Mac OS X (GraphPad Software Inc., La Jolla, CA, USA). Data were first analyzed using the Kruskal–Wallis test, then compared between the selected two groups using the Mann–Whitney U-test with Bonferroni adjustment. Correlation between the obtained values and histological findings was determined using Spearman’s rank coefficient. Values of P < 0.05 were considered statistically significant.
Results
Histological Findings and Stage Classification of 29 NASH Patients
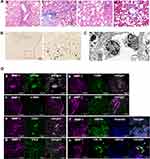
Histopathological findings in 29 patients with NASH are shown in Supporting Table S4. Matteoni’s classification, Brunt’s grading, and NAFLD activity score (NAS) of patients were conducted according to the guidelines of NAFLD diagnosis and management.13,14 We classified NASH patients into two groups: 17 patients with steatosis showing mild inflammation, slight ballooning, and slight-to-moderate fibrosis were diagnosed as early-stage NASH (Figure 1A), whereas 12 patients with steatosis associated with marked inflammation, ballooning, and severe fibrosis or cirrhosis without hepatocellular carcinoma were diagnosed as advanced-stage NASH (Figure 2A).
Comparison of Clinical and Laboratory Data Between NASH and Non-NASH Patients
Clinical and laboratory data of 29 NASH patients (Supporting Table S1) and 26 non-NASH subjects (Supporting Table S2) were compared in Supporting Table S5. Distribution of sex and age was slightly different between the groups. Complications with diabetes mellitus were observed in 7 NASH patients (24%) and 1 non-NASH subject (4%), respectively. The incidence of metabolic syndrome was higher in patients with NASH (15/29 patients, 52%) than in patients without NASH (4/26 patients, 15%). Among 12 advanced-stage NASH patients, 7 patients were complicated with metabolic syndrome (58%). Drugs administered to patients with and without NASH are shown in Supporting Tables S6 and S7, respectively.
Detection of MMP-1-Positive Cells in NASH Liver by Immunohistochemistry, Confocal Laser-Scanning Microscopy, and Immunoelectron Microscopy
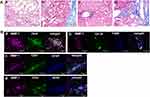
In early-stage NASH, immunohistochemical staining showed clusters of small MMP-1-positive cells around the central vein (Figure 1B). Immunoelectron microscopy revealed the presence of gold grains of MMP-1 on the surface of monocytes migrating into the sinusoid, but not other types of cells including hepatocytes, hepatic stellate cells and sinusoidal endothelial cells (Figure 1C). Confocal laser-scanning microscopy further confirmed co-localization of MMP-1 and CD11b, a representative marker of monocytes (Figure 1Da). In some cases, faint MMP-1 staining was observed in small numbers of F4/80-positive Kupffer cells (Figure 1Db). α-SMA-expressing activated hepatic stellate cells (Figure 1Dc), and GFAP-positive quiescent hepatic stellate cells (Figure 1Dd). CD31-expressing sinusoidal endothelial cells (Figure 1De), HNF4α-positive hepatocytes (Figure 1Df), and OV-6 (Figure 1Dg) or CK19 (Figure 1Dh)-expressing hepatic progenitor cells were also stained positively for MMP-1. As disease progressed, MMP-1 expression in the clusters of OV-6-positive cells became more prominent. They represented a morphological feature of ductular reaction composed of hepatic progenitor cells (Figure 2Ba). Expression of MMP-1 was also strong in CK19-positive cells showing a bile ductular structure (Figure 2Bb). Interestingly, GFAP-positive hepatic stellate cells were observed along the MMP-1-expressing CD31-positive capillary endothelial cells in advanced-stage NASH liver (Figure 2Bc and d), suggesting that MMP-1 up-regulation in endothelial cells might correlate angiogenesis with fibrogenesis. Taken together, the findings obtained using confocal laser-scanning microscopy confirmed MMP-1 up-regulation in infiltrating monocytes as an initial event in early-stage NASH and the subsequent dynamic change in MMP-1 expression in a variety of liver cell types in more advanced stage of NASH.
Flow-Cytometry of Circulating Peripheral Blood Mononuclear Cells in NASH and Non-NASH Patients
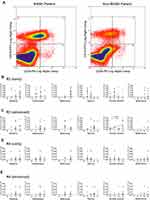
Peripheral blood mononuclear cells were isolated from both groups of patients and subjected to flow cytometric analysis. To identify a small number of hematopoietic progenitor cells present in the peripheral blood, they were divided into the following 4 populations; CD45+CD14−CD34− cells (R1), CD45+CD14+CD34− cells (R2), CD45+CD14+CD34+ cells (R3), and CD45+CD14−CD34+ cells (R4). Proportions of these 4 populations in each patient were shown in Supporting Tables S8 and S9. Overall, the proportions of R1, R2, R3, and R4 fractions in NASH patients were almost identical to those in the control non-NASH subjects, and there were no significant differences between the two groups (Table 1). However, as shown in Figure 3A, a large R3 population that represented CD14+CD34+ monocytic progenitor cells was often observed in advanced-stage NASH patients with exacerbated laboratory data. We, therefore, focused on this R3 population and examined its correlation with histological features such as steatosis, lobular inflammation, ballooning, fibrosis, NAS, and ductular reaction in both early- (Figure 3B) and advanced-stage NASH (Figure 3C). There was a significant correlation between the R3 population and the extent of ductular reaction in advanced-stage NASH (r = 0.6336, P = 0.0179; Figure 3C and Supporting Table S10). On the other hand, R4 population that represented CD14−CD34+ non-monocytic progenitor cell fraction was the same between NASH patients and the control non-NASH subjects (Table 1). There was no correlation between R4 population and any histological features in either early- (Figure 3D) or advanced-stage NASH (Figure 3E and Supporting Table S11).
 |
Table 1 Each Population in Peripheral Blood Mononuclear Cells from Nonalcoholic Steatohepatitis (NASH) Patients and Non-NASH Control Subjects |
Negligible MMP-1 Expression in Peripheral Blood Monocytes
We next assessed MMP-1 expression in circulating monocytic lineage cells using flow cytometry. Few, if any, CD45+CD14+CD34+ cells in the peripheral blood from NASH patients were stained positively for MMP-1 (Table 2). We also examined the presence of MMP1 mRNA in 4 different fractions of peripheral blood mononuclear cells (R1, R2, R3, and R4) using flow cytometry. Compared to definite MMP1 expression in a hepatocellular carcinoma cell line HLE, MMP1 mRNA expression levels were very low in all 4 fractions (Table 3). Furthermore, MMP-1 protein expression was not detected in plasma exosomes extracted from 8 NASH patients (Figure 4A). These results indicated that circulating monocytic lineage cells did not express MMP-1 mRNA or protein prior to their migration into NASH liver tissue.
 |
Table 2 Flow Cytometric Analysis of MMP-1 Expression in CD45+CD14+CD34+ Monocytic Progenitor Cells in NASH and Non-NASH Patients |
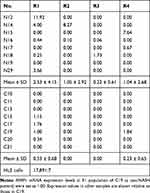 |
Table 3 Relative MMP1 mRNA Expression Levels in Peripheral Blood Mononuclear Cell Fractions from NASH and Non-NASH Patients |
Co-Culture of Steatotic Hepatocytes Separated from NASH Liver Induced MMP-1 Expression in Peripheral Blood Monocytic Lineage Cells
To reveal how MMP-1 expression is induced in infiltrating monocytes, co-culture experiments were performed using steatotic hepatocytes and monocytic lineage cells (R2 and R3 fractions) obtained from the identical patients. After 7-day co-culture, confocal laser-scanning microscopy revealed that MMP-1expression was induced in a certain population of CD11b+ cells (Figure 4B). The proportions of MMP-1-positive cells in R2 and R3 populations were 12.9% to 21.7% and 40.0% to 85.7%, respectively (Supporting Table S12).
Discussion
The present study revealed that MMP-1 expression was up-regulated in a variety of liver cell populations in both early- and advanced-stages of NASH. A combination of confocal laser-scanning microscopy and flow cytometric analysis using the different cohort of NASH and non-NASH patients from those in our previous studies10–12 confirmed dynamic and sequential change in MMP-1 expression during progression of NASH. Given that MMP-1 up-regulation reflects disease progression in NASH,10–12 the results of the present study may implicate MMP-1 in NASH pathogenesis. Three major findings were observed in the present study.
First, immunoelectron microscopy study showed that, in the very early stage of NASH, monocytes infiltrating liver sinusoid harbored gold grains of MMP-1 on their surface, while the other cell types including the neighboring hepatocytes and endothelial cells did not (Figure 1C). These results indicated that monocytes acquired an MMP-1-expressing phenotype at an earlier stage than any other investigated cell types. However, few monocytic progenitor cells present in peripheral blood of NASH patients expressed MMP-1 (Table 2), nor was significant amounts of MMP1 mRNA detected in peripheral blood mononuclear cell fractions (Table 3). Furthermore, MMP-1 protein was not detected in circulating exosomes obtained from NASH patients (Figure 4A). Initially, we hypothesized that circulating monocytic progenitor cells mobilized from bone marrow express MMP-1 and infiltrate NASH liver for tissue repair.23,24 This was based on the results of our previous study demonstrating possible contribution of bone marrow-derived MMP-13- and MMP-9-expressing stem/progenitor cells to the reversal of experimental liver fibrosis in mice chronically intoxicated with carbon tetrachloride.8 However, this hypothesis based on mouse experiments was nullified in the present study using human NASH samples. Further studies are needed to determine whether this discrepancy is attributable to the difference in the causes of liver injury/fibrosis or due to the functional difference between MMP-1 and MMP-13/MMP-9.
Second, co-culture with steatotic hepatocytes induced MMP-1 expression in a significant proportion of CD11b-positive monocytic lineage cells that did not express MMP-1 when present in the circulating blood (Figure 4B and Supporting Table 12). There are several possibilities that account for this in vitro finding. Steatotic hepatocytes may secrete the same humoral factor(s) that induce MMP-1 expression in the neighboring monocytic lineage cells as in NASH liver tissue in vitro. Alternatively, it may merely represent an artificial in vitro phenomenon that several substances released by primary cultures of hepatocytes undergoing severe damage or epithelial-to-mesenchymal transition during 7-day culture stimulate MMP-1 expression in co-cultured monocytes. These possibilities have to be further examined using a large number of samples from both NASH and non-NASH patients.
Finally, substantial MMP-1 up-regulation was observed in large clusters of OV-6- or CK19-positive hepatic progenitor cells in advanced-stage NASH liver (Figure 2Ba and b). These findings represent ductular reaction reflecting regeneration of injured or fibrotic liver, and suggest that MMP-1 up-regulation in hepatic progenitor cells may play an important role in the repair process. As well as in other types of liver diseases, ductular reaction is often observed in advanced-stage NASH liver.25 Kupffer cells, monocytes, and macrophages are reported to stimulate the differentiation of hepatic stem cells into hepatic progenitor cells.26 Consistent with these findings, increased numbers of monocytic progenitor cell population (R3) were significantly correlated with the occurrence of ductular reaction in advanced-stage NASH liver (Figure 3C and Supporting Table S10).
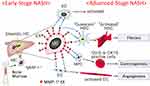
It should be noted that, following a phenotypic change of infiltrating monocytes to express MMP-1, other types of cells including Kupffer cells, hepatic stellate cells, hepatocytes, hepatic progenitor cells, and sinusoidal endothelial cells also became MMP-1-positive at a later stage (Figures 1 and 2). Sequential MMP-1 expression in a variety of cell populations is supposed to play a number of critical roles in liver pathophysiology. For example, the presence of GFAP-positive hepatic stellate cells along the MMP-1-expressing endothelial cells (Figure 2Bc and d) suggests a possible role of this cellular crosstalk in regulating angiogenesis/fibrogenesis. It has been reported that enhanced MMP-1 expression in endothelial cells stimulates capillary tube regression and collagen degradation.27 Along the same line, an adenovirus-mediated MMP1 gene transfer accelerated regression of liver fibrosis.28 Similar effects on fibrosis regression and regeneration of fibrotic liver were also reported using other types of interstitial collagenases such as MMP-139 and MMP-8.29 MMP-1-positive hepatic progenitor cells may be activated and transformed into small hepatocytes, biliary epithelial cells, or hepatic stellate cells via epithelial-mesenchymal transition (EMT) in certain situations.30–32 Meanwhile, they may also represent dysplasia of hepatocytes, leading to cancer development,33,34 as many types of cancer cells increase MMP-1 expression at the mRNA and/or protein levels.4,35–41 From these findings, we speculate that MMP-1-containing exosomes secreted from infiltrating monocytic lineage cells may transfect the neighboring cells, which in turn affects a number of pathophysiological processes in NASH such as angiogenesis and carcinogenesis (Figure 5). Alternatively, these cells changed their phenotypes after coming into direct contact with steatotic hepatocytes. This latter possibility was raised by others who demonstrated hepatic stellate cell activation by steatotic hepatocyte-derived exosomes.42
The present study has several limitations. A small number of subjects included in the non-NASH group were complicated with obesity, impaired glucose tolerance, dyslipidemia, or hypertension. It is well known that obesity or metabolic syndrome per see can be a significant risk factor of liver fibrosis.43 Even though all non-NASH subjects exhibited normal liver function values, we have to be careful when dealing with the obtained results. Ideally, MMP-1 expression should be analyzed and compared between patients with NASH and simple steatosis without any inflammation or fibrosis, both of which are diagnosed by liver biopsy. Such a study will certainly reveal the causal relationship between steatosis/fibrosis and MMP-1 up-regulation and implicate MMP-1 in NASH pathogenesis. Co-culture experiments were conducted using liver specimens obtained from a limited number of patients. This was mostly because of the difficulty obtaining an enough number of hepatocytes from the remaining small liver specimens that had been taken primarily for a diagnostic purpose. Setting of appropriate control liver samples from healthy subjects is also difficult for an ethical reason.
The precise mechanisms of MMP-1 up-regulation in infiltrating monocytic lineage cells and possible transduction of MMP-1-containing exosomes to other types of cells are largely unknown. Immunohistochemical (Figure 1B) and immunoelectron microscopic findings (Figure 1C) showing MMP-1 up-regulation in infiltrating monocytes in a very early stage of NASH suggest that steatosis may be the initial trigger of MMP-1 induction. This possibility has to be validated using appropriate experimental models. Mmp1a and Mmp1b have been identified as homologous genes in mouse genome.44 However, these gene encode different proteins from human MMP-1 in the peptidase domain. Although murine MMP-13 has been considered a homologue of human MMP-1 (86% amino acid identity),9 it is not suitable to apply the results of rodent MMP-13 experiments directly to clinical setting. In addition, there has been no ideal experimental model for human NASH.45 For example, feeding of methionine and choline-deficient diet exhibits similar histopathological findings, but does not have the same mechanistic background as human NASH that is based on excess calorie intake and reduced physical activity as well as a number of genetic factors. A recent study has reported a human NASH model using pluripotent stem cell-derived organoids,46 which sheds an insight into the cellular crosstalk between steatotic hepatocytes and monocytes as well as neighboring other types of cells. Since MMP-1 expression can be manipulated with microRNA,37,47,48 MMP-1 regulation in human NASH liver would be a potential therapeutic option.
In conclusion, monocytic lineage cells acquire an MMP-1-positive phenotype after infiltrating NASH liver, which may be exerted through interaction with steatotic hepatocytes. Subsequent dynamic changes in MMP-1 expression in a variety of liver cell types may modulate a number of pathophysiological aspects of NASH. Despite several limitations described above, our study represents an important first step toward understanding the role of MMP-1 up-regulation as an initial trigger and modulator of human NASH.
Acknowledgments
We are grateful to the staff, especially doctors, nurses, and expert nutritionists of the “NASH Education Admission Program” at the Surgical Department in IUHW Hospital. We thank Ms. Cecilia Hamagami for assistance with the English revision of the manuscript, and Editage for English language editing.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors have declared that no conflicts of interest exist.
References
1. Younossi ZM, Loomba R, Rinella ME, et al. Current and future therapeutic regimens for nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. 2018;68:361–371. doi:10.1002/hep.29724
2. Okazaki I, Noro T, Tsutsui N, et al. Fibrogenesis and carcinogenesis in nonalcoholic steatohepatitis (NASH): involvement of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinase (TIMPs). Cancers. 2014;6:1220–1255. doi:10.3390/cancers6031220
3. Okazaki I, Ninomiya Y, Friedman SL, Tanikawa K, eds, Extracellular Matrix and the Liver. Approach to Gene Therapy. London, New York: Academic Press, Amsterdam, Boston; 2003:1–467.
4. Okazaki I, Nabeshima K. Introduction: MMPs, ADAMs/ADAMTSs research products to achieve big dream. Anticancer Agents Med Chem. 2012;12:688–706. doi:10.2174/187152012802650200
5. Okazaki I, Maruyama K. Collagenase activity in experimental hepatic fibrosis. Nature. 1974;252:49–50. doi:10.1038/252049a0
6. Watanabe T, Niioka M, Hozawa S, et al. Gene expression of interstitial collagenase in both progressive and recovery phase of rat liver fibrosis induced by carbon tetrachloride. J Hepatol. 2000;33:224–235. doi:10.1016/S0168-8278(00)80363-3
7. Watanabe T, Niioka M, Ishikawa A, et al. Dynamic change of cells expressing MMP-2 mRNA and MT1-MMP mRNA in the recovery from liver fibrosis in the rat. J Hepatol. 2001;35:465–473. doi:10.1016/S0168-8278(01)00177-5
8. Higashiyama R, Inagaki Y, Hong YY, et al. Bone marrow-derived cells express matrix metalloproteinases and contribute to regression of liver fibrosis in mice. Hepatology. 2007;45:213–222. doi:10.1002/hep.21477
9. Endo H, Niioka M, Sugioka Y, et al. Matrix metalloproteinase-13 promotes recovery from experimental liver cirrhosis in rats. Pathobiology. 2011;78:239–252. doi:10.1159/000328841
10. Yokomori H, Inagaki Y, Ando W, et al. Spatiotemporal expression of metalloproteinase-1 in progression of nonalcoholic steatohepatitis. J Mod Hum Pathol. 2016;1:11–20. doi:10.14312/2397-6845.2016-3
11. Yokomori H, Ando W, Oda M, Inagaki Y, Okazaki I. Hepatic progenitor cell expansion in early-stage nonalcoholic steatohepatitis: evidence from immunohistochemistry and immunoelectron microscopy of matrix metalloproteinase-1. Med Mol Morphol. 2017;50:238–242. doi:10.1007/.s00795-017-0162-y
12. Ando W, Yokomori H, Tsutsui N, et al. Serum matrix metalloproteinase-1 represents disease activity as opposed to fibrosis in patients with histologically proven nonalcoholic steatohepatitis. Clin Mol Hepatol. 2018;24:61–76. doi:10.3350/cmh.2017.0030
13. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American association for the study of liver diseases. Hepatology. 2018;67:328–357. doi:10.1002/hep.29367
14. Younossi ZM, Loomba R, Anstee QM, et al. Diagnostic modalities for nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, and associated fibrosis. Hepatology. 2018;68:349–360. doi:10.1002/hep.29721
15. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34:S62-S69. doi:10.2337/dc11-S062
16. The Japanese Task Force Committee for Diagnosis Criteria of Metabolic Syndrome. Definition and diagnosis criteria of metabolic syndrome. J Jap Inter Med Ass. 2005;94:794–809.
17. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001;285:2486–2497. doi:10.1001/jama.285.19.2486
18. Morini S, Carotti S, Carpino G, et al. GFAP expression in the liver as an early marker of stellate cells activation. It J Anat Embryol. 2005;110:193–2007.
19. Yang L, Jung Y, Omenetti A, et al. Fate-mapping evidence that hepatic stellate cells are epithelial progenitors in adult mouse livers. Stem Cells. 2008;26:2104–2113. doi:10.1634/stemcells.2008-0115
20. Shibata S, Iseda T, Mitsuhashi T, et al. Large-area fluorescence and electron microscopic correlative imaging with multibeam scanning electron microscopy. Front Neural Circuits. 2019;13:29. doi:10.3389/fncir2019.00029
21. Sugioka Y, Watanabe T, Inagaki Y, et al. c-JUN NH2-terminal kinase pathway is involved in constitutive matrix metalloproteinase-1 expression in a hepatocellular carcinoma-derived cell line. Int J Cancer. 2004;109:867–874. doi:10.1002/ijc.20095
22. Yanagawa T, Sumiyoshi H, Higashi K, et al. Identification of a novel bone marrow cell-derived accelerator of fibrotic liver regeneration through mobilization of hepatic progenitor cells in mice. Stem Cells. 2019;37:89–101. doi:10.1002/stem.2916
23. Heissig B, Hattori K, Dias S, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–637. doi:10.1016/s0092-8674(02)00754-7
24. Bihari C, Anand L, Rooge S, et al. Bone marrow stem cells and their niche components are adversely affected in advanced cirrhosis of the liver. Hepatology. 2016;64:1273–1288. doi:10.1002/hep.28754
25. Boulter L, Govaere O, Bird TG, et al. Macrophage derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat Med. 2012;18:572–579. doi:10.1038/nm.2667
26. Pollard JW. Tropic macrophages in development and disease. Nat Rev Immunol. 2009;9:259–270. doi:10.1038/nri2528
27. Saunders WB, Bayless KJ, Davis GE. MMP-1 activation by serine proteases and MMP-10 induces human capillary tubular network collapse and regression in 3D collagen matrices. J Cell Sci. 2005;118(Pt 10):2325–2340. doi:10.1242/jcs.02360
28. Iimuro Y, Nishio T, Morimoto T, et al. Delivery of matrix metalloproteinase-1 attenuates established liver fibrosis in the rat. Gastroenterology. 2003;124:445–458. doi:10.1053/gast.2003.50063
29. Siller-Lόpez F, Sandoval A, Salgado S, et al. Treatment with human metalloproteinase-8 gene delivery ameliorates experimental rat liver cirrhosis. Gastroenterology. 2004;126:1122–1133. doi:10.1053/j.gastro.2003.12.045
30. Zeisberg M, Yang C, Martino M, et al. Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J Biol Chem. 2007;282(32):23337–23347. doi:10.1074/jbc.M700194200
31. Syn W-K, Jung Y, Omenetti A, et al. Hedgehog-mediated epithelial-to-mesenchymal transition and fibrogenic repair in non-alcoholic fatty liver disease. Gastroenterology. 2009;137:1478–1488. doi:10.1053/j.gastro.2009.06.051
32. Zhao Y-L, Zhu R-T, Sun Y-L. Epithelial-mesenchymal transition in liver fibrosis (Review). Biomed Rep. 2016;4:269–274. doi:10.3892/br.2016.578
33. Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548–558. doi:10.1016/j.ceb.2005.08.001
34. Giannelli G, Koudelkova P, Dituri F, Mikulits W. Role of epithelial to mesenchymal transition in hepatocellular carcinoma. J Hepatol. 2016;65:798–808. doi:10.1016/j.jhep.2016.05.007
35. Okazaki I, Wada N, Nakano M, et al. Difference in gene expression for matrix metalloproteinase-1 between early and advanced hepatocellular carcinomas. Hepatology. 1997;25:580–584. doi:10.1002/hep.510250315
36. Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi:10.1016/j.cell.2010.03.015
37. Ma F, Zhang L, Li M, Zhang Y, Zhang J, Guo B. Mir-361-5p inhibits glycolytic metabolism, proliferation and invasion of breast cancer by targeting FGFR1 and MMP-1. J Exp Clin Cancer Res. 2017;36:158. doi:10.1186/s13046-017-0630-1
38. Ma H, Cai H, Zhang Y, et al. Membrane palmitoylated protein 3 promotes hepatocellular carcinoma cell migration and invasion via up-regulating matrix metalloproteinase 1. Cancer Lett. 2014;344:74–81. doi:10.1016/j.canlet.2013.10.017
39. Kim E, Kim D, Lee J-S, et al. Capicua suppresses hepatocellular carcinoma progression by controlling the ETV4-MMP-1 axis. Hepatology. 2018;67:2287–2301. doi:10.1002/hep.29738
40. Sauter W, Rosenberger A, Beckmann L, et al. Matrix metalloproteinase 1 (MMP-1) is associated with early-onset lung cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:1127–1135. doi:10.1158/1055-9965.EPI-07-2840
41. Saito R, Miki Y, Ishida N, et al. The significance of MMP-1 in EGFR-TKI-resistant lung adenocarcinoma: potential for therapeutic targeting. Int J Mol Sci. 2018;19:609. doi:10.3390/ijms.19020609
42. Lee Y-S, Kim S-Y, Ko E, et al. Exosomes derived from palmitic acid-treated hepatocytes induce fibrotic activation of hepatic stellate cells. Sci Rep. 2017;7:3710. doi:10.1038/s41598-017-03389-2
43. Hohenester S, Christiansen S, Nagel J, et al. Lifestyle intervention for morbid obesity: effects on liver steatosis, inflammation and fibrosis. Am J Physiol Gastrointest Liver Physiol. 2018;315:G329-G338. doi:10.1152/ajpgi.00044.2018
44. Balbin M, Fueyo A, Knauper V, et al. Identification and enzymatic characterization of two diverging murine counterparts of human interstitial collagenase (MMP-1) expressed at sites of embryo implantation. J Biol Chem. 2001;276:10253–10262. doi:10.1074/jbc.M009586200
45. Nevzorova YA, Boyer-Diaz Z, Cubero FJ, Gracia-Sancho J. Animal models for liver disease - a practical approach for translational research. J Hepatol. 2020. doi:10.1016/j.jhep.2020.04.011
46. Ouchi R, Togo S, Kimura M, et al. Modeling steatohepatitis in humans with pluripotent stem cell-derived organoids. Cell Metab. 2019;30:374–384.e6. doi:10.1016/j.cmet.2019.05.007
47. Kim K-H, Jung J-Y, Son ED, Shin DW, Noh M, Lee TR. miR-526b targets 3ʹUTR of MMP-1 mRNA. Exp Mol Med. 2015;47:e178. doi:10.38/emm.2015.52
48. Li W-D, Zhou D-M, Sun -L-L, et al. LncRNA WTAPP1 promotes migration and angiogenesis of endothelial progenitor cells via MMP-1 through microRNA 3,120 and AKT/P13K/autophagy pathways. Stem Cells. 2018. doi:10.1002/stem.2904
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.